Manipulation under Anesthesia versus Non-Surgical Treatment for Patients with Frozen Shoulder Contracture Syndrome: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources and Searches
2.2. Study Selection Criteria
2.2.1. Population
2.2.2. Intervention and Comparator
2.2.3. Outcomes
2.2.4. Study Design
2.3. Data Extraction
2.4. Methodological Quality and Risk of Bias Assessment
2.5. Data Synthesis and Analysis
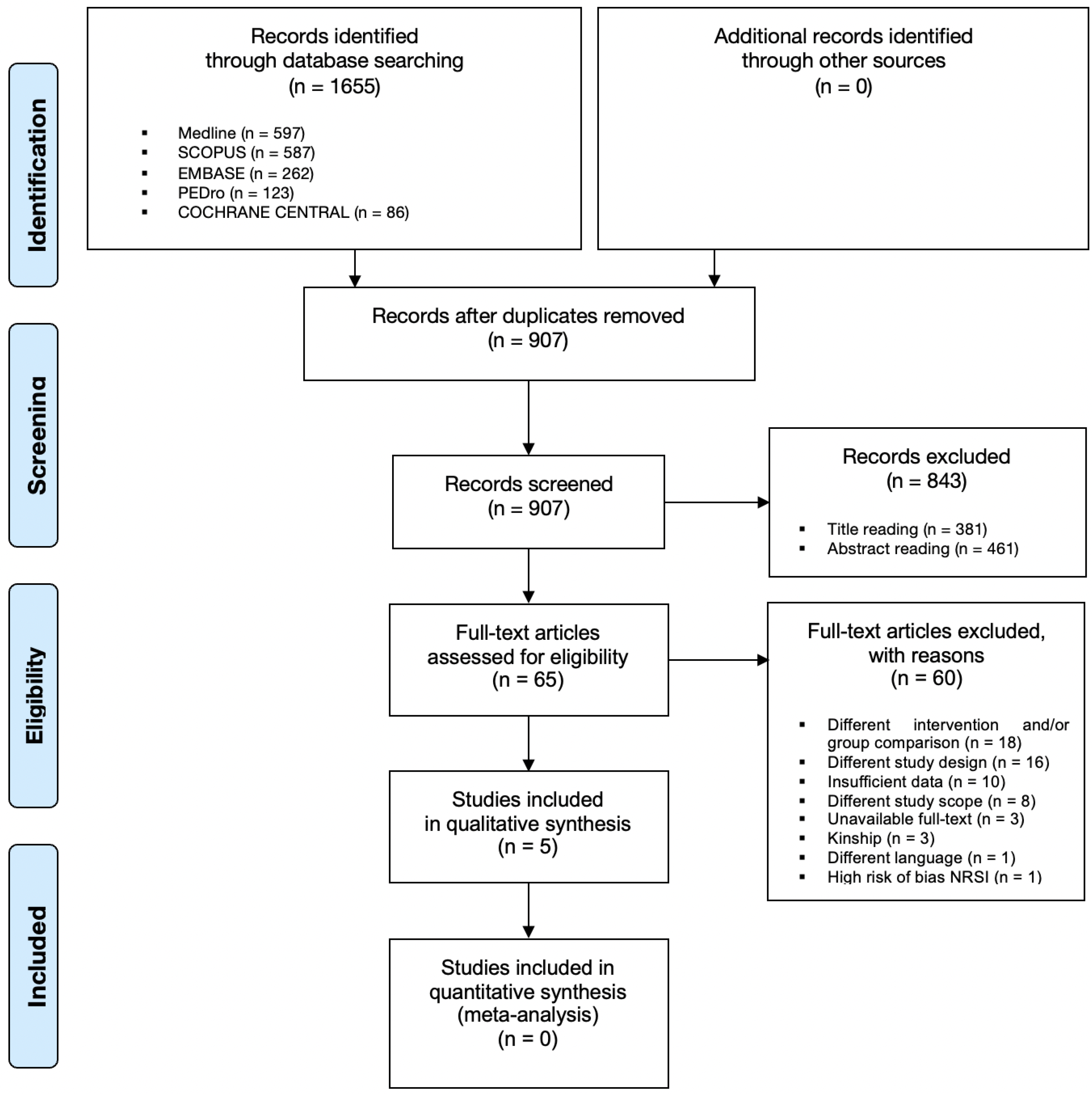
3. Results
3.1. Study Selection and Data Extraction
3.2. Methodological Quality and Risk of Bias Assessment
3.3. Summary of Evidence
3.3.1. Pain
3.3.2. Function
3.3.3. Range of Motion
3.3.4. Disability
3.3.5. Other Outcomes
3.3.6. Adverse Events
4. Discussion
4.1. Strengths and Limitations
4.2. Clinical Implications and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lewis, J. Frozen shoulder contracture syndrome—Aetiology, diagnosis and management. Man. Ther. 2015, 20, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, J.D.; Rokito, A. Frozen shoulder: A consensus definition. J. Shoulder Elb. Surg. 2011, 20, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Reeves, B. The natural history of the frozen shoulder syndrome. Scand. J. Rheumatol. 1975, 4, 193–196. [Google Scholar] [CrossRef]
- Vastamaki, H.; Kettunen, J.; Vastamaki, M. The natural history of idiopathic frozen shoulder: A 2- to 27-year followup study. Clin. Orthop. Relat. Res. 2012, 470, 1133–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hand, C.; Clipsham, K.; Rees, J.L.; Carr, A.J. Long-term outcome of frozen shoulder. J. Shoulder Elb. Surg. 2008, 17, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Hanchard, N.; Hamilton, S.; Rangan, A. A qualitative study of patients’ perceptions and priorities when living with primary frozen shoulder. BMJ Open 2013, 3, e003452. [Google Scholar] [CrossRef] [Green Version]
- Kelley, M.J.; Shaffer, M.A.; Kuhn, J.E.; Michener, L.A.; Seitz, A.L.; Uhl, T.L.; Godges, J.J.; McClure, P.W. Shoulder pain and mobility deficits: Adhesive capsulitis: Clinical practice guidelines linked to the international classification of functioning, disability, and health from the orthopaedic section of the american physical therapy association. J. Orthop. Sports Phys. Ther. 2013, 43, A1–A31. [Google Scholar] [CrossRef] [Green Version]
- Favejee, M.M.; Huisstede, B.M.; Koes, B.W. Frozen shoulder: The effectiveness of conservative and surgical interventions—Systematic review. Br. J. Sports Med. 2011, 45, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Lowe, C.M.; Barrett, E.; McCreesh, K.; De Burca, N.; Lewis, J. Clinical effectiveness of non-surgical interventions for primary frozen shoulder: A systematic review. J. Rehabil. Med. 2019, 51, 539–556. [Google Scholar] [CrossRef] [Green Version]
- Maund, E.; Craig, D.; Suekarran, S.; Neilson, A.; Wright, K.; Brealey, S.; Dennis, L.; Goodchild, L.; Hanchard, N.; Rangan, A.; et al. Management of frozen shoulder: A systematic review and cost-effectiveness analysis. Health Technol. Assess. 2012, 16, 1–264. [Google Scholar] [CrossRef] [Green Version]
- D’Orsi, G.M.; Via, A.G.; Frizziero, A.; Oliva, F. Treatment of adhesive capsulitis: A review. Ligaments Tendons J. 2012, 2, 70–78. [Google Scholar]
- Guyver, P.M.; Bruce, D.J.; Rees, J.L. Frozen shoulder—A stiff problem that requires a flexible approach. Maturitas 2014, 78, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shi, M.; Zhou, C.; Shi, Z.; Cai, X.; Lin, T.; Yan, S. Effectiveness of corticosteroid injections in adhesive capsulitis of shoulder: A meta-analysis. Medicine 2017, 96, e7529. [Google Scholar] [CrossRef] [PubMed]
- Buchbinder, R.; Green, S.; Youd, J.M. Corticosteroid injections for shoulder pain. Cochrane Database Syst. Rev. 2003, CD004016. [Google Scholar] [CrossRef]
- Kitridis, D.; Tsikopoulos, K.; Bisbinas, I.; Papaioannidou, P.; Givissis, P. Efficacy of Pharmacological Therapies for Adhesive Capsulitis of the Shoulder: A Systematic Review and Network Meta-analysis. Am. J. Sports Med. 2019, 47, 3552–3560. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Green, S.; Kramer, S.; Johnston, R.V.; McBain, B.; Chau, M.; Buchbinder, R. Manual therapy and exercise for adhesive capsulitis (frozen shoulder). Cochrane Database Syst. Rev. 2014, CD011275. [Google Scholar] [CrossRef]
- Kelley, M.J.; McClure, P.W.; Leggin, B.G. Frozen shoulder: Evidence and a proposed model guiding rehabilitation. J. Orthop. Sports Phys. Ther. 2009, 39, 135–148. [Google Scholar] [CrossRef] [Green Version]
- Mertens, M.G.; Meert, L.; Struyf, F.; Schwank, A.; Meeus, M. Exercise Therapy Is Effective for Improvement in Range of Motion, Function, and Pain in Patients with Frozen Shoulder: A Systematic Review and Meta-analysis. Arch. Phys. Med. Rehabil. 2022, 103, 998–1012.E14. [Google Scholar] [CrossRef]
- Buchbinder, R.; Green, S.; Youd, J.M.; Johnston, R.V.; Cumpston, M. Arthrographic distension for adhesive capsulitis (frozen shoulder). Cochrane Database Syst. Rev. 2008, CD007005. [Google Scholar] [CrossRef]
- Saltychev, M.; Laimi, K.; Virolainen, P.; Fredericson, M. Effectiveness of Hydrodilatation in Adhesive Capsulitis of Shoulder: A Systematic Review and Meta-Analysis. Scand. J. Surg. 2018, 107, 285–293. [Google Scholar] [CrossRef]
- Farrell, C.M.; Sperling, J.W.; Cofield, R.H. Manipulation for frozen shoulder: Long-term results. J. Shoulder Elb. Surg. 2005, 14, 480–484. [Google Scholar] [CrossRef]
- Vastamaki, H.; Vastamaki, M. Motion and pain relief remain 23 years after manipulation under anesthesia for frozen shoulder. Clin. Orthop. Relat. Res. 2013, 471, 1245–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsubheen, S.A.; Nazari, G.; Bobos, P.; MacDermid, J.C.; Overend, T.J.; Faber, K. Effectiveness of Nonsurgical Interventions for Managing Adhesive Capsulitis in Patients with Diabetes: A Systematic Review. Arch. Phys. Med. Rehabil. 2019, 100, 350–365. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, B.; Lavoie-Gagne, O.; Patel, B.H.; Lu, Y.; Ritz, E.; Chahla, J.; Okoroha, K.R.; Allen, A.A.; Nwachukwu, B.U. Efficacy of Arthroscopic Surgery in the Management of Adhesive Capsulitis: A Systematic Review and Network Meta-analysis of Randomized Controlled Trials. Arthroscopy 2021, 37, 2281–2297. [Google Scholar] [CrossRef] [PubMed]
- Dodenhoff, R.M.; Levy, O.; Wilson, A.; Copeland, S.A. Manipulation under anesthesia for primary frozen shoulder: Effect on early recovery and return to activity. J. Shoulder Elb. Surg. 2000, 9, 23–26. [Google Scholar] [CrossRef]
- Dennis, L.A.; Brealey, S.D.; Rangan, A.; Rookmoneea, M.; Watson, J. Managing idiopathic frozen shoulder: A survey of health professionals’ current practice and research priorities. Shoulder Elb. 2010, 2, 294–300. [Google Scholar] [CrossRef]
- Grant, J.A.; Schroeder, N.; Miller, B.S.; Carpenter, J.E. Comparison of manipulation and arthroscopic capsular release for adhesive capsulitis: A systematic review. J. Shoulder Elb. Surg. 2013, 22, 1135–1145. [Google Scholar] [CrossRef]
- Kraal, T.; Beimers, L.; The, B.; Sierevelt, I.; van den Bekerom, M.; Eygendaal, D. Manipulation under anaesthesia for frozen shoulders: Outdated technique or well-established quick fix? EFORT Open Rev. 2019, 4, 98–109. [Google Scholar] [CrossRef]
- Amir-Us-Saqlain, H.; Zubairi, A.; Taufiq, I. Functional outcome of frozen shoulder after manipulation under anaesthesia. J. Pak. Med. Assoc. 2007, 57, 181–185. [Google Scholar]
- Sasanuma, H.; Sugimoto, H.; Kanaya, Y.; Iijima, Y.; Saito, T.; Saito, T.; Takeshita, K. Magnetic resonance imaging and short-term clinical results of severe frozen shoulder treated with manipulation under ultrasound-guided cervical nerve root block. J. Shoulder Elb. Surg. 2016, 25, e13–e20. [Google Scholar] [CrossRef]
- Kraal, T.; Visser, C.; Sierevelt, I.; Beimers, L. How to treat a frozen shoulder? A survey among shoulder specialists in the Netherlands and Belgium. Acta Orthop. Belg. 2016, 82, 78–84. [Google Scholar] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2021. [Google Scholar]
- Reeves, B.C.; Deeks, J.J.; Higgins, J.P.T.; Shea, B.; Tugwell, P.; Wells, G.A. Including non-randomized studies on intervention effects. In Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Higgins, J.P.; Ramsay, C.; Reeves, B.C.; Deeks, J.J.; Shea, B.; Valentine, J.C.; Tugwell, P.; Wells, G. Issues relating to study design and risk of bias when including non-randomized studies in systematic reviews on the effects of interventions. Res. Synth. Methods 2013, 4, 12–25. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [Green Version]
- Altman, D.G. Practical Statistics for Medical Research, 1st ed.; Hall, C.A., Ed.; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2020, 12, 55–61. [Google Scholar] [CrossRef]
- Thomas, D.; Williams, R.A.; Smith, D.S. The frozen shoulder: A review of manipulative treatment. Rheumatol. Rehabil. 1980, 19, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Kivimaki, J.; Pohjolainen, T.; Malmivaara, A.; Kannisto, M.; Guillaume, J.; Seitsalo, S.; Nissinen, M. Manipulation under anesthesia with home exercises versus home exercises alone in the treatment of frozen shoulder: A randomized, controlled trial with 125 patients. J. Shoulder Elb. Surg. 2007, 16, 722–726. [Google Scholar] [CrossRef]
- Quraishi, N.A.; Johnston, P.; Bayer, J.; Crowe, M.; Chakrabarti, A.J. Thawing the frozen shoulder. A randomised trial comparing manipulation under anaesthesia with hydrodilatation. J. Bone Jt. Surg. 2007, 89, 1197–1200. [Google Scholar] [CrossRef]
- Jacobs, L.G.; Smith, M.G.; Khan, S.A.; Smith, K.; Joshi, M. Manipulation or intra-articular steroids in the management of adhesive capsulitis of the shoulder? A prospective randomized trial. J. Shoulder Elb. Surg. 2009, 18, 348–353. [Google Scholar] [CrossRef]
- Rangan, A.; Brealey, S.D.; Keding, A.; Corbacho, B.; Northgraves, M.; Kottam, L.; Goodchild, L.; Srikesavan, C.; Rex, S.; Charalambous, C.P.; et al. Management of adults with primary frozen shoulder in secondary care (UK FROST): A multicentre, pragmatic, three-arm, superiority randomised clinical trial. Lancet 2020, 396, 977–989. [Google Scholar] [CrossRef]
- Paul, A.; Lewis, M.; Shadforth, M.F.; Croft, P.R.; Van Der Windt, D.A.; Hay, E.M. A comparison of four shoulder-specific questionnaires in primary care. Ann. Rheum. Dis. 2004, 63, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.P.; Turner, J.A.; Romano, J.M.; Fisher, L.D. Comparative reliability and validity of chronic pain intensity measures. Pain 1999, 83, 157–162. [Google Scholar] [CrossRef]
- Dawson, J.; Fitzpatrick, R.; Carr, A. Questionnaire on the perceptions of patients about shoulder surgery. J. Bone Jt. Surg. 1996, 78, 593–600. [Google Scholar] [CrossRef] [Green Version]
- Dawson, J.; Rogers, K.; Fitzpatrick, R.; Carr, A. The Oxford shoulder score revisited. Arch. Orthop. Trauma. Surg. 2009, 129, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Jones, I.A.; Togashi, R.; Heckmann, N.; Vangsness, C.T., Jr. Minimal clinically important difference (MCID) for patient-reported shoulder outcomes. J. Shoulder Elb. Surg. 2020, 29, 1484–1492. [Google Scholar] [CrossRef]
- Constant, C.R.; Murley, A.H. A clinical method of functional assessment of the shoulder. Clin. Orthop. Relat. Res. 1987, 160–164. [Google Scholar] [CrossRef]
- Green, S.; Buchbinder, R.; Glazier, R.; Forbes, A. Systematic review of randomised controlled trials of interventions for painful shoulder: Selection criteria, outcome assessment, and efficacy. BMJ 1998, 316, 354–360. [Google Scholar] [CrossRef] [Green Version]
- Beaton, D.E.; Wright, J.G.; Katz, J.N.; Upper Extremity Collaborative, G. Development of the QuickDASH: Comparison of three item-reduction approaches. J. Bone Jt. Surg. 2005, 87, 1038–1046. [Google Scholar] [CrossRef]
- Herdman, M.; Gudex, C.; Lloyd, A.; Janssen, M.; Kind, P.; Parkin, D.; Bonsel, G.; Badia, X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual. Life Res. 2011, 20, 1727–1736. [Google Scholar] [CrossRef] [Green Version]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Nazareth, I.; Petersen, I. Observational studies of treatment effectiveness: Worthwhile or worthless? Clin. Epidemiol. 2019, 11, 35–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rex, S.S.; Kottam, L.; McDaid, C.; Brealey, S.; Dias, J.; Hewitt, C.E.; Keding, A.; Lamb, S.E.; Wright, K.; Rangan, A. Effectiveness of interventions for the management of primary frozen shoulder: A systematic review of randomized trials. Bone Jt. Open 2021, 2, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; O’Connor, D.A.; Malek, M.; Haas, R.; Beaton, D.; Huang, H.; Ramiro, S.; Richards, P.; Voshaar, M.J.H.; Shea, B.; et al. Patients’ experience of shoulder disorders: A systematic review of qualitative studies for the OMERACT Shoulder Core Domain Set. Rheumatology 2019, 58, 1410–1421. [Google Scholar] [CrossRef]
- Roy, J.S.; MacDermid, J.C.; Woodhouse, L.J. A systematic review of the psychometric properties of the Constant-Murley score. J. Shoulder Elb. Surg. 2010, 19, 157–164. [Google Scholar] [CrossRef]
- Vrotsou, K.; Avila, M.; Machon, M.; Mateo-Abad, M.; Pardo, Y.; Garin, O.; Zaror, C.; Gonzalez, N.; Escobar, A.; Cuellar, R. Constant-Murley Score: Systematic review and standardized evaluation in different shoulder pathologies. Qual. Life Res. 2018, 27, 2217–2226. [Google Scholar] [CrossRef] [Green Version]
- Williams, J.W., Jr.; Holleman, D.R., Jr.; Simel, D.L. Measuring shoulder function with the Shoulder Pain and Disability Index. J. Rheumatol. 1995, 22, 727–732. [Google Scholar]
- Dixon, D.; Johnston, M.; McQueen, M.; Court-Brown, C. The Disabilities of the Arm, Shoulder and Hand Questionnaire (DASH) can measure the impairment, activity limitations and participation restriction constructs from the International Classification of Functioning, Disability and Health (ICF). BMC Musculoskelet. Disord. 2008, 9, 114. [Google Scholar] [CrossRef] [Green Version]
- Tveita, E.K.; Ekeberg, O.M.; Juel, N.G.; Bautz-Holter, E. Responsiveness of the shoulder pain and disability index in patients with adhesive capsulitis. BMC Musculoskelet. Disord. 2008, 9, 161. [Google Scholar] [CrossRef] [Green Version]
- Rodgers, S.; Brealey, S.; Jefferson, L.; McDaid, C.; Maund, E.; Hanchard, N.; Goodchild, L.; Spencer, S. Exploring the outcomes in studies of primary frozen shoulder: Is there a need for a core outcome set? Qual. Life Res. 2014, 23, 2495–2504. [Google Scholar] [CrossRef]
- Hush, J.M.; Cameron, K.; Mackey, M. Patient satisfaction with musculoskeletal physical therapy care: A systematic review. Phys. Ther. 2011, 91, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Ramiro, S.; Page, M.J.; Whittle, S.L.; Huang, H.; Verhagen, A.P.; Beaton, D.E.; Richards, P.; Voshaar, M.; Shea, B.; van der Windt, D.A.; et al. The OMERACT Core Domain Set for Clinical Trials of Shoulder Disorders. J. Rheumatol. 2019, 46, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Woods, D.A.; Loganathan, K. Recurrence of frozen shoulder after manipulation under anaesthetic (MUA): The results of repeating the MUA. Bone Jt. J. 2017, 99, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Hanchard, N.C.; Goodchild, L.; Thompson, J.; O’Brien, T.; Davison, D.; Richardson, C. Evidence-based clinical guidelines for the diagnosis, assessment and physiotherapy management of contracted (frozen) shoulder: Quick reference summary. Physiotherapy 2012, 98, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Sugimoto, H.; Sasanuma, H.; Iijima, Y.; Kanaya, Y.; Fukushima, T.; Watanabe, H.; Kikkawa, I.; Takeshita, K. The course and clinical impact of articular magnetic resonance imaging findings 6 months after shoulder manipulation under ultrasound-guided cervical nerve root block for frozen shoulder. JSES Open Access 2019, 3, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Diercks, R.L.; Stevens, M. Gentle thawing of the frozen shoulder: A prospective study of supervised neglect versus intensive physical therapy in seventy-seven patients with frozen shoulder syndrome followed up for two years. J. Shoulder Elb. Surg. 2004, 13, 499–502. [Google Scholar] [CrossRef]
- Ko, Y.W.; Park, J.H.; Youn, S.M.; Rhee, Y.G.; Rhee, S.M. Effects of comorbidities on the outcomes of manipulation under anesthesia for primary stiff shoulder. J. Shoulder Elb. Surg. 2021, 30, e482–e492. [Google Scholar] [CrossRef]
- Kim, D.H.; Song, K.S.; Min, B.W.; Bae, K.C.; Lim, Y.J.; Cho, C.H. Early Clinical Outcomes of Manipulation under Anesthesia for Refractory Adhesive Capsulitis: Comparison with Arthroscopic Capsular Release. Clin. Orthop. Surg. 2020, 12, 217–223. [Google Scholar] [CrossRef]
- Flannery, O.; Mullett, H.; Colville, J. Adhesive shoulder capsulitis: Does the timing of manipulation influence outcome? Acta Orthop. Belg. 2007, 73, 21–25. [Google Scholar]
- Thomas, W.J.; Jenkins, E.F.; Owen, J.M.; Sangster, M.J.; Kirubanandan, R.; Beynon, C.; Woods, D.A. Treatment of frozen shoulder by manipulation under anaesthetic and injection: Does the timing of treatment affect the outcome? J. Bone Jt. Surg. 2011, 93, 1377–1381. [Google Scholar] [CrossRef] [Green Version]
- Kwaees, T.A.; Charalambous, C.P. Surgical and non-surgical treatment of frozen shoulder. Survey on surgeons treatment preferences. Muscles Ligaments Tendons J. 2014, 4, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Hanchard, N.C.A.; Goodchild, L.; Brealey, S.D.; Lamb, S.E.; Rangan, A. Physiotherapy for primary frozen shoulder in secondary care: Developing and implementing stand-alone and post operative protocols for UK FROST and inferences for wider practice. Physiotherapy 2020, 107, 150–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Ding, H.; Tang, Y.; Xue, Y.; Yang, Z.; Li, Z.; He, D.; Zhao, Y.; Zong, Y. A report on the prevalence of depression and anxiety in patients with frozen shoulder and their relations to disease status. Psychol. Health Med. 2014, 19, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Brindisino, F.; Silvestri, E.; Gallo, C.; Venturin, D.; Di Giacomo, G.; Peebles, A.M.; Provencher, M.T.; Innocenti, T. Depression and Anxiety Are Associated with Worse Subjective and Functional Baseline Scores in Patients with Frozen Shoulder Contracture Syndrome: A Systematic Review. Arthrosc. Sports Med. Rehabil. 2022, 4, e1219–e1234. [Google Scholar] [CrossRef]

| PICO | Features |
|---|---|
| Population | Patient suffering from frozen shoulder contracture syndrome (also called adhesive capsulitis) |
| Intervention | MUA (manipulation under anesthesia) |
| Comparator | Conservative treatment strategies (e.g., physical therapy, exercise, manual therapy, injection) |
| Outcome | Measures of pain, mobility, function, disability and quality of life |
| Author | Participants (n) Inclusion/Exclusion Criteria | Imaging Other Exams | Group of Intervention (GI) | Group of Control (GC) | Outcome Measures (S) Time of Follow-Up | Key Results |
|---|---|---|---|---|---|---|
| Thomas et al. [40] | 30 (17 F, 15 M) Mean age (na) Inclusion criteria:
Exclusion criteria:
| X-ray (shoulder) X-ray (cervical spine) X-ray (thoracic spine) Blood picture, erythrocyte sedimentation rate, serum uric acid, random blood sugar | (na) Mean age 59.3 years (45–76) Mean symptom duration 7.4 months (2–24 months) MUA GROUP MUA with short general anesthesia (20 mg of intravenous valium and injection of 50 mg of hydrocortisone acetate, sub-acromial postero-lateral approach). Patients invited to perform exercises to maintain the improvement (self-induced passive stretching) and to manage residual pain with analgesics. | (na) Mean age 57.9 years (38–73) Mean symptom duration 7.4 months (2–24 months) SJ GROUP Short general anesthesia (20 mg of intravenous valium and injection of 50 mg of hydrocortisone acetate, sub-acromial postero-lateral approach). Patients invited to perform exercises to maintain the improvement (self-induced passive stretching). | Pain 4-point scale † (0–3) 4 weeks 3 months | Pain (day) At 3 months (n = 19) GI: n = 12 (80%) GC: n = 7 (47%) Pain (night) At 3 months (n = 19) GI: n = 12 (80%) GC: n = 7 (47%) |
| ROM active 5-point scale # (0–4) 4 weeks 3 months | At 4 weeks, good response (n = 2) GI: n = 2 GC: n = 0 At 3 months, substantial recovery for 80% of FE and ABD (n = 8) GI: n = 6 (40%) GC: n = 2 (13%) | |||||
| Disability 4-point scale † (0–3) 4 weeks 3 months | At 4 weeks, recovered (n = 2) GI: n = 2 GC: n = 0 At 3 months, completely recovered (n = 9) GI: n = 7 (47%) GC: n = 2 (13%) | |||||
| Kivimäki et al. [41] | 125 (na) Mean age (na) Inclusion criteria:
Exclusion criteria:
| US (rotator cuff) | 65 Mean age 53.0 years (SD = 8.4) Mean symptom duration 7.4 months (SD = 0.3) MUA GROUP MUA with short general anesthesia. The patients received advice in 2 sessions and written instructions for a daily training program (pendulum exercises for the arm and stretching techniques for the shoulder joint) from a trained physical therapist. | 65 Mean age 53.0 years (SD = 8.3) Mean symptom duration 7.0 months (SD = 0.3) HE GROUP Home exercise program. The patients received advice in 2 sessions and written instructions for a daily training program (pendulum exercises for the arm and stretching techniques for the shoulder joint) from a trained physical therapist. | Pain VAS ‡ (0–10) 6 weeks 3 months 6 months 1 year | At 6 weeks (4.9 GI vs. 4.7 GC) MD = 0.2 95% CI (−0.64 to 1.02) At 3 months (3.9 GI vs. 3.7 GC) MD = 0.2 95% CI (−1.06 to 1.10) At 6 months (2.0 GI vs. 2.8 GC) MD = −0.8 95% CI (−1.8 to 0.2) At 1 year (1.5 GI vs. 2.2 GC) MD = −0.7 95% CI (−1.8 to 0.4) |
| ROM * passive goniometer § (°) 6 weeks 3 months 6 months 1 year | Measures of FE: At 6 weeks (133° GI vs. 129° GC) MD = 4° 95% CI (−3.8° to 11.8°) At 3 months (144° GI vs. 136° GC) MD = 8° (0° to 16°); p <0.05 At 6 months (151° GI vs. 146° GC) MD = 5° (−5° to 15°) At 1 year (157° GI vs. 154° GC) MD = 3° (−5° to 11°) Measures of ABD: At 6 weeks (125° GI vs. 112° GC) MD = 10° 95% CI (−3.2° to 23.2°) At 3 months (150° GI vs. 141° GC) MD = 9° (−6 to 24) At 6 months (151° GI vs. 142° GC) MD = 9° (−4° to 22°) At 1 year (161° GI vs. 154° GC) MD = 7° (−5° to 19°) Measures of IR: At 6 weeks (30° GI vs. 34° GC) MD = 4° 95% CI (−1° to 9°) At 3 months (22° GI vs. 25° GC) MD = −3° (−7.4° to 2.4°) At 6 months (16° GI vs. 18° GC) MD = −2° (−7.4° to 3.4°) At 1 year (11° GI vs. 12° GC) MD = −1° (−4.1° to 6.1°) Measures of ER: At 6 weeks (38° GI vs. 33° GC) MD = 5° 95% CI (−2° to 12°) At 3 months (48° GI vs. 42° GC) MD = 6° (−3° to 15°) At 6 months (59° GI vs. 53° GC) MD = 6° (−2° to 14°) At 1 year (65° GI vs. 61° GC) MD = 4° (−4.2° to 12.2°) | |||||
| Disability Modified SDQ †† (0–28) 6 weeks 3 months 6 months 1 year Working ability ‡‡ (0–10) 6 weeks 3 months 6 months 1 year | Disability (SDQ) At 6 weeks (18.9 GI vs. 19.2 GC) MD = −0.3 95% CI (−2.3 to 1.7) At 3 months (14.5 GI vs. 14.2 GC) MD = 0.3 95% CI (−2.69 to 2.75) At 6 months (9.6 GI vs. 11.3 GC) MD = −1.7 95% CI (−5.3 to 1.9) At 1 year (6.6 GI vs. 6.6 GC) MD = 0 95% CI (−3.2 to 3.2) Disability (Working ability) At 6 weeks SDQ (6.6 GI vs. 6.2 GC) MD = −0.4 95% CI (−4.2 to 1.28) At 3 months SDQ (7.1 GI vs. 7.1 GC) MD = 0 95% CI (−0.8 to 0.8) At 6 months SDQ (7.8 GI vs. 7.3 GC) MD = 0.5 95% CI (−0.6 to 1.6) At 1 year SDQ (8.3 GI vs. 8.2 GC) MD = 0.1 95% CI (−0.8 to 1.0) | |||||
| Quraishi et al. [42] | 36 (21 F, 15 M) Mean age 55.2 y (39–70) Inclusion criteria:
Exclusion criteria:
| X-ray (shoulder) | 17 Mean age 54.5 years (39–69) Mean symptom duration 39.8 weeks MUA GROUP MUA with specific protocol and local anesthesia. Protocol to resume normal activities as soon as possible, home self-exercise program (pendular exercises and wall-climbing movements). | 19 Mean age 55.2 years (44–70) Mean symptom duration 37.4 weeks HD GROUP Hydrodilatation by a consultant radiologist (anterior approach with radio-opaque contrast material and normal saline solution, 10 to 55 mL). Protocol to resume normal activities as soon as possible, home self-exercise program (pendular exercises and wall-climbing movements). | Pain VAS ‡ (0–10) 8 weeks 6 months | At 8 weeks GI: 4.7 95% CI (0.0 to 8.5) GC: 2.4 95% CI (0.0 to 8.0) At 6 months GI: 2.7 95% CI (0.0 to 9.0) GC: 1.7 95% CI (0.0 to 7.0) Between-group difference, p < 0.0001 in favor of hydrodilatation group compared to MUA group. |
| ROM na (°) 8 weeks 6 months | Between-group difference in favor of hydrodilatation group compared to MUA group ABD: p < 0.0005 FE: p < 0.0004 IR: p = 0.02 ER: p = 0.004 | |||||
| Function * CS ** (0–100) 8 weeks 6 months | At 8 weeks GI: 58.5 95% CI (24 to 90) GC: 57.4 95% CI (17 to 80) At 6 months GI: 59.5 95% CI (23 to 85) GC: 65.9 95% CI (28 to 92) Between-group difference, p = 0.02 in favor of hydrodilatation group compared to MUA group. | |||||
| Satisfaction level Modified Likert §§ (0–2) 6 months | At 6 months, satisfied or very satisfied GI: 81% (n = 13) GC: 94% (n = 17) | |||||
| Jacobs et al. [43] | 53 (35 F, 18 M) Mean age (na) (40–75) Inclusion criteria:
| X-ray (shoulder) | 28 (15 F, 13 M) Mean age 56.5 years Mean symptom duration 19 weeks MUA GROUP MUA with general anesthesia (day-surgery treatment). Detailed brochure with home exercise delivered by physical therapists (unavailable description of dosage or type of provided home exercise program). | 25 (20 F, 5 M) Mean age 57.0 years Mean symptom duration 16 weeks SJHD GROUP Injection with steroid and capsular distension, 3 treatments at 6-week intervals (40 mg of triamcinolone in 1 mL, 5 mL of 2% lignocaine, 10 mL of 0.25% bupivacaine and 5 mL of air, posterior route). Detailed brochure with home exercise delivered by physical therapists (unavailable description of dosage or type of provided home exercise program). | Pain VAS (0–100) 2 weeks 6 weeks 3 months 6 months 9 months 1 year | Main outcome measures subjected to regression analysis on the first 4 time points (change occurred in the first 16 weeks) and regression coefficients (of time) were compared, with no significant difference between treatment groups (95% CI (−1.11 to 1.15) GI: −2.77 (SE = 0.33) GC: −2.75 (SE = 0.42) |
| Function CS ** (0–100) 2 weeks 6 weeks 3 months 6 months 9 months 1 year | Main outcome measures subjected to regression analysis on the first 4 time points (change occurred in the first 16 weeks) and regression coefficients (of time) were compared, with no significant difference between treatment groups (95% CI (−0.90 to 1.11) GI: 3.13 (SE = 0.24) GC: 3.23 (SE = 0.42) | |||||
| Quality of life SF-36 ## (0–100) 1 year | All components of the SF-36 scores improved for all patients, but no statistically significant difference was found between groups. | |||||
| Rangan et al. [44] | 300 (na) Mean age (na) Inclusion criteria:
| X-ray (shoulder) | 201 (129 F, 72 M) Mean age 54.5 years (SD = 7.7) Mean symptom duration 10.5 months (SD = 8.6) MUA GROUP MUA with general anesthesia (day-surgery treatment). Provided post-surgery analgesia procedures (including nerve block procedures as per usual care). Intra-articular steroid injection of corticosteroid (with or without imaging guidance depending on usual practice of hospital site). Multiple (n = 12) sessions of structured physiotherapy over 12 w within 24 h: (a) focused physiotherapy (information leaflet containing education, advice on pain management and function, “hands-on” mobilization techniques, instruction on a graduated home exercise program progressing from gentle pendular exercises to firm stretching exercises); (b) supplemental physiotherapy (not essential intervention, but considered as permissible addition to allow some physiotherapists flexibility); (c) intra-articular steroid injection of corticosteroid. Treatment features were selected according to potential different stages of FSCS, as stated in systematic reviews, UK guidelines and previous surveys of UK physiotherapists and Delphi consensus methodology. | 99 (64 F, 35 M) Mean age 54.5 years (SD = 7.8) Mean symptom duration 10.8 months (SD = 8.8) SPT GROUP Multiple (n = 12) sessions of structured physiotherapy over 12 w: (a) focused physiotherapy (information leaflet containing education, advice on pain management and function, “hands-on” mobilization techniques, instruction on a graduated home exercise program progressing from gentle pendular exercises to firm stretching exercises); (b) supplemental physiotherapy (not essential intervention, but considered as permissible addition to allow some physiotherapists flexibility); (c) intra-articular steroid injection of corticosteroid. Treatment features were selected according to potential different stages of FSCS, as stated in systematic reviews, UK guidelines and previous surveys of UK physiotherapists and Delphi consensus methodology. | Pain NRS ‡ (0–10) 3 months 6 months 9 months 1 year | At 3 months (4.1 GI vs. 3.7 GC) MD = 0.43 95% CI (−0.17 to 1.03); p = 016 At 6 months (2.8 GI vs. 3.0 GC) MD = −0.19 95% CI (−0.78 to 0.40); p = 0.53 At 1 year (2.4 GI vs. 2.5 GC) MD = −0.08 95% CI (−0.66 to 0.50); p = 0.78 |
| Function OSS *, *** (0–48) 3 months 6 months 9 months 1 year | At 3 months (30.2 GI vs. 31.6 GC) MD = −1.36 95% CI (−3.70 to 0.98); p = 0.25 At 6 months (37.1 GI vs. 34.9 GC) MD = 2.15 95% CI (−0.12 to 4.42); p = 0.064 At 1 year (38.3 GI vs. 37.2 GC) MD = 1.05 95% CI (−1.28 to 3.39); p = 0.38 Median overall OSS of 43 (out of 48) points, compared with an initial median overall OSS of 20 points for both groups. | |||||
| Disability QuickDASH ††† (0–100) 3 months 6 months 9 months 1 year | At 3 months (38.8 GI vs. 37.1 GC) MD = 1.77 95% CI (−3.41 to 6.96); p = 0.50 At 6 months (27.7 GI vs. 29.2 GC) MD = −3.55 95% CI (−8.68 to 1.58); p = 0.18 At 1 year (29.9 GI vs. 23.4 GC) MD = −0.50 95% CI (−5.70 to 4.70); p = 0.85 | |||||
| Quality of life EQ-5D-5L ### (0–5) 3 months 6 months 9 months 1 year | At 3 months (0.63 GI vs. 0.61 GC) MD = 0.03 95% CI (−0.03 to 0.08); p = 0.38 At 6 months (0.73 GI vs. 0.68 GC) MD = 0.05 95% CI (−0.01 to 0.10); p = 0.10 At 1 year (0.73 GI vs. 0.69 GC) MD = 0.04 95% CI (−0.02 to 0.10); p = 0.20 | |||||
| Extent of recovery VAS (0–100) §§§ 3 months 6 months 9 months 1 year | Extent of recovery At 3 months (51.54 GI vs. 53.9 GC) MD = −2.55 95% CI (−11.68 to 6.58); p = 0.58 At 6 months (31.9 GI vs. 38.6 GC) MD = −6.71 95% CI (−15.83 to 2.42); p = 0.15 At 1 year (27.3 GI vs. 26.9 GC) MD = 0.46 95% CI (−7.79 to 8.70); p = 0.91 | |||||
| Economic analysis QALYs Over 1 year | The base-case health economic analysis with multiple imputation showed that MUA was GBP 276.51 (95% CI 65.67 to 487.35) more expensive per participant than was early structured physiotherapy. MUA was the intervention most likely to be cost-effective at a threshold of GBP 20,000 per QALY (GI = 86%; GC = 14%). |
| Author | Type of Anesthesia | Involved Operator | Sequence of Manipulation | Additional Precautions | Adverse Events/Complications |
|---|---|---|---|---|---|
| Thomas et al. [40] | Short general (intravenous 20 mg Valium, 50 mg hydrocortisone acetate) | 1 | 90° ABD (forced) IR ER | (na) | n = 1 (GH dislocation) |
| Kivimäki et al. [41] | Short general | 1 | FE ABD IR (gm) ER (gm) | Supine patient Scapular stabilization | (na) |
| Quraishi et al. [42] | Local (2 mL al 2.00% lignocaine, 30 mg (0.75 mL) triamcinolone acetonide) | 1 | (na) | Short lever | (na) |
| Jacobs et al. [43] | General | 2 | ADD FE IR ER ABD | Supine patient Scapular stabilization Short lever | (na) |
| Rangan et al. [44] | General | (na) | (na) | (na) Additional steroid injection | n = 2 (visual disturbances, headache, numbness, heaviness of the arm; septic joint arthritis) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salomon, M.; Pastore, C.; Maselli, F.; Di Bari, M.; Pellegrino, R.; Brindisino, F. Manipulation under Anesthesia versus Non-Surgical Treatment for Patients with Frozen Shoulder Contracture Syndrome: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 9715. https://doi.org/10.3390/ijerph19159715
Salomon M, Pastore C, Maselli F, Di Bari M, Pellegrino R, Brindisino F. Manipulation under Anesthesia versus Non-Surgical Treatment for Patients with Frozen Shoulder Contracture Syndrome: A Systematic Review. International Journal of Environmental Research and Public Health. 2022; 19(15):9715. https://doi.org/10.3390/ijerph19159715
Chicago/Turabian StyleSalomon, Mattia, Chiara Pastore, Filippo Maselli, Mauro Di Bari, Raffaello Pellegrino, and Fabrizio Brindisino. 2022. "Manipulation under Anesthesia versus Non-Surgical Treatment for Patients with Frozen Shoulder Contracture Syndrome: A Systematic Review" International Journal of Environmental Research and Public Health 19, no. 15: 9715. https://doi.org/10.3390/ijerph19159715
APA StyleSalomon, M., Pastore, C., Maselli, F., Di Bari, M., Pellegrino, R., & Brindisino, F. (2022). Manipulation under Anesthesia versus Non-Surgical Treatment for Patients with Frozen Shoulder Contracture Syndrome: A Systematic Review. International Journal of Environmental Research and Public Health, 19(15), 9715. https://doi.org/10.3390/ijerph19159715











