Fetal Exposure to Air Pollution in Late Pregnancy Significantly Increases ADHD-Risk Behavior in Early Childhood
Abstract
:1. Introduction
2. Methods
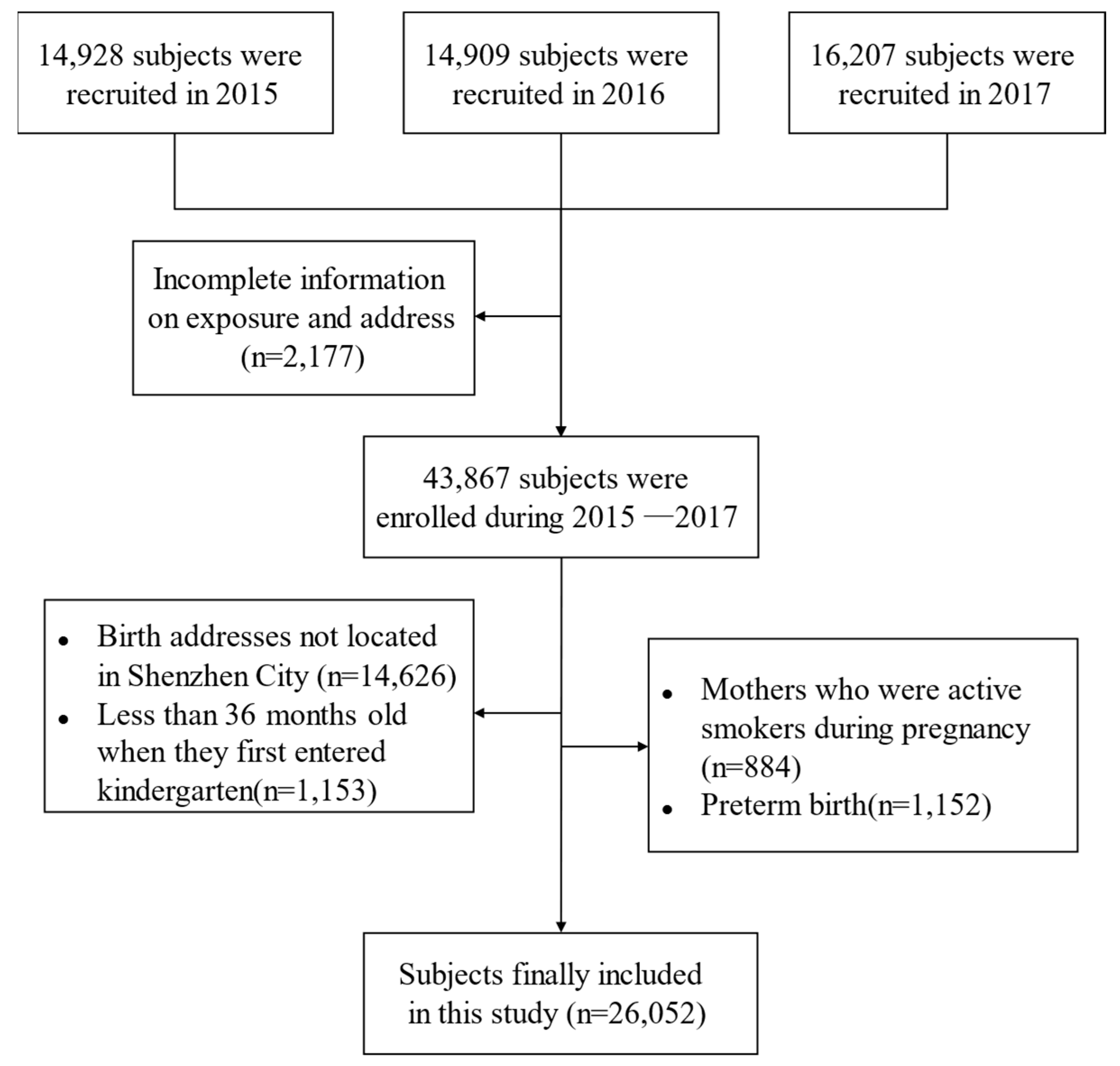
2.1. Study Population and Design
2.2. Questionnaire Survey
2.3. Air Pollution Exposure Assessment
2.4. Measurement of ADHD-like Behaviors
2.5. Statistical Analyses
2.6. Sensitivity Analysis
3. Results
3.1. General Characteristics of Participants
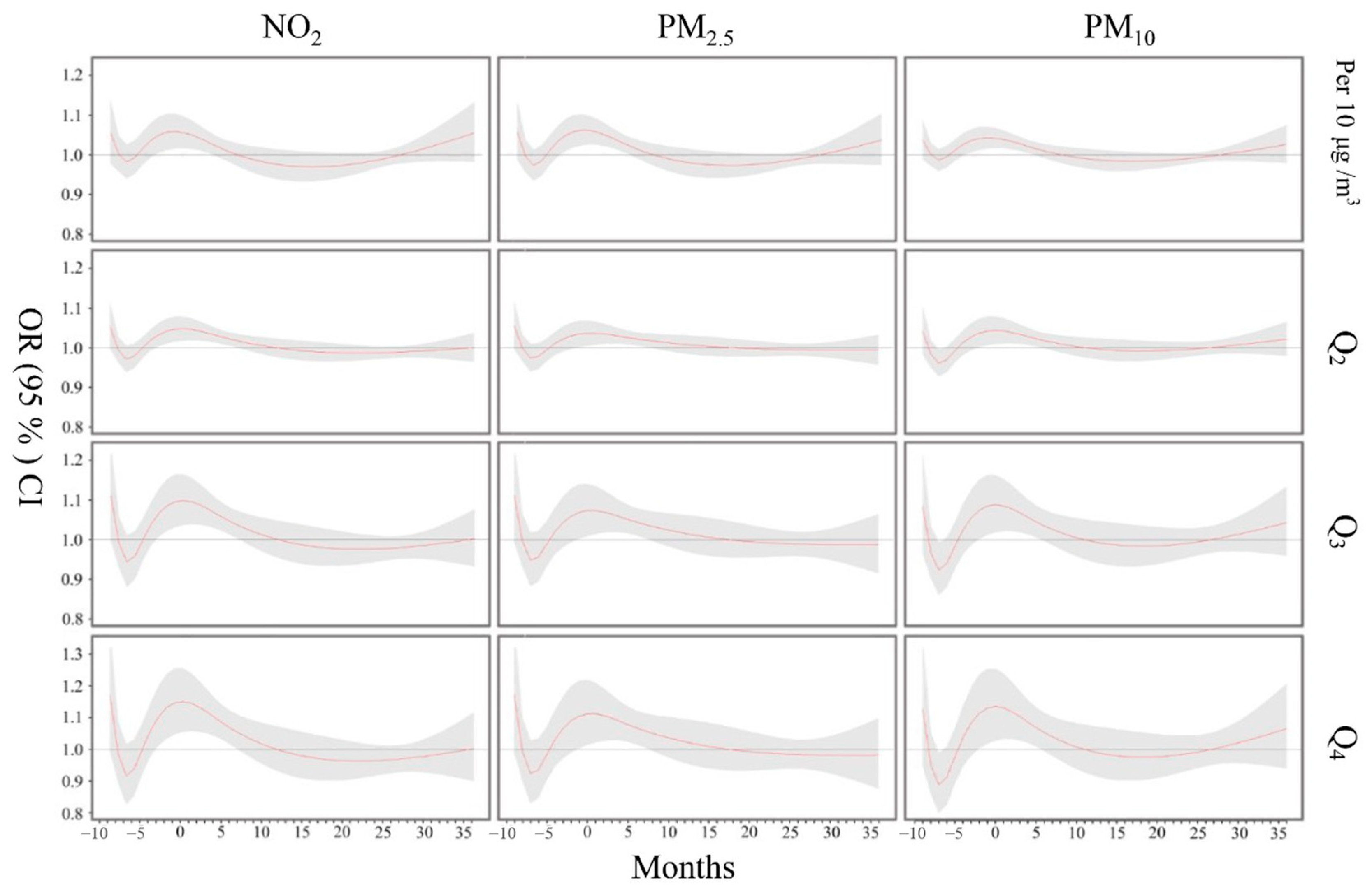
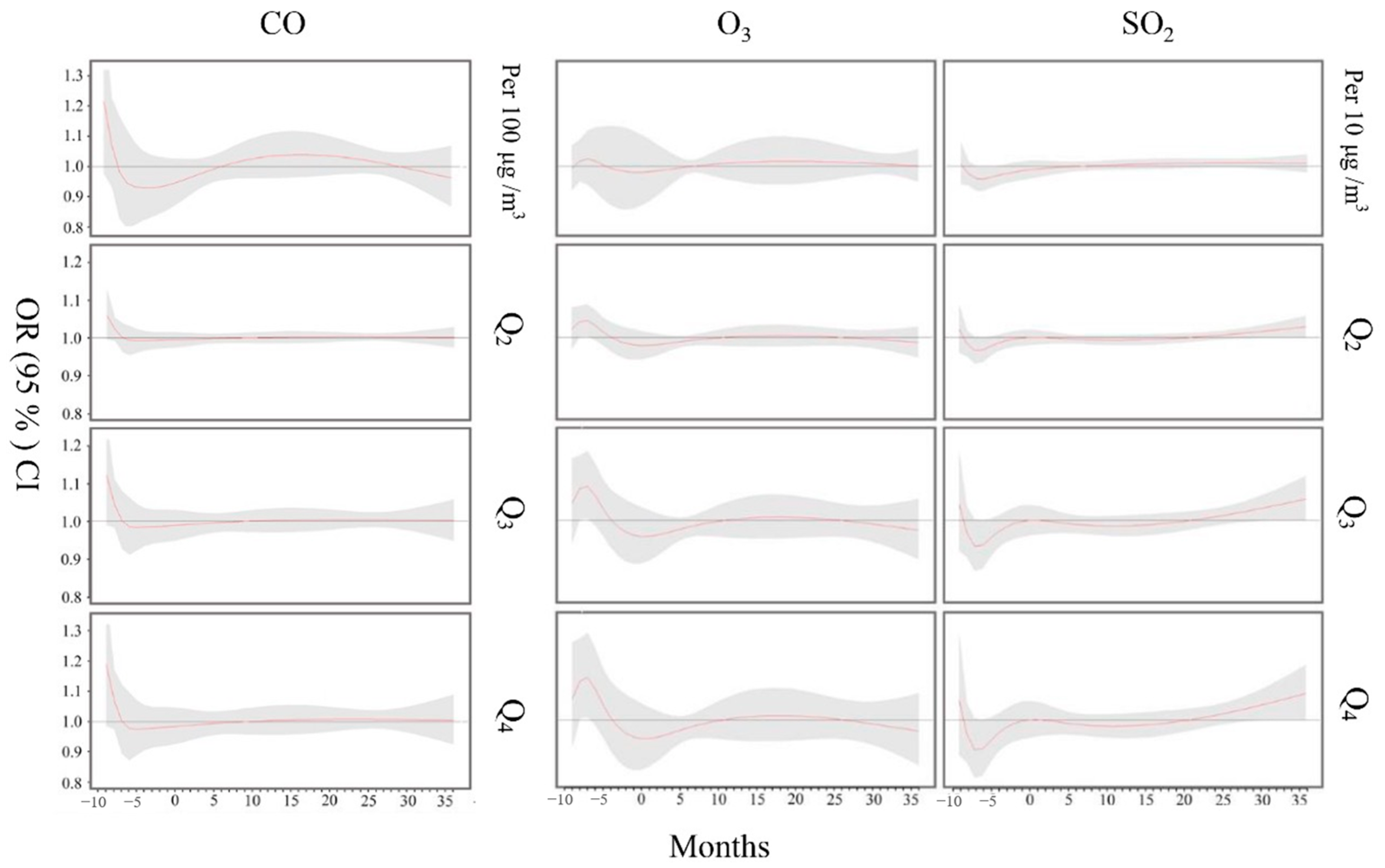
3.2. Effects of Air Pollution Exposure on Child Hyperactivity
3.3. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di, Q.; Wang, Y.; Zanobetti, A.; Wang, Y.; Koutrakis, P.; Choirat, C.; Dominici, F.; Schwartz, J.D. Air Pollution and Mortality in the Medicare Population. N. Engl. J. Med. 2017, 376, 2513–2522. [Google Scholar] [CrossRef] [PubMed]
- Zare Sakhvidi, M.J.; Lequy, E.; Goldberg, M.; Jacquemin, B. Air pollution exposure and bladder, kidney and urinary tract cancer risk: A systematic review. Environ. Pollut. 2020, 267, 115328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, J.; Kwong, J.C.; Burnett, R.T.; van Donkelaar, A.; Hystad, P.; Martin, R.V.; Bai, L.; McLaughlin, J.; Chen, H. Long-term exposure to air pollution and mortality in a prospective cohort: The Ontario Health Study. Environ. Int. 2021, 154, 106570. [Google Scholar] [CrossRef] [PubMed]
- Attademo, L.; Bernardini, F. Air Pollution as Risk Factor for Mental Disorders: In Search for a Possible Link with Alzheimer’s Disease and Schizophrenia. J. Alzheimers Dis. 2020, 76, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Perera, F.P.; Wang, S.; Rauh, V.; Zhou, H.; Stigter, L.; Camann, D.; Jedrychowski, W.; Mroz, E.; Majewska, R. Prenatal exposure to air pollution, maternal psychological distress, and child behavior. Pediatrics 2013, 132, e1284–e1294. [Google Scholar] [CrossRef] [Green Version]
- Sunyer, J.; Dadvand, P. Pre-natal brain development as a target for urban air pollution. Basic Clin. Pharmacol. Toxicol. 2019, 125 (Suppl. S3), 81–88. [Google Scholar] [CrossRef] [Green Version]
- Huff, R.D.; Carlsten, C.; Hirota, J.A. An update on immunologic mechanisms in the respiratory mucosa in response to air pollutants. J. Allergy Clin. Immunol. 2019, 143, 1989–2001. [Google Scholar] [CrossRef]
- Laine, J.E.; Bodinier, B.; Robinson, O.; Plusquin, M.; Scalbert, A.; Keski-Rahkonen, P.; Robinot, N.; Vermeulen, R.; Pizzi, C.; Asta, F.; et al. Prenatal Exposure to Multiple Air Pollutants, Mediating Molecular Mechanisms, and Shifts in Birthweight. Environ. Sci. Technol. 2020, 54, 14502–14513. [Google Scholar] [CrossRef]
- Yi, C.; Wang, Q.; Qu, Y.; Niu, J.; Oliver, B.G.; Chen, H. In-utero exposure to air pollution and early-life neural development and cognition. Ecotoxicol. Environ. Saf. 2022, 238, 113589. [Google Scholar] [CrossRef]
- Allen, J.L.; Oberdorster, G.; Morris-Schaffer, K.; Wong, C.; Klocke, C.; Sobolewski, M.; Conrad, K.; Mayer-Proschel, M.; Cory-Slechta, D.A. Developmental neurotoxicity of inhaled ambient ultrafine particle air pollution: Parallels with neuropathological and behavioral features of autism and other neurodevelopmental disorders. Neurotoxicology 2017, 59, 140–154. [Google Scholar] [CrossRef] [Green Version]
- Costa, L.G.; Chang, Y.C.; Cole, T.B. Developmental Neurotoxicity of Traffic-Related Air Pollution: Focus on Autism. Curr. Environ. Health Rep. 2017, 4, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Garciduenas, L.; Kulesza, R.J.; Doty, R.L.; D’Angiulli, A.; Torres-Jardon, R. Megacities air pollution problems: Mexico City Metropolitan Area critical issues on the central nervous system pediatric impact. Environ. Res. 2015, 137, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Brockmeyer, S.; D’Angiulli, A. How air pollution alters brain development: The role of neuroinflammation. Transl. Neurosci. 2016, 7, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Thapar, A.; Cooper, M. Attention deficit hyperactivity disorder. Lancet 2016, 387, 1240–1250. [Google Scholar] [CrossRef]
- Faraone, S.V.; Biederman, J.; Mick, E. The age-dependent decline of attention deficit hyperactivity disorder: A meta-analysis of follow-up studies. Psychol. Med. 2006, 36, 159–165. [Google Scholar] [CrossRef]
- Charach, A.; Yeung, E.; Climans, T.; Lillie, E. Childhood attention-deficit/hyperactivity disorder and future substance use disorders: Comparative meta-analyses. J. Am. Acad Child. Adolesc. Psychiatry 2011, 50, 9–21. [Google Scholar] [CrossRef]
- Groenman, A.P.; Oosterlaan, J.; Rommelse, N.; Franke, B.; Roeyers, H.; Oades, R.D.; Sergeant, J.A.; Buitelaar, J.K.; Faraone, S.V. Substance use disorders in adolescents with attention deficit hyperactivity disorder: A 4-year follow-up study. Addiction 2013, 108, 1503–1511. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.S.; Humphreys, K.L.; Flory, K.; Liu, R.; Glass, K. Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: A meta-analytic review. Clin. Psychol. Rev. 2011, 31, 328–341. [Google Scholar] [CrossRef] [Green Version]
- Shaw, M.; Hodgkins, P.; Caci, H.; Young, S.; Kahle, J.; Woods, A.G.; Arnold, L.E. A systematic review and analysis of long-term outcomes in attention deficit hyperactivity disorder: Effects of treatment and non-treatment. BMC Med. 2012, 10, 99. [Google Scholar] [CrossRef] [Green Version]
- Teran Prieto, A. Attention-deficit/hyperactivity disorder and substance abuse. Scientific evidence. Medicina (B Aires) 2020, 80 (Suppl. S2), 76–79. [Google Scholar]
- Liu, A.; Xu, Y.; Yan, Q.; Tong, L. The Prevalence of Attention Deficit/Hyperactivity Disorder among Chinese Children and Adolescents. Sci. Rep. 2018, 8, 11169. [Google Scholar] [CrossRef] [PubMed]
- Faraone, S.V.; Larsson, H. Genetics of attention deficit hyperactivity disorder. Mol. Psychiatry 2019, 24, 562–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thapar, A.; Cooper, M.; Jefferies, R.; Stergiakouli, E. What causes attention deficit hyperactivity disorder? Arch. Dis. Child. 2012, 97, 260–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abid, Z.; Roy, A.; Herbstman, J.B.; Ettinger, A.S. Urinary polycyclic aromatic hydrocarbon metabolites and attention/deficit hyperactivity disorder, learning disability, and special education in U.S. children aged 6 to 15. J. Environ. Public Health 2014, 2014, 628508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forns, J.; Sunyer, J.; Garcia-Esteban, R.; Porta, D.; Ghassabian, A.; Giorgis-Allemand, L.; Gong, T.; Gehring, U.; Sorensen, M.; Standl, M.; et al. Air Pollution Exposure During Pregnancy and Symptoms of Attention Deficit and Hyperactivity Disorder in Children in Europe. Epidemiology 2018, 29, 618–626. [Google Scholar] [CrossRef] [Green Version]
- Gong, T.; Almqvist, C.; Bolte, S.; Lichtenstein, P.; Anckarsater, H.; Lind, T.; Lundholm, C.; Pershagen, G. Exposure to air pollution from traffic and neurodevelopmental disorders in Swedish twins. Twin Res. Hum. Genet. 2014, 17, 553–562. [Google Scholar] [CrossRef] [Green Version]
- Maitre, L.; Julvez, J.; Lopez-Vicente, M.; Warembourg, C.; Tamayo-Uria, I.; Philippat, C.; Gutzkow, K.B.; Guxens, M.; Andrusaityte, S.; Basagana, X.; et al. Early-life environmental exposure determinants of child behavior in Europe: A longitudinal, population-based study. Environ. Int. 2021, 153, 106523. [Google Scholar] [CrossRef]
- Min, J.Y.; Min, K.B. Exposure to ambient PM10 and NO2 and the incidence of attention-deficit hyperactivity disorder in childhood. Environ. Int. 2017, 99, 221–227. [Google Scholar] [CrossRef]
- Mortamais, M.; Pujol, J.; van Drooge, B.L.; Macia, D.; Martinez-Vilavella, G.; Reynes, C.; Sabatier, R.; Rivas, I.; Grimalt, J.; Forns, J.; et al. Effect of exposure to polycyclic aromatic hydrocarbons on basal ganglia and attention-deficit hyperactivity disorder symptoms in primary school children. Environ. Int. 2017, 105, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Newman, N.C.; Ryan, P.; Lemasters, G.; Levin, L.; Bernstein, D.; Hershey, G.K.; Lockey, J.E.; Villareal, M.; Reponen, T.; Grinshpun, S.; et al. Traffic-related air pollution exposure in the first year of life and behavioral scores at 7 years of age. Environ. Health Perspect 2013, 121, 731–736. [Google Scholar] [CrossRef]
- Siddique, S.; Banerjee, M.; Ray, M.R.; Lahiri, T. Attention-deficit hyperactivity disorder in children chronically exposed to high level of vehicular pollution. Eur J. Pediatr. 2011, 170, 923–929. [Google Scholar] [CrossRef]
- Yorifuji, T.; Kashima, S.; Diez, M.H.; Kado, Y.; Sanada, S.; Doi, H. Prenatal exposure to outdoor air pollution and child behavioral problems at school age in Japan. Environ. Int. 2017, 99, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Yorifuji, T.; Kashima, S.; Higa Diez, M.; Kado, Y.; Sanada, S.; Doi, H. Prenatal Exposure to Traffic-related Air Pollution and Child Behavioral Development Milestone Delays in Japan. Epidemiology 2016, 27, 57–65. [Google Scholar] [CrossRef]
- Forns, J.; Dadvand, P.; Foraster, M.; Alvarez-Pedrerol, M.; Rivas, I.; Lopez-Vicente, M.; Suades-Gonzalez, E.; Garcia-Esteban, R.; Esnaola, M.; Cirach, M.; et al. Traffic-Related Air Pollution, Noise at School, and Behavioral Problems in Barcelona Schoolchildren: A Cross-Sectional Study. Environ. Health Perspect 2016, 124, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.; Chiu, Y.M.; Hsu, H.L.; Wright, R.O.; Wright, R.J.; Coull, B.A. Potential for Bias When Estimating Critical Windows for Air Pollution in Children’s Health. Am. J. Epidemiol. 2017, 186, 1281–1289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, X.Y.; Strodl, E.; Liu, B.Q.; Liu, L.; Yin, X.N.; Wen, G.M.; Sun, D.L.; Xian, D.X.; Jiang, H.; Jing, J.; et al. Association between prenatal exposure to household inhalants exposure and ADHD-like behaviors at around 3 years of age: Findings from Shenzhen Longhua Child Cohort Study. Environ. Res. 2019, 177, 108612. [Google Scholar] [CrossRef]
- Fang, X.Y.; Strodl, E.; Wu, C.A.; Liu, L.; Yin, X.N.; Wen, G.M.; Sun, D.L.; Xian, D.X.; Jiang, H.; Jing, J.; et al. Maternal cooking during pregnancy may increase hyperactive behaviors among children aged at around 3 years old. Indoor Air 2020, 30, 126–136. [Google Scholar] [CrossRef]
- He, G.H.; Liu, L.; Strodl, E.; Ruan, Z.L.; Jiang, H.; Jing, J.; Jin, Y.; Chen, W.Q. Parental Type D Personality and Children’s Hyperactive Behaviors: The Mediating Role of Parent(-)Child Interactive Activities. Int. J. Environ. Res. Public Health 2019, 16, 1116. [Google Scholar] [CrossRef] [Green Version]
- Araki, S.; Shima, M.; Yamamoto, K. Spatiotemporal land use random forest model for estimating metropolitan NO2 exposure in Japan. Sci. Total Environ. 2018, 634, 1269–1277. [Google Scholar] [CrossRef]
- Goyette, C.H.; Conners, C.K.; Ulrich, R.F. Normative data on revised Conners Parent and Teacher Rating Scales. J. Abnorm. Child Psychol. 1978, 6, 221–236. [Google Scholar] [CrossRef]
- Su, L.; Li, X.; Huang, C.; Luo, X.; Zhang, J. Norms of The Conners Parent Sympt.tom Questionnaire in Chinese Urban Children. Chin. J. Clin. Psychol. 2001, 9, 241–243. [Google Scholar]
- Conners, C.K.; Sitarenios, G.; Parker, J.D.; Epstein, J.N. The revised Conners’ Parent Rating Scale (CPRS-R): Factor structure, reliability, and criterion validity. J. Abnorm. Child Psychol. 1998, 26, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, K.; Raikkonen, K.; Pesonen, A.K.; Andersson, S.; Kajantie, E.; Eriksson, J.G.; Wolke, D.; Lano, A. Behavioural symptoms of attention deficit/hyperactivity disorder in preterm and term children born small and appropriate for gestational age: A longitudinal study. BMC Pediatr. 2010, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Hou, X.Y.; Yin, X.N.; Wen, G.M.; Sun, D.; Xian, D.X.; Fan, L.; Jiang, H.; Jing, J.; Jin, Y.; et al. Prenatal Exposure to Environmental Tobacco Smoke and Hyperactivity Behavior in Chinese Young Children. Int. J. Environ. Res. Public Health 2017, 14, 1132. [Google Scholar] [CrossRef] [Green Version]
- Zhu, P.; Hao, J.H.; Tao, R.X.; Huang, K.; Jiang, X.M.; Zhu, Y.D.; Tao, F.B. Sex-specific and time-dependent effects of prenatal stress on the early behavioral symptoms of ADHD: A longitudinal study in China. Eur. Child Adolesc. Psychiatry 2015, 24, 1139–1147. [Google Scholar] [CrossRef]
- DuPaul, G.J.; McGoey, K.E.; Eckert, T.L.; VanBrakle, J. Preschool children with attention-deficit/hyperactivity disorder: Impairments in behavioral, social, and school functioning. J. Am. Acad Child Adolesc. Psychiatry 2001, 40, 508–515. [Google Scholar] [CrossRef]
- Shojaei, T.; Wazana, A.; Pitrou, I.; Kovess, V. The stre.engths and difficulties questionnaire: Validation study in French school-aged children and cross-cultural comparisons. Soc. Psychiatry Psychiatr. Epidemiol. 2009, 44, 740–747. [Google Scholar] [CrossRef]
- Westerlund, J.; Ek, U.; Holmberg, K.; Naswall, K.; Fernell, E. The Conners’ 10-item scale: Findings in a total population of Swedish 10-11-year-old children. Acta Paediatr. 2009, 98, 828–833. [Google Scholar] [CrossRef]
- Gasparrini, A. Modeling exposure-lag-response associations with distributed lag non-linear models. Stat. Med. 2014, 33, 881–899. [Google Scholar] [CrossRef] [Green Version]
- Sunyer, J.; Esnaola, M.; Alvarez-Pedrerol, M.; Forns, J.; Rivas, I.; Lopez-Vicente, M.; Suades-Gonzalez, E.; Foraster, M.; Garcia-Esteban, R.; Basagana, X.; et al. Association between traffic-related air pollution in schools and cognitive development in primary school children: A prospective cohort study. PLoS Med. 2015, 12, e1001792. [Google Scholar] [CrossRef]
- Gidhagen, L.; Omstedt, G.; Pershagen, G.; Willers, S.; Bellander, T. High-resolution modeling of residential outdoor particulate levels in Sweden. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 306–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Y.; Zhu, Y.; Jang, J.; Wang, S.; Xing, J.; Chiang, P.C.; Zhao, X.; You, Z.; Yuan, Y. Source and sectoral contribution analysis of PM2.5 based on efficient response surface modeling technique over Pearl River Delta Region of China. Sci. Total Environ. 2020, 737, 139655. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, T.K.; Schendel, D. Sociodemographic risk factors for autism in a US metropolitan area. J. Autism. Dev. Disord. 2007, 37, 667–677. [Google Scholar] [CrossRef]
- Flouri, E.; Mavroveli, S.; Tzavidis, N. Cognitive ability, neighborhood deprivation, and young children’s emotional and behavioral problems. Soc. Psychiatry Psychiatr. Epidemiol. 2012, 47, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Kabir, Z.; Connolly, G.N.; Alpert, H.R. Secondhand smoke exposure and neurobehavioral disorders among children in the United States. Pediatrics 2011, 128, 263–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruckerl, R.; Greven, S.; Ljungman, P.; Aalto, P.; Antoniades, C.; Bellander, T.; Berglind, N.; Chrysohoou, C.; Forastiere, F.; Jacquemin, B.; et al. Air pollution and inflammation (interleukin-6, C-reactive protein, fibrinogen) in myocardial infarction survivors. Environ. Health Perspect 2007, 115, 1072–1080. [Google Scholar] [CrossRef] [Green Version]
- Tamagawa, E.; van Eeden, S.F. Impaired lung function and risk for stroke: Role of the systemic inflammation response? Chest 2006, 130, 1631–1633. [Google Scholar] [CrossRef]
- Calderon-Garciduenas, L.; Solt, A.C.; Henriquez-Roldan, C.; Torres-Jardon, R.; Nuse, B.; Herritt, L.; Villarreal-Calderon, R.; Osnaya, N.; Stone, I.; Garcia, R.; et al. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol. Pathol. 2008, 36, 289–310. [Google Scholar] [CrossRef]
- Valavanidis, A.; Fiotakis, K.; Vlachogianni, T. Airborne particulate matter and human health: Toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2008, 26, 339–362. [Google Scholar] [CrossRef]
- Block, M.L.; Elder, A.; Auten, R.L.; Bilbo, S.D.; Chen, H.; Chen, J.C.; Cory-Slechta, D.A.; Costa, D.; Diaz-Sanchez, D.; Dorman, D.C.; et al. The outdoor air pollution and brain health workshop. Neurotoxicology 2012, 33, 972–984. [Google Scholar] [CrossRef] [Green Version]
- Finkelstein, M.M.; Jerrett, M. A study of the relationships between Parkinson’s disease and markers of traffic-derived and environmental manganese air pollution in two Canadian cities. Environ. Res. 2007, 104, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Avila-Costa, M.R.; Colin-Barenque, L.; Fortoul, T.I.; Machado-Salas, P.; Espinosa-Villanueva, J.; Rugerio-Vargas, C.; Rivas-Arancibia, S. Memory deterioration in an oxidative stress model and its correlation with cytological changes on rat hippocampus CA1. Neurosci. Lett. 1999, 270, 107–109. [Google Scholar] [CrossRef]
- Perera, F.P.; Rauh, V.; Whyatt, R.M.; Tsai, W.Y.; Tang, D.; Diaz, D.; Hoepner, L.; Barr, D.; Tu, Y.H.; Camann, D.; et al. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environ. Health Perspect 2006, 114, 1287–1292. [Google Scholar] [CrossRef] [Green Version]
- Rivas-Arancibia, S.; Dorado-Martinez, C.; Colin-Barenque, L.; Kendrick, K.M.; de la Riva, C.; Guevara-Guzman, R. Effect of acute ozone exposure on locomotor behavior and striatal function. Pharmacol. Biochem. Behav. 2003, 74, 891–900. [Google Scholar] [CrossRef]
- Rice, D.; Barone, S., Jr. Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environ. Health Perspect 2000, 108 (Suppl. S3), 511–533. [Google Scholar] [CrossRef] [PubMed]
- Blum, J.L.; Chen, L.C.; Zelikoff, J.T. Exposure to Ambient Particulate Matter during Specific Gestational Periods Produces Adverse Obstetric Consequences in Mice. Environ. Health Perspect 2017, 125, 077020. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.Q.; Chen, Y.; Mi, B.B.; Dang, S.N.; Zhao, D.D.; Liu, R.; Wang, H.L.; Yan, H. Ambient air pollution and adverse birth outcomes: A systematic review and meta-analysis. J. Zhejiang Univ. Sci. B 2019, 20, 238–252. [Google Scholar] [CrossRef]
- Ministry of Environmental Protection of China. Ambient Air Quality Standards. GB 3095-2012; China Environmental Science Press: Beijing, China, 2012. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/dqhjbh/dqhjzlbz/201203/W020120410330232398521.pdf (accessed on 13 May 2020). (In Chinese)
- Chen, L.; Bai, Z.; Kong, S.; Han, B.; You, Y.; Ding, X.; Du, S.; Liu, A. A land use regression for predicting NO2 and PM10 concentrations in different seasons in Tianjin region, China. J. Environ. Sci. 2010, 22, 1364–1373. [Google Scholar] [CrossRef]
- Liu, C.; Henderson, B.H.; Wang, D.F.; Yang, X.Y.; Peng, Z.R. A land use regression application into assessing spatial variation of intra-urban fine particulate matter (PM2.5) and nitrogen dioxide (NO2) concentrations in City of Shanghai, China. Sci. Total Environ. 2016, 565, 607–615. [Google Scholar] [CrossRef]
- Shi, Y.; Lau, K.L.; Ng, E. Incorporating wind availability into land use regression modelling of air quality in mountainous high density urban environment. Environ. Res. 2017, 157, 17–29. [Google Scholar] [CrossRef]
- Hoek, G.; Beelen, R.; de Hoogh, K.; Vienneau, D.; Gulliver, J.; Fischer, P.; Briggs, D. A review of land-use regression models to assess spatial variation of outdoor air pollution. Atmos. Environ. 2008, 42, 7561–7578. [Google Scholar] [CrossRef]


| Variables | † Mean ± SD or n (%) | Variables | Mean ± SD or n (%) |
|---|---|---|---|
| Maternal age at child’s birth | 28.013 ± 3.973 | Gestation drinking | |
| Paternal age at child’s birth | 30.421 ± 4.678 | No | 25,896 (99.4) |
| Maternal education | Yes | 156 (0.6) | |
| Primary or secondary | 3804 (14.6) | Gestational hypertension | |
| High school | 14,250 (54.7) | No | 25,583 (98.2) |
| College and above | 7998 (30.7) | Yes | 469 (1.8) |
| Paternal education | Gestational diabetes mellitus | ||
| Primary or secondary | 3022 (11.6) | No | 24,619 (94.5) |
| High school | 12,244 (47.0) | Yes | 1433 (5.5) |
| College and above | 10,786 (41.4) | Preeclampsia/eclampsia | |
| Maternal occupational | No | 25,948 (99.6) | |
| Housewife | 3673 (14.1) | Yes | 104 (0.04) |
| Employed | 22,379 (85.9) | Taken folic acid during pregnancy | |
| Family income, RMB/month | No | 2293 (8.8) | |
| <5000 | 2319 (8.9) | Yes | 23,759 (91.2) |
| 5001–10,000 | 5497 (21.1) | Taken calcium during pregnancy | |
| 10,001–20,000 | 9170 (35.2) | No | 3595 (13.8) |
| >20,000 | 9066 (34.8) | Yes | 22,457 (86.2) |
| Maternal marital status | Taken vitamins during pregnancy | ||
| Married | 25,609 (98.3) | No | 15,397 (59.1) |
| Unmarried/divorced/windowed | 443 (1.7) | Yes | 10,655 (40.9) |
| Singleton pregnancy | Feeding pattern | ||
| No | 651 (2.5) | Breast feeding | 6721 (25.8) |
| Yes | 25,401 (97.5) | Mixed feeding | 16,621 (63.8) |
| Delivery mode | Artificial feeding | 2710 (10.4) | |
| Eutocia | 14,459 (55.5) | Number of person residence | |
| Caesarean | 11,593 (44.5) | ≤4 | 17,325 (66.5) |
| Child age | 3.506 ± 0.290 | >4 | 8727 (33.5) |
| Child sex | Single child | ||
| Boy | 14,146 (54.3) | No | 12,192 (46.8) |
| Girl | 11,906 (45.7) | Yes | 13,860 (53.2) |
| Air Pollutants | Mean | SD | Min | P25 | P50 | P75 | Max |
|---|---|---|---|---|---|---|---|
| CO (mg/m3) | 0.842 | 0.150 | 0.402 | 0.707 | 0.830 | 0.978 | 1.456 |
| PM2.5 (μg/m3) | 36.161 | 10.276 | 11.439 | 28.248 | 34.972 | 43.040 | 92.666 |
| PM10 (μg/m3) | 54.566 | 15.501 | 18.863 | 43.183 | 52.967 | 64.726 | 113.970 |
| NO2 (μg/m3) | 34.627 | 9.845 | 12.477 | 27.384 | 33.602 | 41.070 | 71.998 |
| O3 (μg/m3) | 93.951 | 25.108 | 26.930 | 74.896 | 91.375 | 109.187 | 197.713 |
| SO2 (μg/m3) | 8.983 | 2.600 | 1.285 | 6.828 | 8.489 | 10.471 | 25.013 |
| Mean temperature (°C) | 22.925 | 5.277 | 11.247 | 18.439 | 23.697 | 27.934 | 29.561 |
| Air Pollutants (10 ug/m3) | Total Cumulative Effects | Cumulative Effects during Sensitive Period | ||
|---|---|---|---|---|
| OR (95% CI) | β (95% CI) | OR (95% CI) | β (95% CI) | |
| PM10 | 1.315 (1.145~1.511) | 0.030 (0.020~0.040) | 1.296 (1.114~1.508) | 0.031 (0.021~0.039) |
| PM2.5 | 1.335 (1.188~1.500) | 0.034 (0.026~0.043) | 1.447 (1.175~1.781) | 0.033 (0.019~0.048) |
| NO2 | 1.385 (1.126~1.703) | 0.038 (0.023~0.052) | 1.387 (1.095~1.757) | 0.039 (0.023~0.056) |
| † CO | 1.046 (0.975~1.122) | 0.006 (−0.001~0.011) | - | - |
| SO2 | 0.863 (0.659~1.131) | 0.023 (−0.003~0.043) | - | - |
| O3 | 0.985 (0.946~1.026) | 0.010 (−0.003~0.003) | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Fang, X.; Strodl, E.; He, G.; Ruan, Z.; Wang, X.; Liu, L.; Chen, W. Fetal Exposure to Air Pollution in Late Pregnancy Significantly Increases ADHD-Risk Behavior in Early Childhood. Int. J. Environ. Res. Public Health 2022, 19, 10482. https://doi.org/10.3390/ijerph191710482
Liu B, Fang X, Strodl E, He G, Ruan Z, Wang X, Liu L, Chen W. Fetal Exposure to Air Pollution in Late Pregnancy Significantly Increases ADHD-Risk Behavior in Early Childhood. International Journal of Environmental Research and Public Health. 2022; 19(17):10482. https://doi.org/10.3390/ijerph191710482
Chicago/Turabian StyleLiu, Binquan, Xinyu Fang, Esben Strodl, Guanhao He, Zengliang Ruan, Ximeng Wang, Li Liu, and Weiqing Chen. 2022. "Fetal Exposure to Air Pollution in Late Pregnancy Significantly Increases ADHD-Risk Behavior in Early Childhood" International Journal of Environmental Research and Public Health 19, no. 17: 10482. https://doi.org/10.3390/ijerph191710482






