Anabasine and Anatabine Exposure Attributable to Cigarette Smoking: National Health and Nutrition Examination Survey (NHANES) 2013–2014
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Correlation of Biomarkers
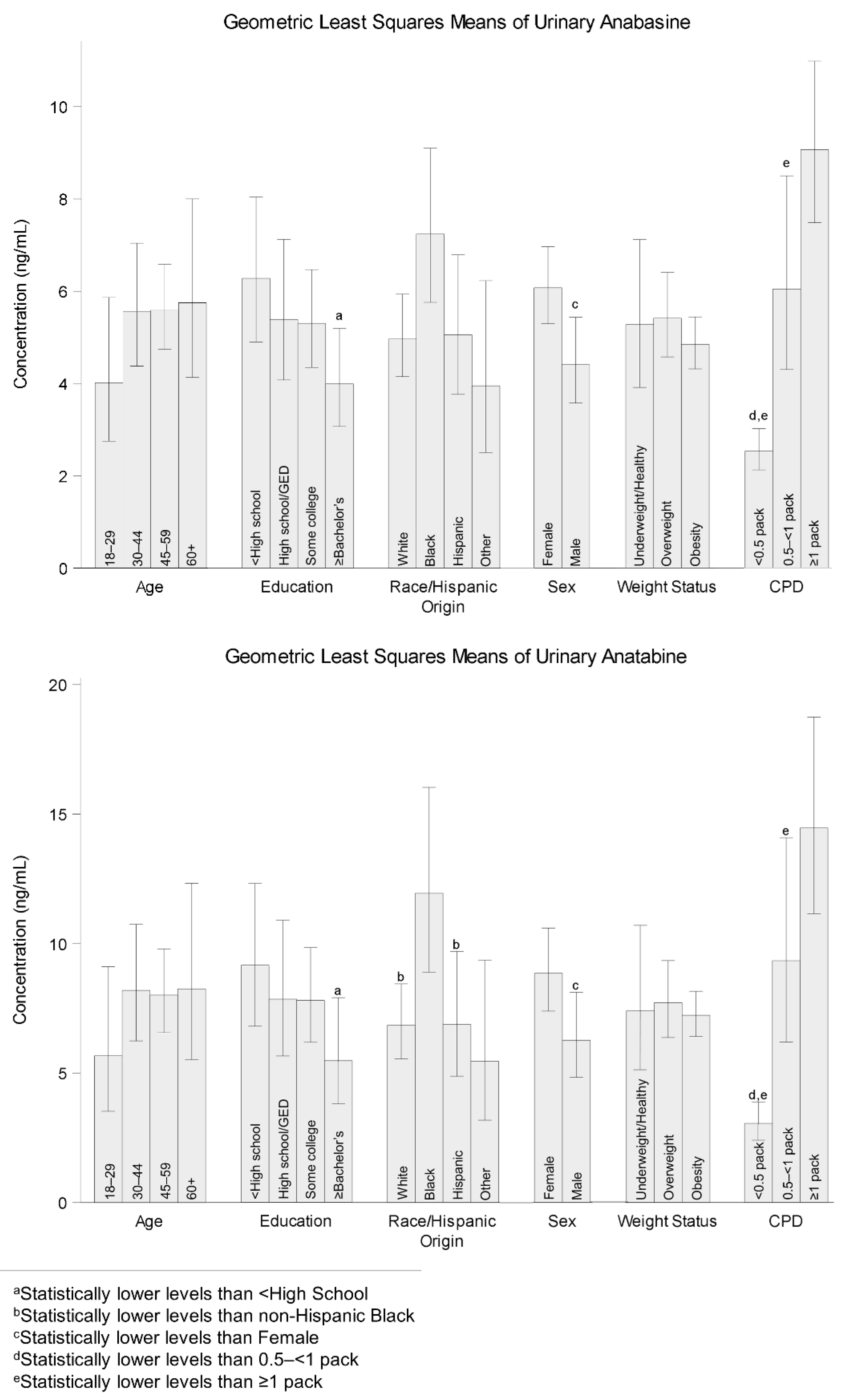
3.2. Comparison of Geometric Least Squares Means (LSM) by Demographic Characteristics
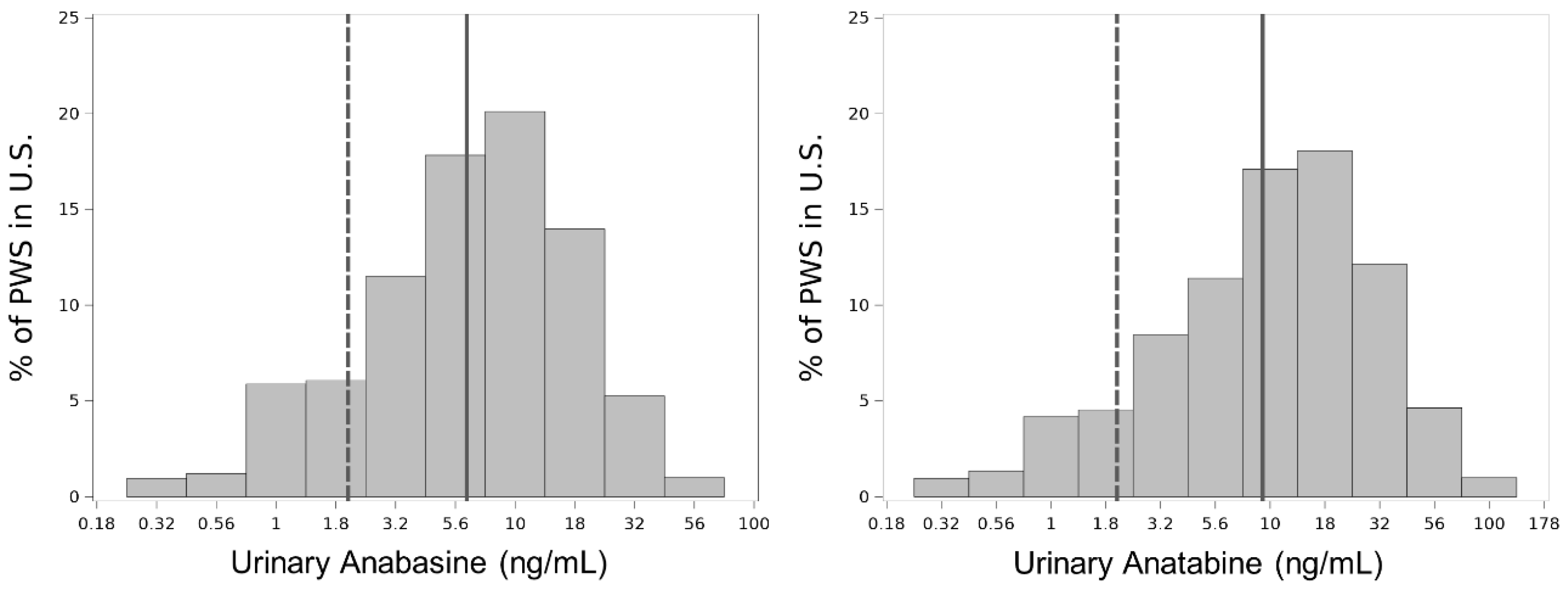
3.3. Biomarker Level Distributions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B

Appendix C
| Smoking Frequency | Sample Size | Urinary Anabasine (% >2 ng/mL) | Urinary Anatabine (% >2 ng/mL) | Serum Cotinine (% >10 ng/mL) |
|---|---|---|---|---|
| Daily + non-daily | 770 | 80% | 85% | 97% |
| Daily | 721 | 86% | 91% | 100% |
| Non-daily | 49 | 45% | 53% | 80% |
References
- Clemens, K.J.; Caillé, S.; Stinus, L.; Cador, M. The addition of five minor tobacco alkaloids increases nicotine-induced hyperactivity, sensitization and intravenous self-administration in rats. Int. J. Neuropsychopharmacol. 2009, 12, 1355–1366. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Dains, K.M.; Dempsey, D.; Wilson, M.; Jacob, P., III. Racial Differences in the Relationship between Number of Cigarettes Smoked and Nicotine and Carcinogen Exposure. Nicotine Tob. Res. 2011, 13, 772–783. [Google Scholar] [CrossRef]
- Tompkins, E.L.; Beltran, T.A.; Bedno, S.A. Differentiating between smokers and nonsmokers using serum cotinine. Biomark. Med. 2019, 13, 1025–1033. [Google Scholar] [CrossRef]
- Lisko, J.G.; Tran, H.; Stanfill, S.B.; Blount, B.C.; Watson, C.H. Chemical Composition and Evaluation of Nicotine, Tobacco Alkaloids, pH, and Selected Flavors in E-Cigarette Cartridges and Refill Solutions. Nicotine Tob. Res. 2015, 17, 1270–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordt, S.-E. Synthetic nicotine has arrived. Tob. Control. 2021, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Jacob, P., III; Hatsukami, R.; Severson, H.; Hall, S.; Yu, L.; Benowitz, N.L. Anabasine and Anatabine as Biomarkers for Tobacco Use during Nicotine Replacement Therapy. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1668–1673. [Google Scholar]
- Scherer, G.; Scherer, M.; Mütze, J.; Hauke, T.; Pluym, N. Assessment of the Exposure to Tobacco-Specific Nitrosamines and Minor Tobacco Alkaloids in Users of Various Tobacco/Nicotine Products. Chem. Res. Toxicol. 2022, 35, 684–693. [Google Scholar] [CrossRef]
- Wagenknecht, L.E.; Perkins, L.L.; Cutter, G.R.; Sidney, S.; Burke, G.L.; Manolio, T.A.; Jacobs, D.R.; Liu, K.; Friedman, G.D.; Hughes, G.H.; et al. Cigarette smoking behavior is strongly related to educational status: The CARDIA study. Prev. Med. 1990, 19, 158–169. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Schultz, K.E.; Haller, C.A.; Wu, A.H.B.; Dains, K.M.; Jacob, P., III. Prevalence of smoking assessed biochemically in an urban public hospital: A rationale for routine cotinine screening. Am. J. Epidemiol. 2009, 170, 885–891. [Google Scholar] [CrossRef] [Green Version]
- Edwards, K.C.; Naz, T.; Stanton, C.A.; Goniewicz, M.L.; Hatsukami, D.K.; Smith, D.M.; Wang, L.; Villanti, A.; Pearson, J.; Blount, B.C.; et al. Urinary Cotinine and Cotinine + Trans-3′-Hydroxycotinine (TNE-2) Cu.-points for Distinguishing Tobacco Use from Nonuse in the United States: PATH Study (2013–2014). Cancer Epidemiol. Biomark. Prev. 2021, 30, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L.; Bernert, J.T.; Foulds, J.; Hecht, S.S.; Jacob, P., III; Jarvis, M.J.; Joseph, A.; Oncken, C.; Piper, M.E. Biochemical Verification of Tobacco Use and Abstinence: 2019 Update. Nicotine Tob. Res. 2019, 22, 1086–1097. [Google Scholar] [CrossRef]
- Kim, S. Overview of Cotinine Cutoff Values for Smoking Status Classification. Int. J. Environ. Res. Public Health 2016, 13, 1236. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.E.; Link, C.A.; Jensen, J.; Le, C.; Puumala, S.S.; Hecht, S.S.; Carmella, S.G.; Losey, L.; Hatsukami, D.K. A Comparison of Urinary Biomarkers of Tobacco and Carcinogen Exposure in Smokers. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1617–1623. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS). About the National Health and Nutrition Examination Survey. 2017. Available online: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm (accessed on 7 April 2021).
- Wei, B.; Feng, J.; Rehmani, I.J.; Miller, S.; McGuffey, J.E.; Blount, B.C.; Wang, L. Ahigh-throughput robotic sample preparation system and HPLC-MS/MS for measuring urinary anatabine, anabasine, nicotine and major nicotine metabolites. Clin. Chim. Acta Int. J. Clin. Chem. 2014, 436, 290–297. [Google Scholar] [CrossRef] [Green Version]
- Bernert, J.; Turner, W.; Pirkle, J.L.; Sosnoff, C.S.; Akins, J.R.; Waldrep, M.K.; Ann, Q.; Covey, T.R.; Whitfield, W.; Gunter, E.W.; et al. Development and validation of sensitive method for determination of serum cotinine in smokers and nonsmokers by liquid chromatography/atmospheric pressure ionization tandem mass spectrometry. Clin. Chem. 1998, 43, 2281–2291. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data. 2014. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/UCOTS_H.htm (accessed on 7 April 2021).
- Parker, J.D.; Talih, M.; Malec, D.J.; Beresovsky, V.; Carroll, M.; Gonzalez, J.F.; Hamilton, B.; Ingram, D.D.; Kochanek, K.; McCarty, F.; et al. National Center for Health Statistics Data Presentation Standards for Proportions. Vital Health Stat. 2 2017, 175, 1–22. [Google Scholar]
- Lisko, J.G.; Stanfill, S.B.; Duncan, B.W.; Watson, C.H. Application of GC-MS/MS for the analysis of tobacco alkaloids in cigarette filler and various tobacco species. Anal. Chem. 2013, 85, 3380–3384. [Google Scholar] [CrossRef] [Green Version]
- Jain, R.B. Revised and extended serum cotinine cut-offs to classify smokers and non-smokers. Biomarkers 2018, 23, 502–507. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Bernert, J.T.; Caraballo, R.S.; Holiday, D.B.; Wang, J. Optimal Serum Cotinine Levels for Distinguishing Cigarette Smokers and Nonsmokers within Different Racial/Ethnic Groups in the United States between 1999 and 2004. Am. J. Epidemiol. 2008, 169, 236–248. [Google Scholar] [CrossRef] [Green Version]
- Caraballo, R.S.; Giovino, G.A.; Pechacek, T.F.; Mowery, P.D.; Richter, P.A.; Strauss, W.J.; Sharp, D.J.; Eriksen, M.P.; Pirkle, J.L.; Maurer, K.R. Racial and Ethnic Differences in Serum Cotinine Levels of Cigarette Smokers: Third National Health and Nutrition Examination Survey, 1988–1991. JAMA 1998, 280, 135–139. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Stable, E.J.; Herrera, B.; Jacob, P., III; Benowitz, N.L. Nicotine Metabolism and Intake in Black and White Smokers. JAMA 1998, 280, 152–156. [Google Scholar] [CrossRef] [Green Version]
- Gan, W.Q.; Cohen, S.B.-Z.; Man, S.F.P.; Sin, D.D. Sex-Related Differences in Serum Cotinine Concentrations in Daily Cigarette Smokers. Nicotine Tob. Res. 2008, 10, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.E. Biochemistry of nicotine metabolism and its relevance to lung cancer. J. Biol. Chem. 2021, 296, 100722. [Google Scholar] [CrossRef] [PubMed]
- von Weymarn, L.B.; Thomson, N.M.; Donny, E.C.; Hatsukami, D.K.; Murphy, S.E. Quantitation of the Minor Tobacco Alkaloids Nornicotine, Anatabine, and Anabasine in Smokers’ Urine by High Throughput Liquid Chromatography-Mass Spectrometry. Chem. Res. Toxicol. 2016, 29, 390–397. [Google Scholar] [CrossRef]

| Demographic Group | Sample Size | Percent (SE) |
|---|---|---|
| All | 770 | 100 |
| Age | ||
| 18–29 | 153 | 25.2 (1.6) |
| 30–44 | 227 | 29.4 (2.5) |
| 45–59 | 239 | 31.4 (3.2) |
| 60+ | 151 | 14.0 (1.0) |
| Education | ||
| <High School | 234 | 25.7 (3.2) |
| High School/GED | 226 | 29.7 (2.5) |
| Some College | 245 | 32.3 (2.2) |
| ≥Bachelor’s | 65 | 12.3 (2.2) |
| Race/Hispanic Origin | ||
| Non-Hispanic White | 410 | 69.8 (3.1) |
| Non-Hispanic Black | 187 | 14.5 (2.3) |
| Hispanic | 99 | 8.6 (2.1) |
| Other/Multiracial | 74 | 7.1 (1.0) |
| Sex | ||
| Female | 352 | 47.3 (2.6) |
| Male | 418 | 52.7 (2.6) |
| Weight Status | ||
| Underweight/Healthy | 277 | 34.8 (2.8) |
| Overweight | 245 | 32.9 (2.0) |
| Obesity | 248 | 32.3 (3.6) |
| CPD | ||
| 1–9 (<0.5 pack) | 289 | 37.2 (2.9) |
| 10–19 (0.5–<1 pack) | 287 | 35.8 (1.6) |
| 20+ (≥1 pack) | 194 | 27.0 (2.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bendik, P.B.; Rutt, S.M.; Pine, B.N.; Sosnoff, C.S.; Blount, B.C.; Zhu, W.; Feng, J.; Wang, L. Anabasine and Anatabine Exposure Attributable to Cigarette Smoking: National Health and Nutrition Examination Survey (NHANES) 2013–2014. Int. J. Environ. Res. Public Health 2022, 19, 9744. https://doi.org/10.3390/ijerph19159744
Bendik PB, Rutt SM, Pine BN, Sosnoff CS, Blount BC, Zhu W, Feng J, Wang L. Anabasine and Anatabine Exposure Attributable to Cigarette Smoking: National Health and Nutrition Examination Survey (NHANES) 2013–2014. International Journal of Environmental Research and Public Health. 2022; 19(15):9744. https://doi.org/10.3390/ijerph19159744
Chicago/Turabian StyleBendik, Patrick B., Sharyn M. Rutt, Brittany N. Pine, Connie S. Sosnoff, Benjamin C. Blount, Wanzhe Zhu, June Feng, and Lanqing Wang. 2022. "Anabasine and Anatabine Exposure Attributable to Cigarette Smoking: National Health and Nutrition Examination Survey (NHANES) 2013–2014" International Journal of Environmental Research and Public Health 19, no. 15: 9744. https://doi.org/10.3390/ijerph19159744
APA StyleBendik, P. B., Rutt, S. M., Pine, B. N., Sosnoff, C. S., Blount, B. C., Zhu, W., Feng, J., & Wang, L. (2022). Anabasine and Anatabine Exposure Attributable to Cigarette Smoking: National Health and Nutrition Examination Survey (NHANES) 2013–2014. International Journal of Environmental Research and Public Health, 19(15), 9744. https://doi.org/10.3390/ijerph19159744






