Application of an Ultrasonic Nebulizer Closet in the Disinfection of Textiles and Footwear
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbiological Indicators Production and Preservation
2.2. Disinfectant and Ultrasonic Nebulizer Closets
2.3. Experimental Design
2.4. Determination of Cell Survival
2.5. Data Analysis
2.6. Criteria for Disinfection Acceptance
3. Results
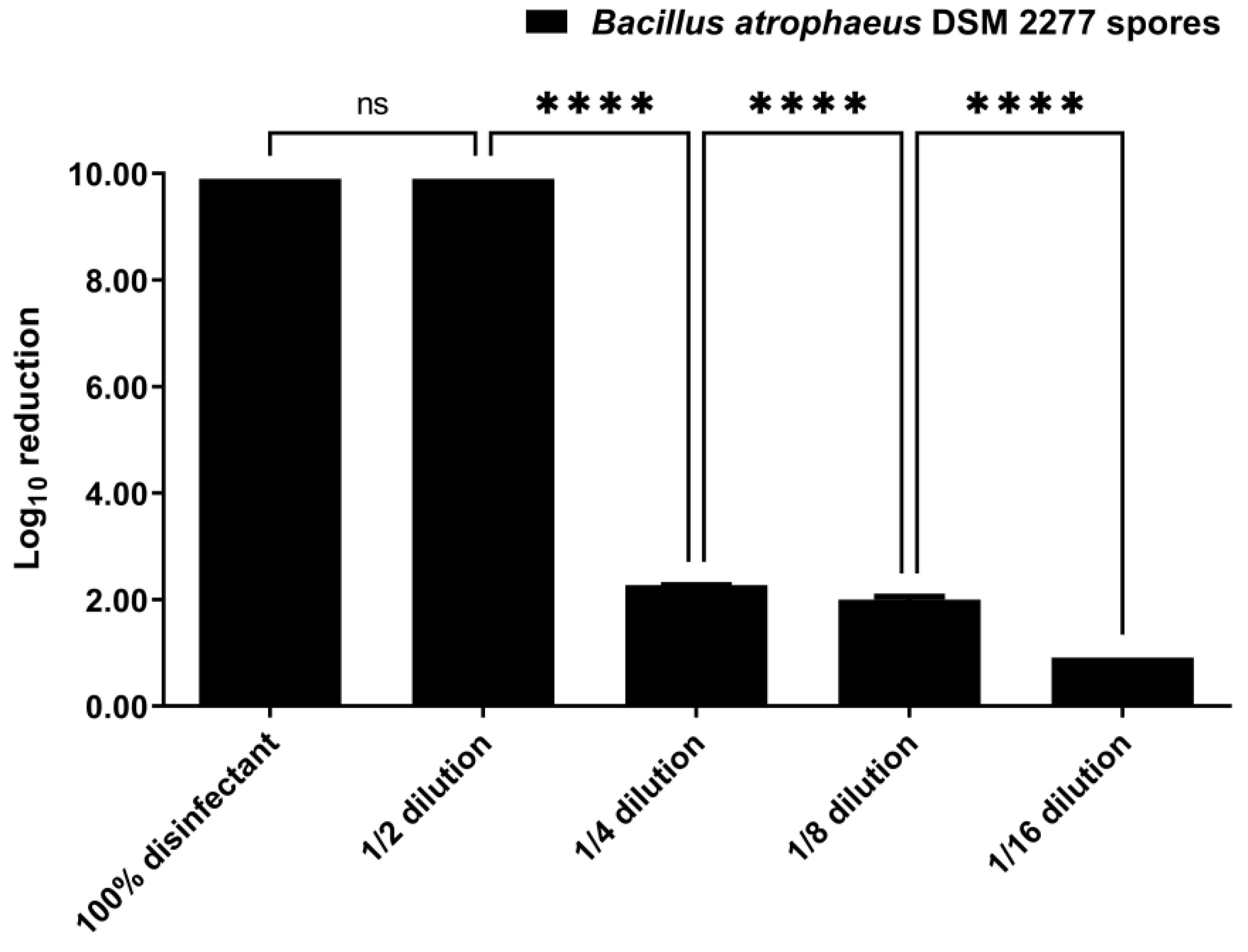
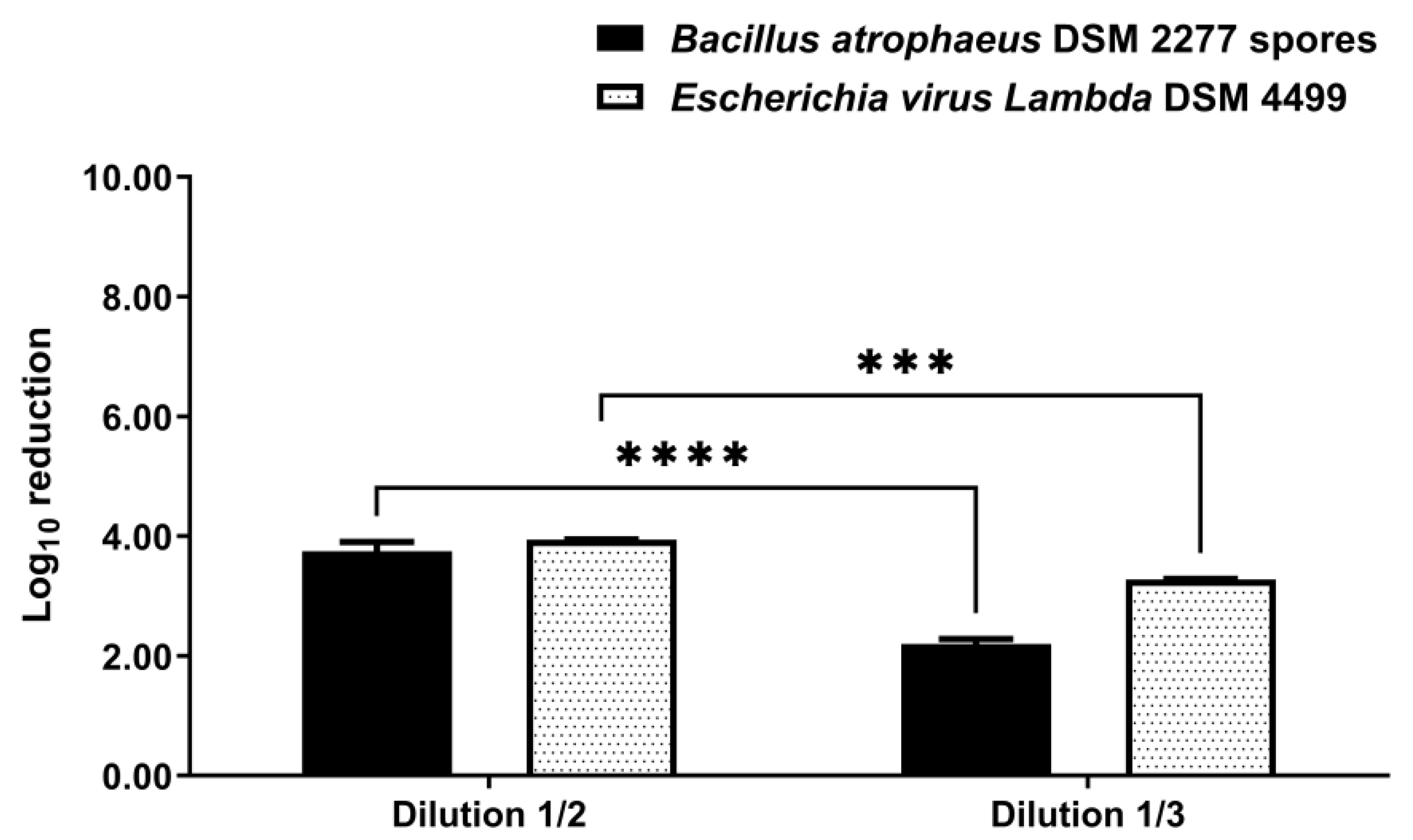
3.1. Disinfection by Suspension Test
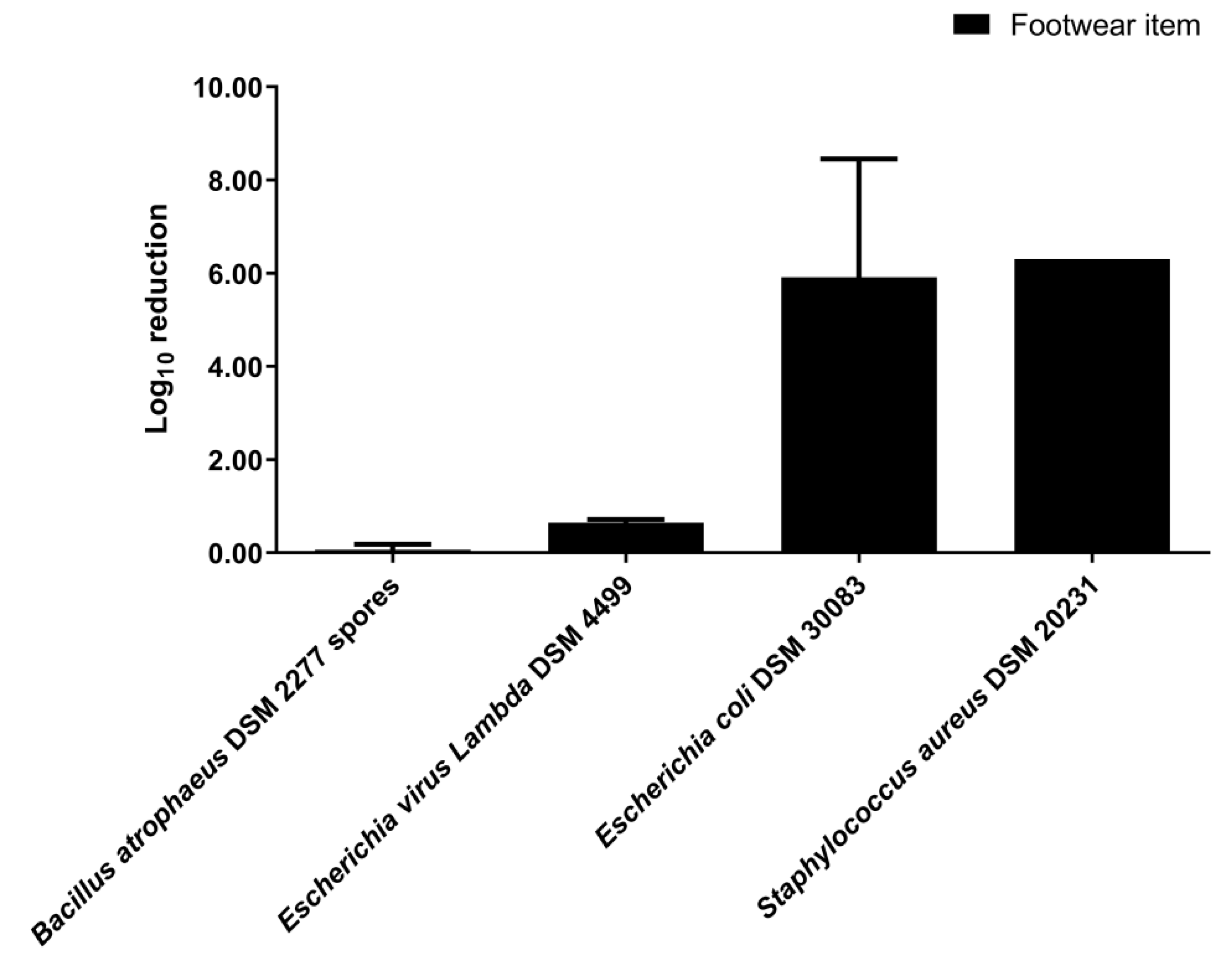
3.2. Disinfection by Nebulization in a 0.08 m3 Closet
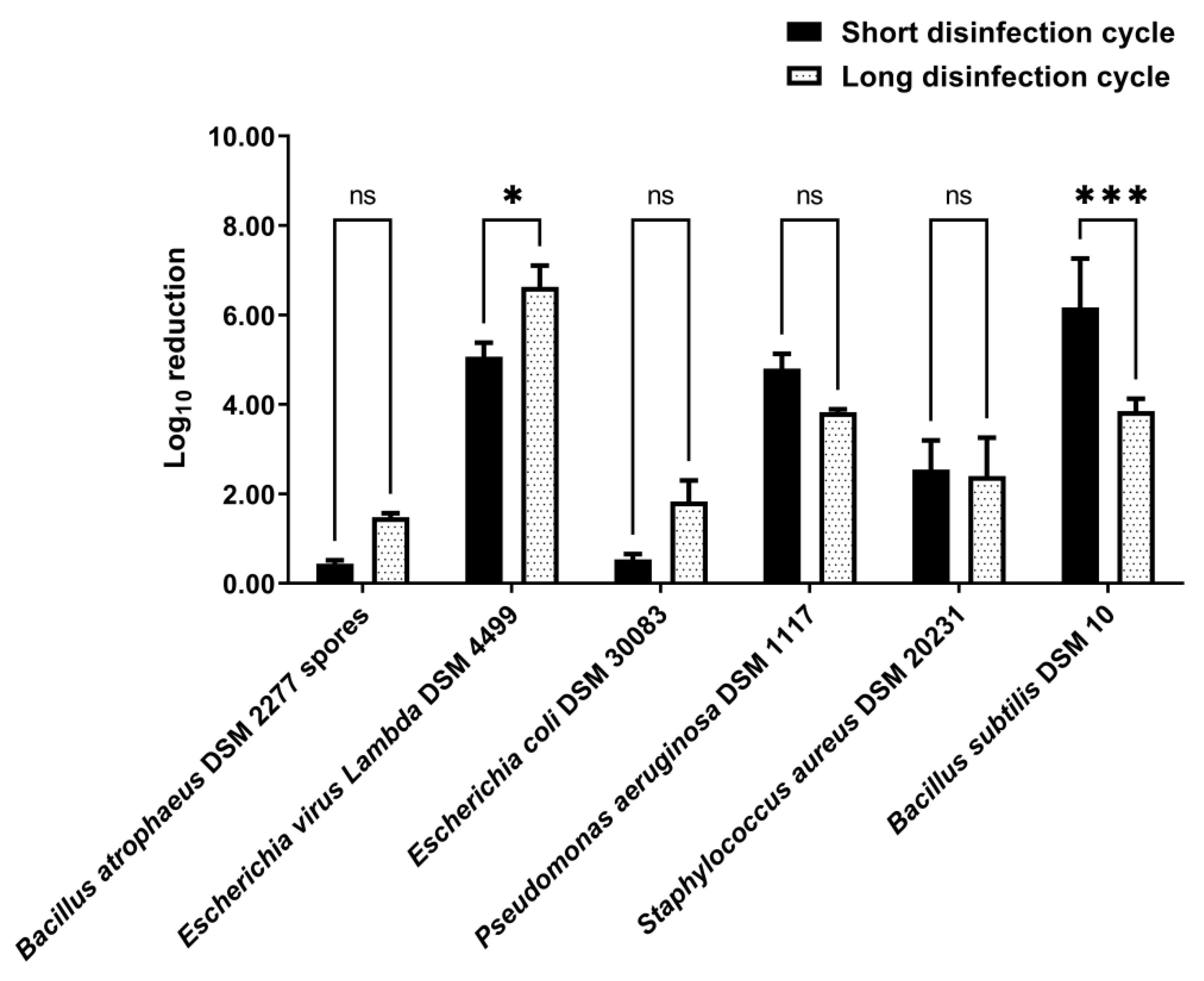
3.3. Disinfection by Nebulization in a 0.58 m3 Closet
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Høiby, N. Pandemics: Past, present, future: That is like choosing between cholera and plague. APMIS 2021, 129, 352–371. [Google Scholar] [CrossRef] [PubMed]
- Grossi, P.A.; Tebini, A.; Dalla Gasperina, D. Novel multidrug resistant microorganisms in critically ill: A potential threat. Minerva Anestesiol. 2015, 81, 52–64. [Google Scholar] [PubMed]
- Boyce, J.M. Modern technologies for improving cleaning and disinfection of environmental surfaces in hospitals. Antimicrob. Resist. Infect. Control 2016, 5, 10. [Google Scholar] [CrossRef]
- Wolfgruber, S.; Loibner, M.; Puff, M.; Melischnig, A.; Zatloukal, K. SARS-CoV2 neutralizing activity of ozone on porous and non-porous materials. New Biotechnol. 2022, 66, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Unal, N.; Yanik, K.; Karadag, A.; Odabasi, H.; Esen, S.; Günaydin, M. Evaluation of the efficacy of akacid plus® fogging in eradicating causative microorganism in nosocomial infections. Int. J. Clin. Exp. Med. 2014, 7, 5867–5871. [Google Scholar]
- Murugan, S.S.; Vijayakumar, P. Identification of ultrasonic frequency for water mist generation using piezoelectric transducer. Arch. Mater. Sci. Eng. 2017, 83, 74–78. [Google Scholar] [CrossRef]
- Bockmühl, D.P.; Schages, J.; Rehberg, L. Laundry and textile hygiene in healthcare and beyond. Microb. Cell 2019, 6, 299–306. [Google Scholar] [CrossRef]
- Fijan, S.; Turk, S.S. Hospital textiles, are they a possible vehicle for healthcare-associated infections? Int. J. Environ. Res. Public Health 2012, 9, 3330–3343. [Google Scholar] [CrossRef]
- Bloomfield, S.F.; Exner, M.; Signorelli, C.; Nath, K.J.; Scott, E.A. The Infection Risks Associated with Clothing and Household Linens in Home and Everyday Life Settings, and the Role of Laundry; International Scientific Forum on Home Hygiene (IFH): Somerset, UK, 2011; pp. 1–43. [Google Scholar]
- Bockmühl, D.P. Laundry hygiene—how to get more than clean. J. Appl. Microbiol. 2017, 122, 1124–1133. [Google Scholar] [CrossRef]
- Gupta, A.K.; Versteeg, S.G. The role of shoe and sock sanitization in the management of superficial fungal infections of the feet. J. Am. Podiatr. Med. Assoc. 2019, 109, 141–149. [Google Scholar] [CrossRef]
- Aumeran, C.; Henquell, C.; Brebion, A.; Noureddine, J.; Traore, O.; Lesens, O. Isolation gown contamination during healthcare of confirmed SARS-CoV-2-infected patients. J. Hosp. Infect. 2021, 107, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Redmond, S.N.; Dousa, K.M.; Jones, L.D.; Li, D.F.; Cadnum, J.L.; Navas, M.E.; Kachaluba, N.M.; Silva, S.Y.; Zabarsky, T.F.; Eckstein, E.C.; et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) nucleic acid contamination of surfaces on a coronavirus disease 2019 (COVID-19) ward and intensive care unit. Infect. Control Hosp. Epidemiol. 2021, 42, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Owen, L.; Laird, K. The role of textiles as fomites in the healthcare environment: A review of the infection control risk. PeerJ 2020, 8, e9790. [Google Scholar] [CrossRef]
- Owen, L.; Shivkumar, M.; Laird, K. The stability of model human coronaviruses on textiles in the environment and during health care laundering. mSphere 2021, 6, e00316-21. [Google Scholar] [CrossRef] [PubMed]
- Shivkumar, M.; Adkin, P.; Owen, L.; Laird, K. Investigation of the stability and risks of fomite transmission of human coronavirus OC43 on leather. FEMS Microbiol. Lett. 2021, 368, fnab112. [Google Scholar] [CrossRef]
- Katoh, T. Dermatomycosis and environment. Jpn. J. Med. Mycol. 2006, 47, 63–67. [Google Scholar] [CrossRef]
- Harbourt, D.E.; Haddow, A.D.; Piper, A.E.; Bloomfield, H.; Kearney, B.J.; Fetterer, D.; Gibson, K.; Minogue, T. Modeling the stability of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on skin, currency, and clothing. PLoS Negl. Trop. Dis. 2020, 14, e0008831. [Google Scholar] [CrossRef]
- Chin, A.W.H.; Chu, J.T.S.; Perera, M.R.A.; Hui, K.P.Y.; Yen, H.-L.; Chan, M.C.W.; Peiris, M.; Poon, L.L.M. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe 2020, 1, e10. [Google Scholar] [CrossRef]
- Fijan, S.; Pahor, D.; Šostar Turk, S. Survival of Enterococcus faecium, Staphylococcus aureus and Pseudomonas aeruginosa on cotton. Text. Res. J. 2017, 87, 1711–1721. [Google Scholar] [CrossRef]
- Riley, K.; Williams, J.; Owen, L.; Shen, J.; Davies, A.; Laird, K. The effect of low-temperature laundering and detergents on the survival of Escherichia coli and Staphylococcus aureus on textiles used in healthcare uniforms. J. Appl. Microbiol. 2017, 123, 280–286. [Google Scholar] [CrossRef]
- Hanczvikkel, A.; Tóth, Á. Quantitative study about the role of environmental conditions in the survival capability of multidrug-resistant bacteria. J. Infect. Public Health 2018, 11, 801–806. [Google Scholar] [CrossRef]
- Agarwal, M.; Hamilton-Stewart, P.; Dixon, R.A. Contaminated operating room boots: The potential for infection. Am. J. Infect. Control 2002, 30, 179–183. [Google Scholar] [CrossRef][Green Version]
- Tanaka, K.; Katoh, T.; Irimajiri, J.; Taniguchi, H.; Yokozeki, H. Preventive effects of various types of footwear and cleaning methods on dermatophyte adhesion. J. Dermatol. 2006, 33, 528–536. [Google Scholar] [CrossRef]
- Aljohani, Y.; Almutadares, M.; Alfaifi, K.; El Madhoun, M.; Albahiti, M.H.; Al-Hazmi, N. Uniform-related infection control practices of dental students. Infect. Drug Resist. 2017, 10, 135–142. [Google Scholar] [CrossRef]
- Lucassen, R.; Blümke, H.; Born, L.; Fritz, A.; Geurtz, P.; Hofmann, N.; Hoffmann, L.; Steiner, R.; Merettig, N.; Bockmühl, D. The washing machine as a source of microbial contamination of domestic laundry—A case study. Househ. Pers. Care. Today 2014, 9, 54–57. [Google Scholar]
- Koganti, S.; Alhmidi, H.; Tomas, M.E.; Cadnum, J.L.; Sass, C.; Jencson, A.L.; Donskey, C.J. Evaluation of an ethanol-based spray disinfectant for decontamination of cover gowns prior to removal. Infect. Control Hosp. Epidemiol. 2017, 38, 364–366. [Google Scholar] [CrossRef]
- Robinson, G.L.; Hitchcock, S.; Kpadeh-Rogers, Z.; Karikari, N.; Johnson, J.K.; Blanco, N.; Morgan, D.J.; Harris, A.D.; Leekha, S. Preventing viral contamination: Effects of wipe and spray-based decontamination of gloves and gowns. Clin. Infect. Dis. 2019, 69, S228–S230. [Google Scholar] [CrossRef]
- Neely, A.N.; Maley, M.P. 3% hydrogen peroxide for the Gram-positive disinfection of fabrics. J. Burn Care Rehabil. 1999, 20, 471–477. [Google Scholar] [CrossRef]
- DeQueiroz, G.A.; Day, D.F. Disinfection of Bacillus subtilis spore-contaminated surface materials with a sodium hypochlorite and a hydrogen peroxide-based sanitizer. Lett. Appl. Microbiol. 2008, 46, 176–180. [Google Scholar] [CrossRef]
- String, G.M.; Gutiérrez, E.V.; Lantagne, D.S. Laboratory efficacy of surface disinfection using chlorine against Vibrio cholerae. J. Water Health 2020, 18, 1009–1019. [Google Scholar] [CrossRef]
- Chabrelie, A.; Mitchell, J.; Rose, J.; Charbonneau, D.; Ishida, Y. Evaluation of the influenza risk reduction from antimicrobial spray application on porous surfaces. Risk Anal. 2018, 38, 1502–1517. [Google Scholar] [CrossRef]
- Moriello, K.A.; Kunder, D.; Hondzo, H. Efficacy of eight commercial disinfectants against Microsporum canis and Trichophyton spp. infective spores on an experimentally contaminated textile surface. Vet. Dermatol. 2013, 24, 621-e152. [Google Scholar] [CrossRef]
- Burnett, S.L.; Egland, S.J.; McKelvey, P.J.; Cook, F.K. Chemical decontamination of footwear soles to limit microbial transfer in a dry environment. Food Prot. Trends 2013, 33, 74–81. [Google Scholar]
- Feuilhade De Chauvin, M. A study on the decontamination of insoles colonized by Trichophyton rubrum: Effect of terbinafine spray powder 1% and terbinafine spray solution 1%. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 875–878. [Google Scholar] [CrossRef]
- Alhmidi, H.; Koganti, S.; Cadnum, J.L.; Rai, H.; Jencson, A.L.; Donskey, C.J. Evaluation of a novel alcohol-based surface disinfectant for disinfection of hard and soft surfaces in healthcare facilities. Open Forum Infect. Dis. 2017, 4, ofx054. [Google Scholar] [CrossRef]
- Abu-Zidan, Y.; Nguyen, K.; Mendis, P.; Setunge, S.; Adeli, H. Design of a smart prefabricated sanitising chamber for COVID-19 using computational fluid dynamics. J. Civ. Eng. Manag. 2021, 27, 139–148. [Google Scholar] [CrossRef]
- Khan, O.; Khan, M.Z.; Khan, M.E.; Goyal, A.; Bhatt, B.K.; Khan, A.; Parvez, M. Experimental analysis of solar powered disinfection tunnel mist spray system for coronavirus prevention in public and remote places. Mater. Today Proc. 2020, 46, 6852–6858. [Google Scholar] [CrossRef]
- Callahan, K.L.; Beck, N.K.; Duffield, E.A.; Shin, G.; Meschke, J.S. Inactivation of methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus faecium (VRE) on various environmental surfaces by mist application of a stabilized chlorine dioxide and quaternary ammonium compound-based disinfectant. J. Occup. Environ. Hyg. 2010, 7, 529–534. [Google Scholar] [CrossRef]
- Berentsveig, V.; Weinberger, R.; Potas, M. Sub-cycle based aerosol disinfection system. US 9358315 B2, 7 June 2016. [Google Scholar]
- Berentsveig, V.; Erickson, G.; Weinberger, R. Aerosol. US 9241491 B2, 26 January 2016. [Google Scholar]
- Kritzler, S.; Sava, A. Improved Disinfection. AU 2010200764 B2, 1 March 2011. [Google Scholar]
- Ricciardi, J.J.; Ricciardi, C.L.; Swidler, H.J. Methods and Apparatuses for Applying Agent to Objects. US 8062590 B1, 22 November 2011. [Google Scholar]
- Klaassen, F.O.; Alma, S.A.; Musters, C.B.H.; Plantinga, P.; Timmerman, C.P. Method and Device for Disinfecting a Space. US 9616148 B2, 11 April 2017. [Google Scholar]
- Pendred, C.J. Method of Generating a Dry Fog. GB 2483552 A, 14 March 2012. [Google Scholar]
- Wypych, A.; Wypych, G. Databook of Biocides: Biocides Included in Article 95 List; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 1–464. [Google Scholar]
- ECHA. Guidance on the BPR: Volume II Efficacy—Assessment and Evaluation (Parts B+C)—Version 4.1; European Chemicals Agency: Helsinki, Finland, 2022. [Google Scholar] [CrossRef]
- EN 13727:2012+A2:2015; Chemical Disinfectants and Antiseptics—Quantitative Suspension Test for the Evaluation of Bactericidal Activity in the Medical Area—Test Method and Requirements (Phase 2, Step 1). European Committee for Standardization: Brussels, Belgium, 2015.
- EN 1276:2019; Chemical Disinfectants and Antiseptics—Quantitative Suspension Test for the Evaluation of Bactericidal Activity of Chemical Disinfectants and Antiseptics Used in Food, Industrial, Domestic and Institutional Areas—Test Method and Requirements (Phase 2, Step 1). European Committee for Standardization: Brussels, Belgium, 2019.
- EN 14476:2013+A2:2019; Chemical Disinfectants and Antiseptics—Quantitative Suspension Test for the Evaluation of Virucidal Activity in the Medical Area—Test method and Requirements (Phase 2/Step 1). European Committee for Standardization: Brussels, Belgium, 2019.
- Rabenau, H.F.; Schwebke, I.; Blümel, J.; Eggers, M.; Glebe, D.; Rapp, I.; Sauerbrei, A.; Steinmann, E.; Steinmann, J.; Willkommen, H.; et al. Guideline for testing chemical disinfectants regarding their virucidal activity within the field of human medicine: As of December 1st, 2014 Prepared by the German Association for the Control of Virus Diseases (DVV) and the Robert Koch Institute (RKI). Bundesgesundheitsblatt Gesundh. Gesundh. 2020, 63, 645–655. [Google Scholar] [CrossRef]
- EN 16616:2015; Chemical Disinfectants and Antiseptics—Chemical-Thermal Textile Disinfection—Test Method and Requirements (Phase 2, Step 2). European Committee for Standardization: Brussels, Belgium, 2015.
- ASTM E2406-16; Standard Test Method for Evaluation of Laundry Sanitizers and Disinfectants for Use in High Efficiency Washing Operations. ASTM International: West Conshohocken, PA, USA, 2016.
- ASTM E2274-16; Standard Test Method for Evaluation of Laundry Sanitizers and Disinfectants. ASTM International: West Conshohocken, PA, USA, 2016.
- EN 13704:2018; Chemical Disinfectants—Quantitative Suspension Test for the Evaluation of Sporicidal Activity of Chemical Disinfectants Used in Food, Industrial, Domestic and Institutional Areas—Test Method and Requirements (Phase 2, Step 1). European Committee for Standardization: Brussels, Belgium, 2018.
- Humphreys, P.N. Testing standards for sporicides. J. Hosp. Infect. 2011, 77, 193–198. [Google Scholar] [CrossRef]
- General Chapter <1072> Disinfectants and Antiseptics. In U.S. Pharmacopeia 42—NF 37; The United States Pharmacopeial Convention: North Bethesda, MD, USA, 2019.
- Hoseinzadeh, E.; Makhdoumi, P.; Taha, P.; Hossini, H.; Pirsaheb, M.; Omid Rastegar, S.; Stelling, J. A review of available techniques for determination of nano-antimicrobials activity. Toxin Rev. 2017, 36, 18–32. [Google Scholar] [CrossRef]
- Dafale, N.A.; Semwal, U.P.; Rajput, R.K.; Singh, G.N. Selection of appropriate analytical tools to determine the potency and bioactivity of antibiotics and antibiotic resistance. J. Pharmaceut. Analy. 2016, 6, 207–213. [Google Scholar] [CrossRef]
- Sella, S.R.B.R.; Vandenberghe, L.P.S.; Soccol, C.R. Bacillus atrophaeus: Main characteristics and biotechnological applications—A review. Crit. Rev. Biotechnol. 2015, 35, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Nazmul, T.; Yoshimoto, R.; Higashiura, A.; Oda, K.; Sakaguchi, T. Ethanol susceptibility of SARS-CoV-2 and other enveloped viruses. Biocontrol Sci. 2021, 26, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Jamsransuren, D.; Makita, Y.; Kaneko, A.; Matsuda, S.; Ogawa, H.; Oh, H. Inactivation activities of ozonated water, slightly acidic electrolyzed water and ethanol against SARS-CoV-2. Molecules 2021, 26, 5465. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, S.; Song, D.; Yuan, Z. Evaluating the virucidal activity of four disinfectants against SARS-CoV-2. Am. J. Infect. Control 2022, 50, 319–324. [Google Scholar] [CrossRef]
- Xiling, G.; Yin, C.; Ling, W.; Xiaosong, W.; Jingjing, F.; Fang, L.; Xiaoyan, Z.; Yiyue, G.; Ying, C.; Lunbiao, C.; et al. In vitro inactivation of SARS-CoV-2 by commonly used disinfection products and methods. Sci. Rep. 2021, 11, 2418. [Google Scholar] [CrossRef]
- Hirose, R.; Bandou, R.; Ikegaya, H.; Watanabe, N.; Yoshida, T.; Daidoji, T.; Naito, Y.; Itoh, Y.; Nakaya, T. Disinfectant effectiveness against SARS-CoV-2 and influenza viruses present on human skin: Model-based evaluation. Clin. Microbiol. Infect. 2021, 27, 1042.e1041–1042.e1044. [Google Scholar] [CrossRef]
- Herdt, B.L.; Black, E.P.; Zhou, S.S.; Wilde, C.J. Inactivation of SARS-CoV-2 by 2 commercially available Benzalkonium chloride-based hand sanitizers in comparison with an 80% ethanol-based hand sanitizer. Infect. Preven. Prac. 2021, 3, 100191. [Google Scholar] [CrossRef]
- Ijaz, M.K.; Nims, R.W.; Zhou, S.S.; Whitehead, K.; Srinivasan, V.; Kapes, T.; Fanuel, S.; Epstein, J.H.; Daszak, P.; Rubino, J.R.; et al. Microbicidal actives with virucidal efficacy against SARS-CoV-2 and other beta- and alpha-coronaviruses and implications for future emerging coronaviruses and other enveloped viruses. Sci. Rep. 2021, 11, 5626. [Google Scholar] [CrossRef]
- Ogilvie, B.H.; Solis-Leal, A.; Lopez, J.B.; Poole, B.D.; Robison, R.A.; Berges, B.K. Alcohol-free hand sanitizer and other quaternary ammonium disinfectants quickly and effectively inactivate SARS-CoV-2. J. Hosp. Infect. 2021, 108, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Welch, J.L.; Xiang, J.; MacKin, S.R.; Perlman, S.; Thorne, P.; O’Shaughnessy, P.; Strzelecki, B.; Aubin, P.; Ortiz-Hernandez, M.; Stapleton, J.T. Inactivation of severe acute respiratory coronavirus virus 2 (SARS-CoV-2) and diverse RNA and DNA viruses on three-dimensionally printed surgical mask materials. Infect. Control Hosp. Epidemiol. 2021, 42, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Catalano, C.E. Bacteriophage lambda: The path from biology to theranostic agent. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1517. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Y.; Zhao, R.; Gao, L.J.; Gao, X.F.; Wang, D.P.; Cao, J.M. SARS-CoV-2: Structure, biology, and structure-based therapeutics development. Front. Cell. Infect. Microbiol. 2020, 10, 587269. [Google Scholar] [CrossRef] [PubMed]
- Bayarri, B.; Cruz-Alcalde, A.; López-Vinent, N.; Micó, M.M.; Sans, C. Can ozone inactivate SARS-CoV-2? A review of mechanisms and performance on viruses. J. Hazard. Mater. 2021, 415, 125658. [Google Scholar] [CrossRef]
- Filipe, H.A.L.; Fiuza, S.M.; Henriques, C.A.; Antunes, F.E. Antiviral and antibacterial activity of hand sanitizer and surface disinfectant formulations. Int. J. Pharm. 2021, 609, 121139. [Google Scholar] [CrossRef]
- Nims, R.W.; Zhou, S.S. Intra-family differences in efficacy of inactivation of small, non-enveloped viruses. Biologicals 2016, 44, 456–462. [Google Scholar] [CrossRef]
- Leggett, M.J.; McDonnell, G.; Denyer, S.P.; Setlow, P.; Maillard, J.Y. Bacterial spore structures and their protective role in biocide resistance. J. Appl. Microbiol. 2012, 113, 485–498. [Google Scholar] [CrossRef]
- Zonta, W.; Mauroy, A.; Farnir, F.; Thiry, E. Comparative virucidal efficacy of seven disinfectants against murine norovirus and feline calicivirus, surrogates of human norovirus. Food Environ. Virol. 2016, 8, 1–12. [Google Scholar] [CrossRef]

| Bacterium | Agar Growing Medium | Incubation Temperature (°C) | Incubation Time (h) |
|---|---|---|---|
| Bacillus atrophaeus DSM 2277 | NA 1 | 30 | 48 |
| Escherichia coli DSM 4230 | LB agar 2 | 37 | 24 |
| Escherichia coli DSM 30083 | NA 1 | 37 | 24 |
| Pseudomonas aeruginosa DSM 1117 | NA 1 | 37 | 24 |
| Staphylococcus aureus DSM 20231 | NA 1 | 37 | 48 |
| Bacillus subtilis DSM 10 | NA 1 | 30 | 24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henriques, T.M.; Rito, B.; Proença, D.N.; Morais, P.V. Application of an Ultrasonic Nebulizer Closet in the Disinfection of Textiles and Footwear. Int. J. Environ. Res. Public Health 2022, 19, 10472. https://doi.org/10.3390/ijerph191710472
Henriques TM, Rito B, Proença DN, Morais PV. Application of an Ultrasonic Nebulizer Closet in the Disinfection of Textiles and Footwear. International Journal of Environmental Research and Public Health. 2022; 19(17):10472. https://doi.org/10.3390/ijerph191710472
Chicago/Turabian StyleHenriques, Tiago M., Beatriz Rito, Diogo N. Proença, and Paula V. Morais. 2022. "Application of an Ultrasonic Nebulizer Closet in the Disinfection of Textiles and Footwear" International Journal of Environmental Research and Public Health 19, no. 17: 10472. https://doi.org/10.3390/ijerph191710472
APA StyleHenriques, T. M., Rito, B., Proença, D. N., & Morais, P. V. (2022). Application of an Ultrasonic Nebulizer Closet in the Disinfection of Textiles and Footwear. International Journal of Environmental Research and Public Health, 19(17), 10472. https://doi.org/10.3390/ijerph191710472









