Adapted Physical Activity Protocol for Lower Limb Functional and Strength Recovery in a Young Athlete with Cutaneous Melanoma: Feasibility and Efficacy during COVID-19 Pandemic
Abstract
:1. Introduction
2. Materials and Methods
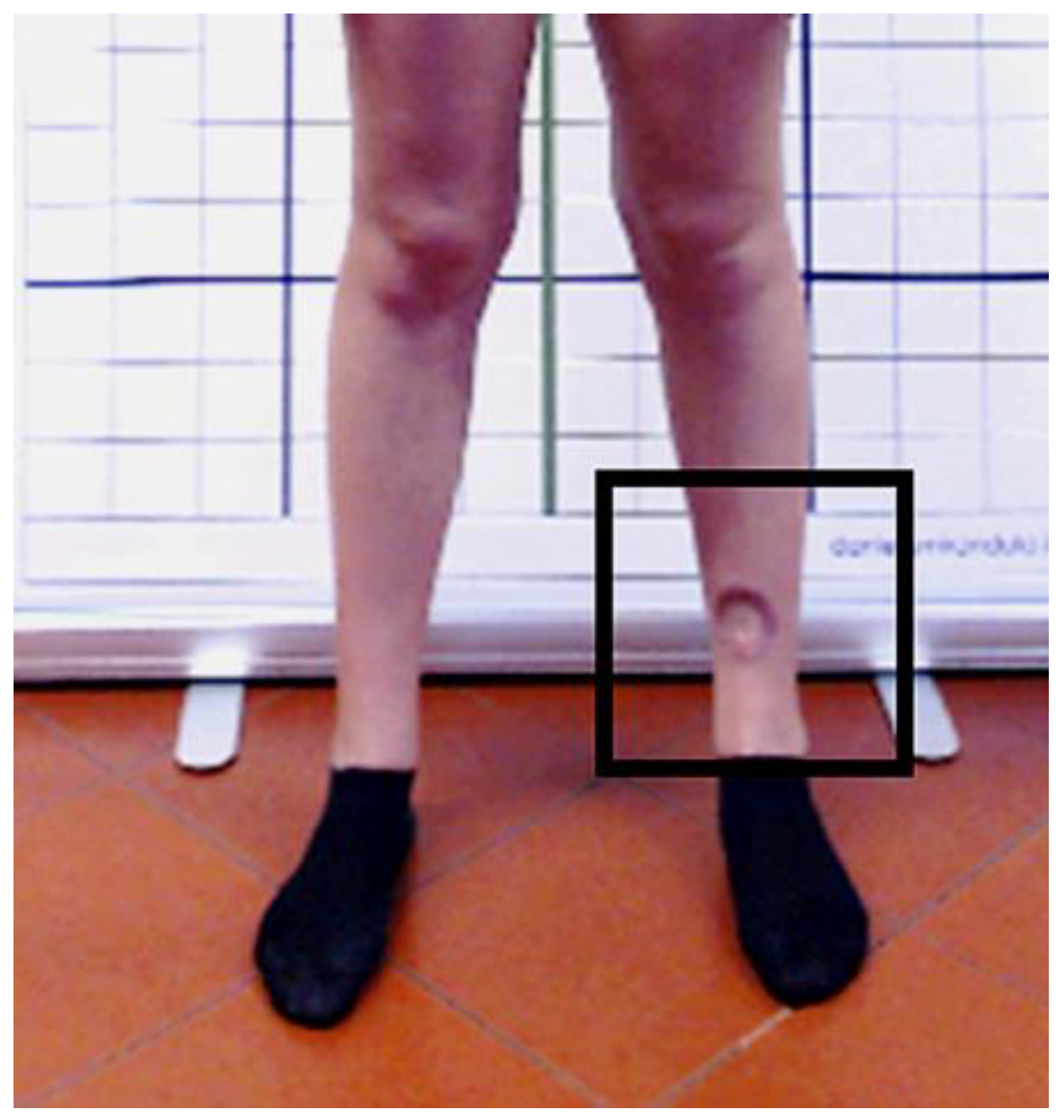
2.1. Case Description
2.2. Structured Physical Activity Protocol
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rösel, I.; Bauer, L.L.; Seiffer, B.; Deinhart, C.; Atrott, B.; Sudeck, G.; Hautzinger, M.; Wolf, S. The effect of exercise and affect regulation skills on mental health during the COVID-19 pandemic: A cross-sectional survey. Psychiatry Res. 2022, 312, 114559. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Overview of Public Health and Social Measures in the Context of COVID-19: Interim Guidance. Available online: https://www.who.int/publications/i/item/overview-of-public-health-and-social-measures-in-the-context-of-covid-19 (accessed on 30 May 2022).
- Brooks, S.K.; Webster, R.K.; Smith, L.E.; Woodland, L.; Wessely, S.; Greenberg, N.; Rubin, G.J. The psychological impact of quarantine and how to reduce it: Rapid review of the evidence. Lancet 2020, 395, 912–920. [Google Scholar] [CrossRef] [Green Version]
- Holmes, E.A.; O’Connor, R.C.; Perry, V.H.; Tracey, I.; Wessely, S.; Arseneault, L.; Ballard, C.; Christensen, H.; Cohen Silver, R.; Everall, I.; et al. Multidisciplinary research priorities for the COVID-19 pandemic: A call for action for mental health science. Lancet Psychiatry 2020, 7, 547–560. [Google Scholar] [CrossRef]
- Mattioli, A.V.; Sciomer, S.; Cocchi, C.; Maffei, S.; Gallina, S. Quarantine during COVID-19 outbreak: Changes in diet and physical activity increase the risk of cardiovascular disease. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1409–1417. [Google Scholar] [CrossRef]
- Meyer, J.; McDowell, C.; Lansing, J.; Brower, C.; Smith, L.; Tully, M.; Herring, M. Changes in Physical Activity and Sedentary Behavior in Response to COVID-19 and Their Associations with Mental Health in 3052 US Adults. Int. J. Environ. Res. Public Health 2020, 17, 6469. [Google Scholar] [CrossRef] [PubMed]
- Ammar, A.; Mueller, P.; Trabelsi, K.; Chtourou, H.; Boukhris, O.; Masmoudi, L.; Bouaziz, B.; Brach, M.; Schmicker, M.; Bentlage, E.; et al. Psychological consequences of COVID-19 home confinement: The ECLB-COVID19 multicenter study. PLoS ONE 2020, 15, e0240204. [Google Scholar] [CrossRef] [PubMed]
- Graupensperger, S.; Benson, A.J.; Kilmer, J.R.; Evans, M.B. Social (Un)distancing: Teammate interactions, athletic identity, and mental health of student-athletes during the COVID-19 pandemic. J. Adolesc. Health 2020, 67, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Sarto, F.; Impellizzeri, F.M.; Spörri, J.; Porcelli, S.; Olmo, J.; Requena, B.; Suarez-Arrones, L.; Arundale, A.; Bilsborough, J.; Buchheit, M.; et al. Impact of potential physiological changes due to COVID-19 home confinement on athlete health protection in elite sports: A call for awareness in sports programming. Sports Med. 2020, 50, 1417–1419. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Garcia, E.; Chrysikou, E.; Kalea, A.Z. The interplay between housing environmental attributes and design exposures and psychoneuroimmunology profile-an exploratory review and analysis paper in the cancer survivors’ mental health morbidity context. Int. J. Environ. Res. Public Health 2021, 18, 1089. [Google Scholar]
- Vyas, A.; Babcock, Z.; Kogut, S. Impact of depression treatment on health-related quality of life among adults with cancer and depression: A population-level analysis. J. Cancer Surviv. 2017, 11, 624–633. [Google Scholar] [CrossRef] [Green Version]
- Keller, M.; Henrich, G. Illness-related distress: Does it mean the same for men and women? Gender aspects in cancer patients’ distress and adjustment. Acta Oncol. 1999, 38, 747–755. [Google Scholar] [PubMed]
- Pigozzi, E.; Tregnago, D.; Costa, L.; Insolda, J.; Turati, E.; Rimondini, M.; Donisi, V.; Madera, P.; Fiorica, F.; Giuliani, J.; et al. Psychological impact of COVID-19 pandemic on oncological patients: A survey in Northern Italy. PLoS ONE 2021, 16, e0248714. [Google Scholar] [CrossRef] [PubMed]
- Kneier, A.W. Coping with melanoma—Ten strategies that promote psychological adjustment. Surg. Clin. N. Am. 2003, 83, 17–30. [Google Scholar] [CrossRef]
- Lichtenthal, W.G.; Cruess, D.G.; Clark, V.L.; Ming, M.E. Investment in body image among patients diagnosed with or at risk for malignant melanoma. Body Image 2005, 2, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Stamataki, Z.; Brunton, L.; Lorigan, P.; Green, A.C.; Newton-Bishop, J.; Molassiotis, A. Assessing the impact of diagnosis and the related supportive care needs in patients with cutaneous melanoma. Support. Care Cancer 2015, 23, 779–789. [Google Scholar] [CrossRef]
- Brown, B.C.; McKenna, S.P.; Siddhi, K.; McGrouther, D.A.; Bayat, A. The hidden cost of skin scars: Quality of life after skin scarring. J. Plast. Reconstr. Aesthetic Surg. 2008, 61, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.B.; Askew, R.L.; Xing, Y.; Weaver, S.; Gershenwald, J.E.; Lee, J.E.; Royal, R.; Lucci, A.; Ross, M.I.; Cormier, J.N. Prospective assessment of postoperative complications and associated costs following inguinal lymph node dissection (ILND) in melanoma patients. Ann. Surg. Oncol. 2010, 17, 2764–2772. [Google Scholar] [CrossRef] [PubMed]
- Tonouchi, H.; Ohmori, Y.; Kobayashi, M.; Konishi, N.; Tanaka, K.; Mohri, Y.; Mizutani, H.; Kusunoki, M. Operative morbidity associated with groin dissections. Surg. Today 2004, 34, 413–418. [Google Scholar] [CrossRef]
- de Vries, M.; Vonkeman, W.G.; van Ginkel, R.J.; Hoekstra, H.J. Morbidity after inguinal sentinel lymph node biopsy and completion lymph node dissection in patients with cutaneous melanoma. Eur. J. Surg. Oncol. 2006, 32, 785–789. [Google Scholar] [CrossRef]
- Gjorup, C.A.; Groenvold, M.; Hendel, H.W.; Dahlstroem, K.; Drzewiecki, K.T.; Klausen, T.W.; Hölmich, L.R. Health-related quality of life in melanoma patients: Impact of melanoma-related limb lymphoedema. Eur. J. Cancer 2017, 85, 122–132. [Google Scholar] [CrossRef]
- Bordoni, B.; Zanier, E. Skin, fascias, and scars: Symptoms and systemic connections. J. Multidiscip. Healthc. 2013, 7, 11–24. [Google Scholar] [CrossRef] [Green Version]
- van der Wal, J. The architecture of the connective tissue in the musculoskeletal system-an often overlooked functional parameter as to proprioception in the locomotor apparatus. Int. J. Ther. Massage Bodyw. 2009, 2, 9–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willard, F.H.; Vleeming, A.; Schuenke, M.D.; Danneels, L.; Schleip, R. The thoracolumbar fascia: Anatomy, function and clinical considerations. J. Anat. 2012, 221, 507–536. [Google Scholar] [CrossRef] [Green Version]
- Skraba, J.S.; Greenwald, A.S. The role of the interosseous membrane on tibiofibular weightbearing. Foot Ankle 1984, 4, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Rowe, M.J.; Tracey, D.J.; Mahns, D.A.; Sahai, V.; Ivanusic, J.J. Mechanosensory perception: Are there contributions from bone-associated receptors? Clin. Exp. Pharmacol. Physiol. 2005, 32, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Henderson, L.A.; Stathis, A.; James, C.; Brown, R.; McDonald, S.; Macefield, V.G. Real-time imaging of cortical areas involved in the generation of increases in skin sympathetic nerve activity when viewing emotionally charged images. Neuroimage 2012, 62, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, B.; Zanier, E. Anatomic connections of the diaphragm: Influence of respiration on the body system. J. Multidiscip. Healthc. 2013, 6, 281–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warburton, D.E.R.; Bredin, S.S.D. Health benefits of physical activity: A systematic review of current systematic reviews. Curr. Opin. Cardiol. 2017, 32, 541–556. [Google Scholar] [CrossRef]
- Coletta, A.M.; Basen-Engquist, K.M.; Schmitz, K.H. Exercise across the cancer care continuum: Why it matters, how to implement it, and motivating patients to move. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 1–7. [Google Scholar] [CrossRef]
- Mirandola, D.; Miccinesi, G.; Muraca, M.G.; Belardi, S.; Giuggioli, R.; Sgambati, E.; Manetti, M.; Monaci, M.; Marini, M. Longitudinal assessment of the impact of adapted physical activity on upper limb disability and quality of life in breast cancer survivors from an Italian cohort. Support. Care Cancer 2018, 26, 329–332. [Google Scholar] [CrossRef]
- Carretti, G.; Mirandola, D.; Maestrini, F.; Sequi, L.; Germano, S.; Muraca, M.G.; Miccinesi, G.; Manetti, M.; Marini, M. Quality of life improvement in breast cancer survivors affected by upper limb lymphedema through a novel multiperspective physical activity methodology: A monocentric pilot study. Breast Cancer 2022, 29, 437–449. [Google Scholar] [CrossRef]
- Kapandji, A. The Physiology of the Joints: The Spinal Column, Pelvic Girdle and Head, 7th ed.; Handspring Publishing: London, UK, 2019. [Google Scholar]
- Kapandji, A. The Physiology of the Joints: The Upper Limb, 7th ed.; Handspring Publishing: London, UK, 2019. [Google Scholar]
- Kapandji, A. The Physiology of the Joints: The Lower Limb, 7th ed.; Handspring Publishing: London, UK, 2019. [Google Scholar]
- Karagiannopoulos, C.; Griech, S.; Leggin, B. Reliability and validity of the ActivForce Digital Dynamometer in assessing shoulder muscle force across different user experience levels. Int. J. Sports Phys. Ther. 2022, 17, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Mirandola, D.; Muraca, M.G.; Sgambati, E.; Manetti, M.; Marini, M. Role of a structured physical activity pathway in improving functional disability, pain and quality of life in a case of breast and gynecological cancer survivorship. J. Clin. Med. 2019, 8, 531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagge, A.S.L.; Wesslau, H.; Cizek, R.; Holmberg, C.J.; Moncrieff, M.; Katsarelias, D.; Carlander, A.; Bagge, R.O. Health-related quality of life using the FACT-M questionnaire in patients with malignant melanoma: A systematic review. Eur. J. Surg. Oncol. 2022, 48, 312–319. [Google Scholar] [CrossRef]
- Askew, R.L.; Xing, Y.; Palmer, J.L.; Cella, D.; Moye, L.A.; Cormier, J.N. Evaluating minimal important differences for the FACT-Melanoma quality of life questionnaire. Value Health 2009, 12, 1144–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornish, D.; Holterhues, C.; van de Poll-Franse, L.V.; Coebergh, J.W.; Nijsten, T. A systematic review of health-related quality of life in cutaneous melanoma. Ann. Oncol. 2009, 20, vi51–vi58. [Google Scholar] [CrossRef]
- Rishi, P.; Rishi, E.; Maitray, A.; Agarwal, A.; Nair, S.; Gopalakrishnan, S. Hospital anxiety and depression scale assessment of 100 patients before and after using low vision care: A prospective study in a tertiary eye-care setting. Indian J. Ophthalmol. 2017, 65, 1203–1208. [Google Scholar] [CrossRef]
- Toquero, P.; Blanco Fernández, C.; López Martí, M.P.; Hernández Marín, B.; Vera Cea, E.B.; Garrido García, A.; Méndez Carrascosa, E.; Bañón Torres, D.; Donnay Candil, O.; Ballesteros García, A.I.; et al. Emotional distress in cancer patients during the first wave of the COVID-19 pandemic. Front. Psychol. 2021, 12, 755965. [Google Scholar] [CrossRef] [PubMed]
- Mirandola, D.; Maestrini, F.; Carretti, G.; Manetti, M.; Marini, M. Effectiveness of an adapted physical activity protocol for upper extremity recovery and quality of life improvement in a case of seroma after breast cancer treatment. Int. J. Environ. Res. Public Health. 2020, 17, 7727. [Google Scholar] [CrossRef]
- Hyatt, A.; Drosdowsky, A.; Williams, N.; Paton, E.; Bennett, F.; Andersen, H.; Mathai, J.; Milne, D. Exercise behaviors and fatigue in patients receiving immunotherapy for advanced melanoma: A cross-sectional survey via social media. Integr. Cancer Ther. 2019, 18, 1534735419864431. [Google Scholar] [CrossRef] [Green Version]
- Douglass, J.; Graves, P.; Gordon, S. Self-care for management of secondary lymphedema: A systematic review. PLoS Negl. Trop. Dis. 2016, 10, e0004740. [Google Scholar] [CrossRef] [PubMed]
- Vitali, F.; Bisagno, E.; Coco, M.; Cadamuro, A.; Maldonato, N.M.; Di Corrado, D. A Moderated mediation analysis of the effects of the COVID-19 pandemic on well-being and sport readiness of Italian team sports players: The role of perceived safety of the training environment. Int. J. Environ. Res. Public Health 2022, 19, 2764. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, H.T.; Rubin, S.T.; Fitzgerald, D.A.; Rubin, B.K. COVID-19 and the impact on young athletes. Paediatr. Respir. Rev. 2021, 39, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Irvin, L.; Severin, R.; Ellis, B. Return-to-play considerations following a COVID-19 infection in elite athletes. J. Athl. Train. 2021, 56, 1061–1063. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.G.; Hull, J.H.; Rogers, J.; Pollock, N.; Dodd, M.; Haines, J.; Harris, S.; Loosemore, M.; Malhotra, A.; Pieles, G.; et al. Cardiorespiratory considerations for return-to-play in elite athletes after COVID-19 infection: A practical guide for sport and exercise medicine physicians. Br. J. Sports Med. 2020, 54, 1157–1161. [Google Scholar] [CrossRef]
- Erdmann, F.; Lortet-Tieulent, J.; Schüz, J.; Zeeb, H.; Greinert, R.; Breitbart, E.W.; Bray, F. International trends in the incidence of malignant melanoma 1953–2008—Are recent generations at higher or lower risk? Int. J. Cancer 2013, 132, 385–400. [Google Scholar] [CrossRef]
- Siegel, R.; DeSantis, C.; Virgo, K.; Stein, K.; Mariotto, A.; Smith, T.; Cooper, D.; Gansler, T.; Lerro, C.; Fedewa, S.; et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J. Clin. 2012, 62, 220–241. [Google Scholar] [CrossRef] [Green Version]






| Variables | Baseline | Post-APA |
|---|---|---|
| ROM trunk, degrees | ||
| Flexion | 38.6 | 44.7 |
| Extension | 17 | 17 |
| Left lateral inclination | 42.2 | 38.7 |
| Right lateral inclination | 30.4 | 31 |
| Left rotation | 55.4 | 59.4 |
| Right rotation | 56.9 | 64.3 |
| ROM right upper limb, degrees | ||
| Flexion | 152.5 | 163.8 |
| Extension | 59.5 | 50 |
| Abduction | 160.6 | 180.5 |
| Adduction | 45.5 | 51.7 |
| ROM left upper limb, degrees | ||
| Flexion | 183.6 | 182 |
| Extension | 48.6 | 66 |
| Abduction | 153.4 | 143.6 |
| Adduction | 40.4 | 60.8 |
| ROM right hip, degrees | ||
| Flexion | 86.8 | 95.3 |
| Extension | 43.5 | 41.8 |
| Abduction | 43.9 | 51.3 |
| Adduction | 25 | 27.3 |
| Internal rotation | 53.6 | 39.4 |
| External rotation | 35.9 | 39 |
| ROM left hip, degrees | ||
| Flexion | 100.4 | 115.5 |
| Extension | 41.4 | 32.5 |
| Abduction | 39.4 | 56.4 |
| Adduction | 37.8 | 39.1 |
| Internal rotation | 41.7 | 42.5 |
| External rotation | 44.4 | 55.6 |
| Variables | Baseline | Post-APA |
|---|---|---|
| Muscle strength, kilograms | ||
| Right hip flexion | 6.5 | 8.5 |
| Right hip extension | 7.1 | 15.5 |
| Right hip abduction | 4.9 | 7.6 |
| Right hip adduction | 6 | 9.9 |
| Right knee extension | 13.5 | 17.2 |
| Right ankle plantar flexion | 5.7 | 7 |
| Right ankle plantar dorsiflexion | 5.5 | 7 |
| Right ankle inversion | 3.4 | 6.6 |
| Right ankle eversion | 2.5 | 5.9 |
| Left hip flexion | 5.5 | 14.5 |
| Left hip extension | 7.8 | 14.4 |
| Left hip abduction | 5.2 | 11.1 |
| Left hip adduction | 5.8 | 9.4 |
| Left knee extension | 8.7 | 15.9 |
| Left ankle plantar flexion | 5.5 | 7.6 |
| Left ankle plantar dorsiflexion | 4.9 | 7.6 |
| Left ankle inversion | 4 | 7.2 |
| Left ankle eversion | 3.8 | 7.7 |
| Variables | Baseline | Post-APA | ||||
|---|---|---|---|---|---|---|
| Lower Limb Circumference, mm | Right | Left | Difference | Right | Left | Difference |
| Groin level | 680 | 680 | 0 | 640 | 645 | 0.5 |
| Midline of thigh | 550 | 560 | 1 | 535 | 540 | 0.5 |
| Knee | 430 | 430 | 0 | 425 | 435 | 1 |
| Half leg | 380 | 380 | 0 | 390 | 395 | 0.5 |
| Ankle | 240 | 260 | 2 | 240 | 245 | 0.5 |
| Midline of foot | 220 | 240 | 2 | 230 | 245 | 1.5 |
| Variables | Baseline | Post-APA |
|---|---|---|
| FACT-M | ||
| Physical wellbeing | 27 | 28 |
| Social/family wellbeing | 28 | 28 |
| Emotional wellbeing | 20 | 21 |
| Functional wellbeing | 21 | 21 |
| Melanoma subscale | 59 | 59 |
| FACT-M total | 155 | 157 |
| Melanoma surgery scale | 28 | 27 |
| Anxiety/depression status (HADS) | ||
| Anxiety | 2 | 2 |
| Depression | 2 | 6 |
| Distress thermometer | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carretti, G.; Mirandola, D.; Germano, S.; Manetti, M.; Marini, M. Adapted Physical Activity Protocol for Lower Limb Functional and Strength Recovery in a Young Athlete with Cutaneous Melanoma: Feasibility and Efficacy during COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2022, 19, 9590. https://doi.org/10.3390/ijerph19159590
Carretti G, Mirandola D, Germano S, Manetti M, Marini M. Adapted Physical Activity Protocol for Lower Limb Functional and Strength Recovery in a Young Athlete with Cutaneous Melanoma: Feasibility and Efficacy during COVID-19 Pandemic. International Journal of Environmental Research and Public Health. 2022; 19(15):9590. https://doi.org/10.3390/ijerph19159590
Chicago/Turabian StyleCarretti, Giuditta, Daniela Mirandola, Sara Germano, Mirko Manetti, and Mirca Marini. 2022. "Adapted Physical Activity Protocol for Lower Limb Functional and Strength Recovery in a Young Athlete with Cutaneous Melanoma: Feasibility and Efficacy during COVID-19 Pandemic" International Journal of Environmental Research and Public Health 19, no. 15: 9590. https://doi.org/10.3390/ijerph19159590
APA StyleCarretti, G., Mirandola, D., Germano, S., Manetti, M., & Marini, M. (2022). Adapted Physical Activity Protocol for Lower Limb Functional and Strength Recovery in a Young Athlete with Cutaneous Melanoma: Feasibility and Efficacy during COVID-19 Pandemic. International Journal of Environmental Research and Public Health, 19(15), 9590. https://doi.org/10.3390/ijerph19159590








