Outcomes of COVID-19 Patients Admitted to the Intermediate Respiratory Care Unit: Non-Invasive Respiratory Therapy in a Sequential Protocol
Abstract
:Highlights
- In our patient population, 57% of patients improved during an Intermediate Respiratory Care Unit stay without Intensive Care Unit admission.
- Age and lack of corticosteroid treatment were associated with higher mortality regardless of the severity of hypoxic respiratory failure and the non-invasive therapy applied.
- A rapid respiratory worsening despite maximal non-invasive therapy involves bad outcomes being mandatory not to delay intubation in this scenario.
- Starting non-invasive ventilation as the first line of non-invasive therapy does not always mean bad outcomes (further intubation).
Abstract
1. Background
2. Methods
3. Results
3.1. Characterization Depending on Severity: Moderate vs. Severe HRF
3.2. Mortality, ICU Transfer, and Intubation Rates in NIV Group (Severe HRF)
3.3. Mortality, ICU Transfer and Intubation Rates in Non-NIV Group (Moderate HRF)
3.4. Predicting Factors of Patient Outcomes: Survival, ICU Transfer, and Intubation Rates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CPAP | continuous positive airway pressure |
| CRP | serum C-reactive protein |
| EPAP | expiratory positive airway pressure |
| FiO2 | inspired oxygen fraction |
| HCO3 | arterial bicarbonate levels |
| HFNC | high flow nasal cannula |
| HRF | hypoxemic respiratory failure |
| IRCU | Intermediate respiratory care unit |
| ICU | Intensive care unit |
| IPAP | inspiratory positive airway pressure |
| LDH | serum lactate dehydrogenase |
| NIV | non-invasive ventilation |
| NRT | non-invasive respiratory therapy |
| PaCO2 | partial arterial pressure of carbon dioxide |
| PaO2 | partial arterial pressure of oxygen |
| PaFiO2 | partial arterial pressure of oxygen divided by inspired oxygen fraction |
| PEEP | positive end-expiratory pressure |
| RR | respiratory rate |
| SaO2 | arterial oxygen saturation |
| SaFiO2 | oxygen saturation by pulse oximetry divided by inspired oxygen fraction |
| SpO2 | oxygen saturation by pulse oximetry |
References
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72,314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2019, 7, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Plate, J.D.J.; Leenen, L.P.H.; Houwert, M.; Hietbrink, F. Utilisation of Intermediate Care Units: A Systematic Review. Crit. Care Res. Pr. 2017, 2017, 8038460. [Google Scholar] [CrossRef] [PubMed]
- Raoof, S.; Nava, S.; Carpati, C.; Hill, N.S. High-Flow, Noninvasive Ventilation and Awake (Nonintubation) Proning in Patients with Coronavirus Disease 2019 With Respiratory Failure. Chest 2020, 158, 1992–2002. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.C.; Geoghegan, L.; Arbyn, M.; Mohammed, Z.; McGuinness, L.; Clarke, E.L.; Wade, R.G. The prevalence of symptoms in 24,410 adults infected by the novel coronavirus (SARS-CoV-2; COVID-19): A systematic review and meta-analysis of 148 studies from 9 countries 2020. PLoS ONE 2020, 15, e0234765. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Living Guidance for Clinical Management of COVID-19: Living Guidance; World Health Organization (WHO): Geneva, Switzerland, 23 November 2021. [Google Scholar]
- Maves, R.C.; Downar, J.; Dichter, J.R.; Hick, J.L.; Devereaux, A.; Geiling, J.A.; Kissoon, N.; Hupert, N.; Niven, A.S.; King, M.A.; et al. Triage of Scarce Critical Care Resources in COVID-19 An Implementation Guide for Regional Allocation an Expert Panel Report of the Task Force for Mass Critical Care and the American College of Chest Physicians. Chest 2020, 158, 212–225. [Google Scholar] [CrossRef]
- Leclerc, T.; Donat, N.; Donat, A.; Pasquier, P.; Libert, N.; Schaeffer, E.; D’Aranda, E.; Cotte, J.; Fontaine, B.; Perrigault, P.-F.; et al. Prioritisation of ICU treatments for critically ill patients in a COVID-19 pandemic with scarce resources. Anaesth. Crit. Care Pain Med. 2020, 39, 333–339. [Google Scholar] [CrossRef]
- The RECOVERY Collaborative Group. Dexamethasone in Hospitalized patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Suarez-Cuartin, G.; Gasa, M.; Bermudo, G.; Ruiz, Y.; Hernandez-Argudo, M.; Marin, A.; Trias-Sabria, P.; Cordoba, A.; Cuevas, E.; Sarasate, M.; et al. Clinical Outcomes of Severe COVID-19 Patients Admitted to an Intermediate Respiratory Care Unit. Front. Med. 2021, 8, 711027. [Google Scholar] [CrossRef]
- Carpagnano, G.E.; Migliore, G.; Grasso, S.; Procacci, V.; Resta, E.; Panza, F.; Resta, O. More skilled clinical management of COVID-19 patients modified mortality in an intermediate respiratory intensive care unit in Italy 2020. Respir. Res. 2021, 22, 16. [Google Scholar] [CrossRef]
- Ferreyro, B.L.; Angriman, F.; Munshi, L.; Del Sorbo, L.; Ferguson, N.D.; Rochwerg, B.; Ryu, M.J.; Saskin, R.; Wunsch, H.; da Costa, B.R.; et al. Association of Noninvasive Oxygenation Strategies With All-Cause Mortality in Adults With Acute Hypoxemic Respiratory Failure A Systematic Review and Meta-analysis. JAMA 2020, 324, 57–67. [Google Scholar] [CrossRef]
- Crimi, C.; Noto, A.; Cortegiani, A.; Impellizzeri, P.; Elliott, M.; Ambrosino, N.; Gregoretti, C. Noninvasive respiratory support in acute hypoxemic respiratory failure associated with COVID-19 and other viral infections. Minerva Anestesiol. 2020, 86, 1190–1204. [Google Scholar] [CrossRef] [PubMed]
- Crimi, C.; Noto, A.; Madotto, F.; Ippolito, M.; Nolasco, S.; Campisi, R.; De Vuono, S.; Fiorentino, G.; Pantazopoulos, I.; Chalkias, A.; et al. High-flow nasal oxygen versus conventional oxygen therapy in patients with COVID-19 pneumonia and mild hypoxaemia: A randomised controlled trial. Thorax 2022. [Google Scholar] [CrossRef] [PubMed]
- Ospina-Tascon, G.A.; Calderón-Tapia, L.E.; Garcia, A.F.; Zarama, V.; Gomez-Alvarez, F.; Alvarez-Saa, T.; Pardo-Otálvaro, S.; Bautista-Rincón, D.F.; Vargas, M.P.; Aldana-Díaz, J.L.; et al. Effect of High-Flow Oxygen Therapy vs. Conventional Oxygen Therapy on Invasive Mechanical Ventilation and Clinical Recovery in Patients With Severe COVID-19 A Randomized Clinical Trial. JAMA 2021, 326, 2161–2171. [Google Scholar] [CrossRef]
- Grieco, D.L.; Menga, L.S.; Cesarano, M.; Rosà, T.; Spadaro, S.; Bitondo, M.M.; Montomoli, J.; Falò, G.; Tonetti, T.; Cutuli, S.L.; et al. Effect of Helmet Noninvasive Ventilation vs High-Flow Nasal Oxygen on Days Free of Respiratory Support in Patients With COVID-19 and Moderate to Severe Hypoxemic Respiratory Failure the HENIVOT Randomized Clinical Trial. JAMA 2021, 325, 1731–1743. [Google Scholar] [CrossRef] [PubMed]
- Perkins, G.D.; Ji, C.; Connolly, B.A.; Couper, K.; Lall, R.; Baillie, J.K.; Bradley, J.M.; Dark, P.; Dave, C.; de Soyza, A.; et al. Effect of Noninvasive Respiratory Strategies on Intubation or Mortality Among Patients with Acute Hypoxemic Respiratory Failure and COVID-19 The RECOVERY-RS Randomized Clinical Trial. JAMA 2022, 327, 546–558. [Google Scholar] [CrossRef]
- Hentsch, L.; Cocetta, S.; Allali, G.; Santana, I.; Eason, R.; Adam, E.; Janssens, J.P. Breathlessness and COVID-19: A Call for Research. Respiration 2021, 100, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Fuehner, T.; Renger, I.; Welte, T.; Freundt, T.; Gottlieb, J. Clinical Investigations Silent Hypoxia in COVID-19: A Case Series. Respiration 2019, 101, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Dhont, S.; Derom, E.; Van Braeckel, E.; Depuydt, P.; Lambrecht, B.N. The pathophysiology of “happy” hypoxemia in COVID-19. Respir Res. 2020, 21, 198. [Google Scholar] [CrossRef]
- Nair, P.R.; Haritha, D.; Behera, S.; Kayina, C.A.; Maitra, S.; Anand, R.K.; Ray, B.R.; Soneja, M.; Subramaniam, R.; Baidya, D.K. Comparison of High-Flow Nasal Cannula and Noninvasive Ventilation in Acute Hypoxemic Respiratory Failure Due to Severe COVID-19 Pneumonia. Respir. Care 2021, 66, 1824–1830. [Google Scholar] [CrossRef]
- Duca, A.; Memaj, I.; Zanardi, F.; Preti, C.; Alesi, A.; Della Bella, L.; Ghezzia, E.; di Marco, F.; Lorini, F.L.; Venturelli, S.; et al. Severity of respiratory failure and outcome of patients needing a ventilatory support in the Emergency Department during Italian novel coronavirus SARS-CoV2 outbreak: Preliminary data on the role of Helmet CPAP and Non-Invasive Positive Pressure Ventilation. EClinicalMedicine 2020, 24, 100419. [Google Scholar]
- Gorman, E.; Connolly, B.; Couper, K.; Perkins, G.D.; McAuley, D.F. Non-invasive respiratory support strategies in COVID-19. Lancet Respir. Med. 2021, 9, 553–556. [Google Scholar] [CrossRef]
- Arabi, Y.; Aldekhyl, S.; Al Qahtani, S.; Al-Dorzi, H.M.; Abdukahil, S.A.; Jose, J.; Al Harbi, M.K.; Al Haji, H.; Al Mutairi, M.; Al Zumai, O.; et al. Helmet noninvasive ventilation for COVID-19 patients (Helmet-COVID): Statistical analysis plan for a randomized controlled trial. Trials 2022, 23, 105. [Google Scholar] [CrossRef] [PubMed]
- Friedman, E.; Franzone, J.; Ko, E.R.; Corey, K.; Mock, J.; Alavian, N.; Schwartz, A.; Drummond, M.B.; Suber, T.; Linstrum, K.; et al. Rationale and design of the Prone Position and Respiratory Outcomes in Non-intubated COVID-19 Patients: The “PRONE” Study. Contemp. Clin. Trials 2021, 109, 106541. [Google Scholar] [CrossRef] [PubMed]
- Tomazini, B.M.; Maia, I.S.; Cavalcanti, A.B.; Berwanger, O.; Rosa, R.G.; Veiga, V.C.; Avezum, A.; Lopes, R.D.; Bueno, F.R.; Silva, M.V.A.O.; et al. Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients with Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19 The CoDEX Randomized Clinical Trial. JAMA 2020, 324, 1307–1316. [Google Scholar] [CrossRef]
- Cano, E.J.; Fuentes, X.F.; Campioli, C.C.; O’Horo, J.C.; Abu Saleh, O.; Odeyemi, Y.; Yadav, H.; Temesgen, Z. Impact of Corticosteroids in Coronavirus Disease 2019 Outcomes Systematic Review and Meta-analysis. Chest 2021, 159, 1019–1040. [Google Scholar] [CrossRef]
- Malik, P.; Patel, U.; Mehta, D. Biomarkers and outcomes of COVID-19 hospitalisations: Systematic review and meta-analysis. BMJ Evid.-Based Med. 2021, 26, 107–108. [Google Scholar] [CrossRef]
- Burke, H.; Freeman, A.; Cellura, D.C.; Stuart, B.L.; Brendish, N.J.; Poole, S.; Borca, F.; Phan, H.T.T.; Sheard, N.; Williams, S.; et al. Inflammatory phenotyping predicts clinical outcome in COVID-19. Respir. Res. 2020, 21, 245. [Google Scholar] [CrossRef]
- Liao, D.; Zhou, F.; Luo, L.; Xu, M.; Wang, H.; Xia, J.; Gao, Y.; Cai, L.; Wang, Z.; Yin, P.; et al. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: A retrospective cohort study. Lancet Haematol. 2020, 7, e671–e678. [Google Scholar] [CrossRef]
- Smilowitz, N.R.; Kunichoff, D.; Garshick, M.; Shah, B.; Pillinger, M.; Hochman, J.S.; Berger, J.S. C-reactive protein and clinical outcomes in patients with COVID-19. Eur. Heart J. 2021, 42, 2270–2279. [Google Scholar] [CrossRef]
- Gómez-Mesa, J.E.; Galindo-Coral, S.; Montes, M.C.; Muñoz Martin, A.J.M. Thrombosis and Coagulopathy in COVID-19. Curr. Probl. Cardiol. 2021, 46, 100742. [Google Scholar] [CrossRef]
- Bivona, G.; Agnello, L.; Ciaccio, A.M. Biomarkers for Prognosis and Treatment Response in COVID-19 Patients. Ann. Lab. Med. 2021, 1, 540–548. [Google Scholar] [CrossRef] [PubMed]

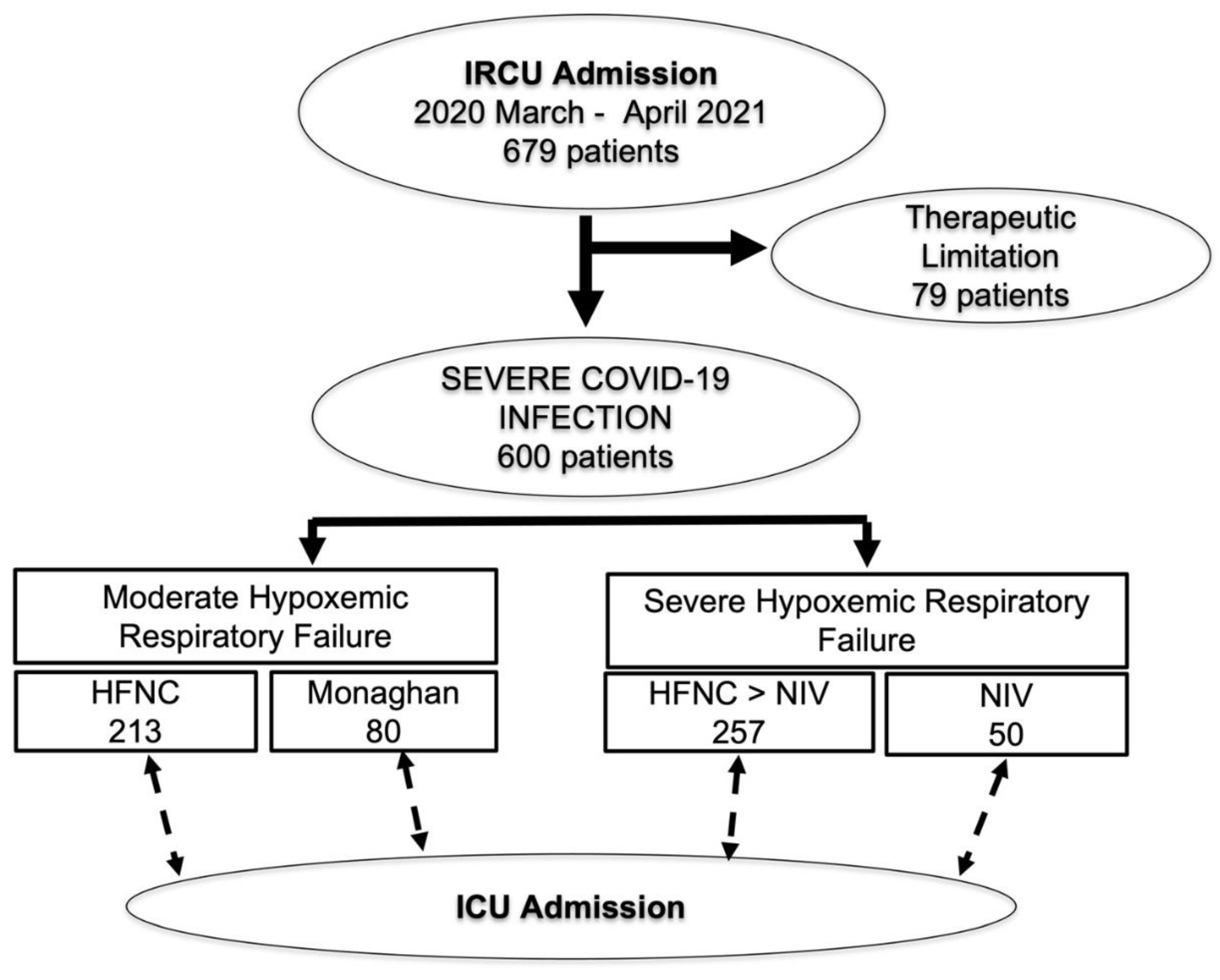



| TOTAL n = 600 | NIV Not Required n = 293 | NIV Required n = 307 | p | |
|---|---|---|---|---|
| SaFiO2 | 158.0 (68) | 173 (76) | 139 (51) | <0.001 |
| PaFiO2 | 156.4 (83) | 184 (93) n = 123 | 137 (68) n = 172 | <0.001 |
| Age (years) | 61 (11) | 59 (11) | 63 (11) | <0.001 |
| Female (n, %) | 193 (32%) | 96 (33%) | 97 (31%) | NS |
| HTA (n, %) | 276 (48%) | 116 (49%) | 160 (55%) | NS |
| Dyslipidemia (n, %) | 242 (42%) | 102 (45%) | 142 (49%) | NS |
| Diabetes (n, %) | 145(25%) | 58 (27%) | 87 (31%) | NS |
| Obesity (n, %) | 176 (30%) | 72 (32%) | 104 (37%) | NS |
| Cardiovascular disease (n, %) | 71 (12%) | 27 (13%) | 44 (16%) | NS |
| Respiratory disease (n, %) None OSA COPD Asthma | 493 (82%) 53(10%) 26 (4%) 24 (4%) | 243 (83%) 24 (8%) 12 (4%) 12 (4%) | 250 (81%) 29 (9%) 14 (4%) 12 (4%) | NS |
| Chronic kidney failure (n, %) | 56 (9%) | 18 (6%) | 38 (12%) | 0.026 |
| History of malignancy (n, %) | 71 (12%) | 37 (17%) | 34 (12%) | NS |
| Chronic liver disease (n, %) | 42 (7%) | 22 (10%) | 20 (7%) | NS |
| Chronic IS therapy (n, %) | 30 (5%) | 10 (3%) | 20 (7%) | NS |
| Length of stay (days) | 29.5 (30.0) | 20.1 (19.2) | 34.7 (31.0) | <0.001 |
| Length pre-IRCU stay (days) | 4.8 (13.5) | 3.0 (6.1) | 2.5 (6.5) | NS |
| Length of IRCU stay (days) | 8.7 (12.2) | 7.9 (9.1) | 8.6 (13.2) | NS |
| Length post-IRCU stay (days) | 14.5 (24.4) | 9.3 (16.3) | 24.0 (30.9) | <0.001 |
| Wave of Hospital Admission (n, %) 1st (March 20–August 20) 2nd (Sep 20–Dec 20) 3rd (Jan 2021–June 21) | 256 (42%) 144 (24%) 202 (34%) | 136 (46%) 74 (25%) 83 (28%) | 120 (39%) 70 (23%) 119 (38%) | 0.029 |
| Setting of Hospital admission (n, %) General Ward IRCU Advanced IRCU | 274 (44%) 307 (50%) 21 (6%) | 162 (55%) 125 (43%) 6 (20%) | 112 (36%) 182 (59%) 15 (5%) | <0.001 |
| Length IRCU Admission—OT (days) | 5.7 (4.5) | 4.8 (6.9) n = 29 | 5.9 (4.0) n = 165 | NS |
| Length ICU admission—OT (days) | 1.7 (2.9) | 1.4 (2.4) | 1.8 (3.0) | NS |
| RR (breaths/minute) | 23.9 (5.5) | 23.2 (4.9) n = 147 | 24.4 (5.9) n = 190 | 0.045 |
| PaCO2 (mmHg) | 36.3 (9.7) | 35.3 (5.4) n = 124 | 37.0 (11.9) n = 173 | NS |
| Seric Bicarbonate (mEq/L) | 28.4 (26.3) | 29.3 (31.7) | 27.8 (21.7) | NS |
| Ferritin (ng/L) | 1745 (2690) | 1753 (2854) | 1764 (2629) | NS |
| LDH (U/L) | 434 (181) | 392 (134) | 472 (203) | <0.001 |
| D-dimer (mcg/L) | 1831 (5897) | 1137 (3147) | 2386 (7459) | 0.014 |
| C-reactive protein (mg/L) | 135 (146) | 123 (106) | 151 (175) | 0.019 |
| NRT in IRCU (n, %) Monaghan HFNC HFNC→NIV Initial NIV | 80 (13%) 213 (33%) 257 (41%) 50 (8%) | 80 (27%) 213 (73%) - - | - - 257 (84%) 50 (16%) | - |
| FiO2 HFNC (%) | 90.3 (50.8) | 80.7 (13.4) n = 135 | 97.6 (6.5) n = 175 | 0.003 |
| Time on HFNC (days) | 4.0 (3.8) | 6.0 (3.5) n = 135 | 2.6 (3.3) n = 188 | <0.001 |
| Corticosteroids (n, %) No treatment Bolus Bolus + tapering Low-dose | 75 (12%) 193 (32%) 316 (53%) 19 (3%) | 50 (17%) 83 (28%) 150 (51%) 10 (3%) | 25 (8%) 110 (36%) 166 (54%) 9 (3%) | 0.005 |
| Tocilizumab (n, %) | 242 (41%) | 108 (37%) | 134 (43%) | 0.068 |
| Remdesivir (n, %) | 93 (17%) | 52 (18%) | 41 (13%) | NS |
| ICU transfer rate (n,%) | 258 (43%) | 46 (16%) | 212 (69%) | <0.001 |
| Intubation rate (n, %) | 220 (36%) | 44 (15%) | 176 (57%) | <0.001 |
| Survival rate (n, %) | 469 (78%) | 279 (95%) | 205 (67%) | <0.001 |
| NIV Required n = 307 | DEAD n = 102 | ALIVE n = 205 | p | OT n = 176 | NO OT n = 132 | p | |
|---|---|---|---|---|---|---|---|
| Age (years) | 63 (11) | 68 (8) | 60 (11) | <0.001 | 64 (11) | 61 (11) | 0.008 |
| Female (n, %) | 97 (31%) | 37 (36%) | 60 (29%) | NS | 59 (33%) | 38 (29%) | NS |
| HTA (n, %) | 160 (55%) | 55 (55%) | 105 (55%) | NS | 90 (53%) | 70 (58%) | NS |
| Dyslipidemia (n, %) | 142 (49%) | 51 (50%) | 89 (48%) | NS | 88 (51%) | 53 (46%) | NS |
| Diabetes (n, %) | 87 (31%) | 26 (26%) | 61 (33%) | NS | 54 (32%) | 33 (29%) | NS |
| Obesity (n, %) | 104 (37%) | 29 (30%) | 74 (41%) | 0.076 | 53 (31%) | 50 (45%) | 0.026 |
| Cardiovascular disease (n, %) | 44 (16%) | 20 (20%) | 23 (13%) | NS | 22 (13%) | 21 (19%) | NS |
| Respiratory disease (n, %) None OSA COPD Asthma | 250 (81%) 29 (9%) 14 (4%) 12 (4%) | 76 (74%) 11 (11%) 8 (8%) 4 (45) | 172 (84%) 18 (9%) 6 (3%) 8 (4%) | NS | 146 (83%) 12 (7%) 9 (5%) 6 (3%) | 103 (78%) 17 (13%) 5 (4%) 6 (4%) | NS |
| Chronic kidney failure (n, %) | 38 (12%) | 25 (24%) | 13 (6%) | <0.001 | 24 (14%) | 15 (12%) | NS |
| History of malignancy (n, %) | 34 (12%) | 14 (14%) | 20 (11%) | NS | 24 (14%) | 10 (9%) | NS |
| Chronic liver disease (n, %) | 20 (7%) | 4 (4%) | 16 (9%) | NS | 9 (5%) | 11 (10%) | NS |
| Chronic IS therapy (n, %) | 20 (7%) | 13 (13%) | 7 (3%) | 0.014 | 13 (7%) | 7 (5%) | NS |
| Length of hospital stay (days) | 34.7 (31.0) | 28.3 (22.4) | 37.4 (33.4) | 0.013 | 44.0 (36.9) | 22.5 (12.8) | <0.001 |
| Length of pre-IRCU stay (days) | 2.5 (6.5) | 2.0 (5.1) | 2.8 (7.1) | NS | 2.7 (7.6) | 2.3 (4.6) | NS |
| Length of IRCU stay (days) | 8.6 (13.2) | 6.0 (4.8) | 9.9 (15.7) | 0.003 | 7.3 (15.1) | 10.2 (10.0) | 0.048 |
| Length of post-IRCU stay (days) | 24.0 (30.9) | 20.3 (22.1) | 24.8 (33.7) | NS | 34.0 (36.9) | 10.0 (10.1) | <0.001 |
| Wave at H. admission (n, %) 1st (March 20–August 20) 2nd (September 20–December 20) 3rd (January 21–June 21) | 120 (39%) 70 (23%) 119 (38%) | 36 (35%) 34 (33%) 32 (31%) | 84 (41%) 36 (18%) 85 (41%) | 0.007 | 77 (44%) 39 (22%) 60 (34%) | 43 (33%) 32 (23%) 58 (44%) | NS |
| Setting at H. admission (n, %) General Ward IRCU Advanced IRCU | 112 (36%) 182 (59%) 15 (5%) | 38 (37%) 63 (62%) 1 (1%) | 74 (36%) 117 (57%) 14 (7%) | 0.080 | 53 (30%) 113 (64%) 10 (6%) | 59 (45%) 86 (51%) 5 (4%) | 0.030 |
| ICU transfer (n, %) | 210 (69%) | 96 (94%) | 114 (57%) | <0.001 | 176 (100%) | 36 (27%) | <0.001 |
| Intubation rate (n, %) | 175 (57%) | 89 (87%) | 86 (42%) | <0.001 | 176 (100%) | - | - |
| Length IRCU Adm.—OT (days) | 5.9 (4.0) n = 165 | 7.2 (4.1) n = 89 | 4.3 (3.3) n = 75 | <0.001 | 5.9 (4.0) n = 165 | 5.9 (4.0) n = 165 | - |
| Length ICU Adm.—OT (days) | 1.8 (3.0) | 2.1 (3.1) | 1.4 (3.0) | NS | 1.8 (3.0) | 1.8 (3.0) | - |
| SaFiO2 | 139 (51) | 135 (47) | 140 (53) | NS | 138 (51) | 140 (51) | NS |
| PaFiO2 | 137 (68) n = 172 | 135 (64) n = 58 | 138 (71) n = 124 | NS | 126 (55) n = 92 | 138 (51) n = 79 | 0.021 |
| RR (breaths/minute) | 24.4 (5.9) n = 190 | 24.7 (5.7) n = 64 | 24.4 (5.9) n = 124 | NS | 25.0 (5.9) n = 100 | 23.9 (5.7) n = 90 | NS |
| PaCO2 (mmHg) | 37.0 (11.9) n = 173 | 36.4 (10.6) n = 58 | 37.4 (12.6) n = 113 | NS | 35.6 (8.4) n = 92 | 38.6 (14.8) n = 80 | NS |
| Seric Bicarbonate (mEq/L) | 27.8 (21.7) | 25.1 (6.8) | 27.5 (19.3) | NS | 28.9 (19.1) | 26.4 (6.3) | NS |
| Ferritin (ng/L) | 1764 (2629) | 1704 (1709) | 1599 (1672) | NS | 1748 (2957) | 1772 (2173) | NS |
| LDH (U/L) | 472 (203) | 500 (211) | 457 (198) | NS | 501 (217) | 433 (178) | 0.004 |
| D-dimer (mcg/L) | 2386 (7459) | 4130 (10,801) | 1540 (4920) | 0.004 | 2999 (8637) | 1601 (5550) | 0.009 |
| C-reactive protein (mg/L) | 151 (175) | 147 (115) | 148 (183) | NS | 178 (212) | 116 (99) | 0.002 |
| NRT in IRCU (n, %) HFNC -> NIV Initial NIV | 257 (84%) 50 (16%) | 86 (84%) 16 (16%) | 171 (83%) 34 (17%) | NS | 145 (84%) 31 (18%) | 113 (86%) 19 (14%) | NS |
| FiO2 HFNC (%) | 97.6 (6.5) n = 175 | 93.2 (4.5) n = 60 | 98.0 (8.1) n = 113 | NS | 98.8 (9.1) n = 89 | 91.2 (8.4) n = 85 | NS |
| FiO2 NIV (%) | 94.3 (12.1) n = 181 | 97.4 (7.9) n = 64 | 92.5 (13.8) n = 115 | 0.009 | 97.7 (6.5) n = 96 | 90.3 (15.5) n = 84 | <0.001 |
| IPAP (cmH2O) | 14.5 (1.9) n = 190 | 15.0 (2.0) n = 65 | 14.2 (1.8) n = 120 | 0.008 | 14.4 (1.8) n = 100 | 14.7 (2.0) n = 86 | NS |
| EPAP (cmH2O) | 8.4 (1.5) n = 190 | 8.4 (1.6) n = 65 | 8.4 (1.5) n = 123 | NS | 8.4 (1.4) n = 100 | 8.4 (1.7) n = 89 | NS |
| Time on HFNC (days) | 2.6 (3.3) n = 188 | 2.2 (2.8) n = 64 | 3.0 (3.6) n = 122 | NS | 1.8 (2.6) n = 98 | 3.6 (3.8) n = 89 | <0.001 |
| Time on intermittent NIV (days) | 2.7 (3.2) n = 189 | 2.1 (2.3) n = 62 | 3.0 (3.6) n = 125 | NS | 1.6 (1.7) n = 97 | 3.8 (4.0) n = 91 | <0.001 |
| Time on continuous NIV (days) | 1.6 (2.7) n = 189 | 2.5 (3.9) n = 60 | 1.1 (1.2) n = 95 | 0.002 | 1.5 (1.3) n = 92 | 1.8 (3.9) n = 64 | NS |
| Corticosteroids (n, %) No treatment Bolus Bolus +Tapering Tapering | 25 (8%) 110 (36%) 166 (54%) 9 (3%) | 5 (5%) 65 (64%) 27 (26%) 5 (5%) | 20 (10%) 44 (21%) 138 (67%) 3 (2%) | <0.001 | 16 (9%) 76 (43%) 78 (44%) 6 (3%) | 9 (7%) 33 (25%) 88 (67%) 2 (1%) | 0.001 |
| Corticosteroids (H + T) n, % | 166 (54%) | 27 (26%) | 138 (67%) | <0.001 | 78 (44%) | 88 (67%) | <0.001 |
| Tocilizumab (n, %) | 134 (43%) | 49 (48%) | 95 (46%) | NS | 68 (39%) | 65 (49%) | NS |
| Remdesivir (n, %) | 41 (13%) | 11 (11%) | 30 (15%) | NS | 16 (9%) | 25 (19%) | 0.012 |
| ICU transfer rate (n, %) | 210 (69%) | 96 (94%) | 114 (57%) | <0.001 | 176 (100%) | 36 (27%) | <0.001 |
| Intubation rate (n, %) | 175 (57%) | 89 (87%) | 86 (42%) | <0.001 | 176 (100%) | - | - |
| Survival rate (n, %) | 205 (67%) | - | - | - | 86 (49%) | 119 (90%) | <0.001 |
| NIV Not Required n = 293 | DEAD n = 14 | ALIVE n = 279 | p | OT n = 44 | No OT n = 249 | p | |
|---|---|---|---|---|---|---|---|
| Age (years) | 59 (11) | 65 (6) | 59 (11) | 0.027 | 60 (10) | 59 (11) | NS |
| Female (n, %) | 96 (33%) | 3 (21%) | 93 (33%) | NS | 9 (20%) | 87 (35%) | 0.059 |
| HTA (n, %) | 116 (49%) | 8 (57% | 108 (48%) | NS | 16 (42%) | 100 (50%) | NS |
| Dyslipidemia (n, %) | 102 (45%) | 9 (64%) | 94 (44%) | NS | 20 (51%) | 82 (44%) | NS |
| Diabetes (n, %) | 58 (27%) | 5 (36%) | 53 (26%) | NS | 9 (24%) | 49 (27%) | NS |
| Obesity (n, %) | 72 (32%) | 5 (36%) | 67 (32%) | NS | 13 (34%) | 59 (32%) | NS |
| Cardiovascular disease (n, %) | 27 (13%) | 4 (31%) | 23 (12%) | 0.048 | 6 (16%) | 21 (12%) | NS |
| Respiratory disease (n, %) None OSA COPD Asthma | 243 (83%) 24 (8%) 12 (4%) 12 (4%) | 10 (71%) 2 (14%) 0% 2 (14%) | 233 (83%) 24 (8%) 12 (4%) 10 (4%) | NS | 34 (77%) 5 (11%) 1 (2%) 4 (9%) | 209 (84%) 21 (8%) 11 (4%) 8 (3%) | NS |
| Chronic kidney failure (n, %) | 18 (6%) | 2 (15%) | 16 (6%) | NS | 2 (5%) | 16 (6%) | NS |
| History of malignancy (n, %) | 37 (17%) | 4 (31%) | 33 (16%) | NS | 6 (16%) | 31 (17%) | NS |
| Chronic liver disease (n, %) | 22 (10%) | 2 (15%) | 20 (10%) | NS | 2 (5%) | 20 (11%) | NS |
| Chronic IS therapy (n, %) | 10 (3%) | 1 (7%) | 9 (3%) | NS | 0% | 10 (4%) | NS |
| Length of hospital stay (days) | 20.1 (19.2) | 22.1 (17.6) | 20.0 (19.3) | NS | 39.2 (27.5) | 16.7 (15.1) | <0.001 |
| Length of pre-IRCU stay (days) | 3.0 (6.1) | 2.5 (3.5) | 3.1 (6.3) | NS | 9.2 (12.5) | 1.9 (3.0) | <0.001 |
| Length of IRCU stay (days) | 7.9 (9.1) | 3.8 (3.3) | 8.1 (9.3) | NS | 8.1 (14.8) | 7.9 (7.8) | NS |
| Length of post-IRCU stay (days) | 9.3 (16.3) | 15.8 (18.9) | 9.0 (16.2) | NS | 21.9 (26.7) | 7.1 (12.6) | <0.001 |
| Wave at H. admission (n, %) 1st (March 20–August 20) 2nd (September 20–December 20) 3rd (January 21–June 21) | 136 (46%) 74 (25%) 83 (28%) | 9 (64%) 5 (36%) 0% | 127 (45%) 69 (25%) 83 (30%) | 0.055 | 28 (64%) 11 (25%) 5 (11%) | 108 (43%) 63 (25%) 78 (31%) | 0.014 |
| Setting at H. admission (n, %) General Ward IRCU Advanced IRCU | 162 (55%) 125 (43%) 6 (20%) | 7 (50%) 7 (50%) 0% | 155 (56%) 118 (42%) 6 (2%) | NS | 25 (57%) 13 (29%) 6 (14%) | 137 (55%) 112 (45%) 0% | <0.001 |
| ICU transfer (n, %) | 46 (16%) | 10 (71%) | 36 (13%) | <0.001 | 38 (86%) | 8 (3%) | <0.001 |
| Intubation rate (n, %) | 44 (15%) | 10 (71%) | 34 (12%) | <0.001 | 44 (100%) | - | - |
| Length IRCU Adm.—OT (days) | 4.8 (6.9) n = 29 | 5.9 (10.7) n = 10 | 4.2 (4.0) n = 19 | NS | 4.8 (6.9) n = 29 | - | - |
| Length ICU Adm.—OT (days) | 1.4 (2.4) n = 29 | 1.6 (3.3) n = 10 | 1.3 (1.7) n = 19 | NS | 1.4 (2.4) | - | - |
| SaFiO2 | 173 (76) | 171 (103) n = 12 | 173 (75) n = 257 | NS | 171 (66) | 173 (77) | NS |
| PaFiO2 | 184 (93) n = 123 | 105 (21) n = 3 | 186 (94) n = 120 | 0.004 | 171 (79) n = 9 | 185 (94) n = 115 | NS |
| RR (breaths/minute) | 23.2 (4.9) | 24.7 (6.1) n = 3 | 23.2(4.9) n = 144 | NS | 25.1 (4.8) n = 9 | 23.1 (4.9) n = 138 | NS |
| PaCO2 (mmHg) | 35.3 (5.4) n = 124 | 34.3 (4.2) n = 3 | 35.3 (5.4) n =121 | NS | 36.5 (7.7) n = 9 | 35.2 (5.2) n = 115 | NS |
| Seric Bicarbonate (mEq/L) | 29.3 (31.7) | 24.2 (2.8) | 29.4 (32.1) | NS | 25.2 (3.2) n = 9 | 29.6 (32.9) | NS |
| Ferritin (ng/L) | 1753 (2854) | 992 (967) | 1784 (2904) | NS | 1720 (1604) | 1757 (2987) | NS |
| LDH (U/L) | 392 (134) | 429 (139) | 391 (134) | NS | 453 (135) | 383 (132) | 0.011 |
| D-dimer (mcg/L) | 1137 (3147) | 740 (765) | 1155 (3214) | NS | 789 (1036) | 1188 (3345) | NS |
| C-reactive protein (mg/L) | 123 (106) | 196 (135) | 119 (103) | 0.010 | 182 (137) | 115 (98) | 0.001 |
| NRT in IRCU (n, %) Monaghan HFNC | 80 (27%) 213 (73%) | 4 (29%) 10 (71%) | 76 (27%) 203 (73%) | NS | 15 (34%) 29 (66%) | 65 (26%) 184 (74%) | NS |
| FiO2 HFNC (%) | 80.7 (13.4) n = 135 | 83.3 (16.1) n = 3 | 80.6 (13.4) n = 132 | NS | 95.0 (2.8) n = 9 | 79.9 (13.3) n = 128 | 0.001 |
| Time on HFNC (days) | 6.0 (3.5) n = 135 | 5.0 (6.1) n = 3 | 6.0 (3.5) n = 132 | NS | 2.4 (1.9) n = 9 | 6.1 (3.5) n = 128 | 0.006 |
| Corticosteroids (n, %) None Bolus Bolus + Tapering Tapering | 50 (17%) 83 (28%) 150 (51%) 10 (3%) | 5 (36%) 8 (57%) 1 (7%) 0% | 45 (16%) 75 (27%) 149 (53%) 10 (4%) | 0.004 | 8 (18%) 14 (32%) 18 (41%) 4 (9%) | 42 (17%) 69 (28%) 132 (53%) 6 (2%) | NS |
| Corticosteroids (H + T) n, % | 150 (51%) | 1 (7%) | 149 (53%) | <0.001 | 18 (41%) | 132 (53%) | NS |
| Tocilizumab (n, %) | 108 (37%) | 4 (29%) | 104 (37%) | NS | 9 (20%) | 99 (40%) | 0.050 |
| Remdesivir (n, %) | 52 (18%) | 1 (7%) | 51 (18%) | NS | 6 (14%) | 46 (18%) | NS |
| ICU transfer rate (n,%) | 46 (16%) | 10 (71%) | 36 (13%) | <0.001 | 44 (100%) | 2(<1%) | <0.001 |
| Intubation rate (n, %) | 44 (15%) | 10 (71%) | 34 (12%) | <0.001 | - | - | - |
| Survival rate (n, %) | 279 (95%) | - | - | - | 34 (77%) | 245 (98%) | - |
| Primary Outcomes | Monaghan n = 80 | HFNC n = 213 | HFNC→NIV n = 257 | Initial NIV n = 50 | p |
|---|---|---|---|---|---|
| Age (years) | 60 (7) | 60 (11) | 63 (11) | 65 (50) | 0.007 |
| SaFiO2 | 217 (63) | 151 (37) | 142 (46) | 120 (38) | <0.001 a |
| ICU transfer rate (n, %) | 17 (21%) | 32 (15%) | 167 (64%) | 31 (62%) | <0.001 |
| Intubation rate (n, %) | 15 (19%) | 29 (14%) | 145 (56%) | 31 (62%) | <0.001 |
| Survival rate (n, %) Global Not ICU-transfer ICU-transfer patients Intubated patients | 76 (95%) 62 (99%) 14 (82%) 12 (80%) | 203/213 (95%) 181 (98%) 22 (76%) | 173 (64%) 78 (94%) 93 (54%) 69 (48%) | 34 (68%) 13 (93%) 21 (58%) 18 (52%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gasa, M.; Ruiz-Albert, Y.; Cordoba-Izquierdo, A.; Sarasate, M.; Cuevas, E.; Suarez-Cuartin, G.; Méndez, L.; Alfaro-Álvarez, J.-C.; Sabater-Riera, J.; Pérez-Fernández, X.L.; et al. Outcomes of COVID-19 Patients Admitted to the Intermediate Respiratory Care Unit: Non-Invasive Respiratory Therapy in a Sequential Protocol. Int. J. Environ. Res. Public Health 2022, 19, 10772. https://doi.org/10.3390/ijerph191710772
Gasa M, Ruiz-Albert Y, Cordoba-Izquierdo A, Sarasate M, Cuevas E, Suarez-Cuartin G, Méndez L, Alfaro-Álvarez J-C, Sabater-Riera J, Pérez-Fernández XL, et al. Outcomes of COVID-19 Patients Admitted to the Intermediate Respiratory Care Unit: Non-Invasive Respiratory Therapy in a Sequential Protocol. International Journal of Environmental Research and Public Health. 2022; 19(17):10772. https://doi.org/10.3390/ijerph191710772
Chicago/Turabian StyleGasa, Mercè, Yolanda Ruiz-Albert, Ana Cordoba-Izquierdo, Mikel Sarasate, Ester Cuevas, Guillermo Suarez-Cuartin, Lidia Méndez, Julio-César Alfaro-Álvarez, Joan Sabater-Riera, Xosé L. Pérez-Fernández, and et al. 2022. "Outcomes of COVID-19 Patients Admitted to the Intermediate Respiratory Care Unit: Non-Invasive Respiratory Therapy in a Sequential Protocol" International Journal of Environmental Research and Public Health 19, no. 17: 10772. https://doi.org/10.3390/ijerph191710772
APA StyleGasa, M., Ruiz-Albert, Y., Cordoba-Izquierdo, A., Sarasate, M., Cuevas, E., Suarez-Cuartin, G., Méndez, L., Alfaro-Álvarez, J.-C., Sabater-Riera, J., Pérez-Fernández, X. L., Molina-Molina, M., & Santos, S. (2022). Outcomes of COVID-19 Patients Admitted to the Intermediate Respiratory Care Unit: Non-Invasive Respiratory Therapy in a Sequential Protocol. International Journal of Environmental Research and Public Health, 19(17), 10772. https://doi.org/10.3390/ijerph191710772






