Falls at the Geriatric Hospital Ward in the Context of Risk Factors of Falling Detected in a Comprehensive Geriatric Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Prevention Strategies and Interventions Implemented at Geriatric Ward
2.2. Data Collected
2.3. Statistical Analysis
3. Results
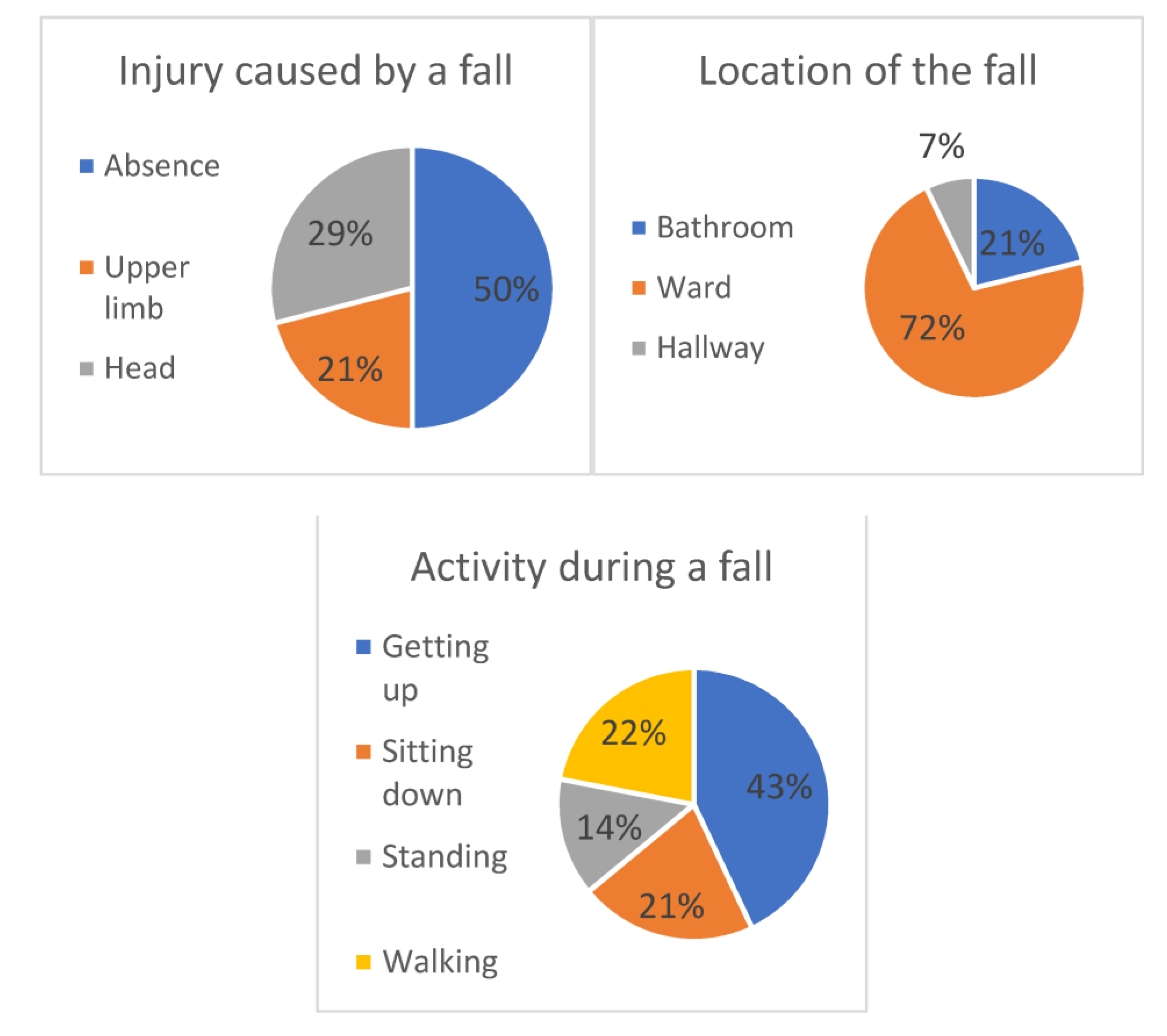
3.1. Prevalence and Circumstances of In-Hospital Falls and Their Consequences
3.2. Study Cohort Characteristics—Sociodemographic and Medical Correlates of Falls
3.3. Study Cohort Characteristics—Pharmacotherapy Correlates of Falls
3.4. Study Cohort Characteristics—Mental and Physical Abilities and Nutritional Correlates of Falls
3.5. Study Cohort Characteristics—Abilities in Performing Activities of Daily Living Correlates of Falls
3.6. Prediction of Falls in a Multivariable Logistic Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Huang, P.; Dou, Q.; Wang, C.; Zhang, W.; Yang, Y.; Wang, J.; Xie, X.; Zhou, J.; Zeng, Y. Falls among older adults with sarcopenia dwelling in nursing home or community: A meta-analysis. Clin. Nutr. 2020, 39, 33–39. [Google Scholar] [CrossRef]
- Wojszel, Z.B.; Bien, B. Falls amongst older people living in the community. Rocz. Akad. Med. Bialymst. 2004, 49, 280–284. [Google Scholar]
- Nyberg, L.; Gustafson, Y.; Janson, A.; Sandman, P.-O.; Eriksson, S. Incidence of falls in three different types of geriatric care. A Swedish prospective study. Scand. J. Soc. Med. 1997, 25, 8–13. [Google Scholar] [CrossRef]
- Heinze, C.; Lahmann, N.; Dassen, T. Frequency of falls in german hospitals. Gesundheitswesen 2002, 64, 598–601. [Google Scholar] [CrossRef]
- Mazur, K.; Wilczynski, K.; Szewieczek, J. Geriatric falls in the context of a hospital fall prevention program: Delirium, low body mass index, and other risk factors. Clin. Interv. Aging 2016, 11, 1253–1261. [Google Scholar]
- Burton, E.; Lewin, G.; O’Connell, H.; Hill, K.D. Falls prevention in community care: 10 years on. Clin. Interv. Aging 2018, 13, 261–269. [Google Scholar] [CrossRef]
- Galeska-Sliwka, A.; Sliwka, M. Liability for damages resulting from hospital falls. Wiad. Lek. 2017, 70, 128–132. [Google Scholar]
- Inouye, S.K.; Brown, C.J.; Tinetti, M.E. Medicare nonpayment, hospital falls, and unintended consequences. N. Engl. J. Med. 2009, 360, 2390–2933. [Google Scholar] [CrossRef]
- Mikos, M.; Banas, T.; Czerw, A.; Banas, B.; Strzępek, Ł.; Curyło, M. Hospital Inpatient Falls across Clinical Departments. Int. J. Environ. Res. Public Health 2021, 18, 8167. [Google Scholar] [CrossRef] [PubMed]
- Magnuszewski, L.; Swietek, M.; Kasiukiewicz, A.; Kuprjanowicz, B.; Baczek, J.; Wojszel, Z.B. Health, Functional and Nutritional Determinants of Falls Experienced in the Previous Year-A Cross-Sectional Study in a Geriatric Ward. Int. J. Environ. Res. Public Health 2020, 17, 4768. [Google Scholar] [CrossRef]
- van Loon, I.N.; Joosten, H.; Iyasere, O.; Johansson, L.; Hamaker, M.E.; Brown, E.A. The prevalence and impact of falls in elderly dialysis patients: Frail elderly Patient Outcomes on Dialysis (FEPOD) study. Arch. Gerontol. Geriatr. 2019, 83, 285–291. [Google Scholar] [CrossRef]
- de Sousa Costa, A.G.; de Araujo, T.L.; Cavalcante, T.F.; Lopes, M.V.O.; de Souza Oliveira-Kumakura, A.R.; Costa, F.B.C. Clinical validation of the nursing outcome falls prevention behavior in people with stroke. Appl. Nurs. Res. 2017, 33, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Wojszel, Z.B.; Magnuszewski, L. Type 2 Diabetes Correlates with Comorbidity and Nutritional Status but Not with Functional Health in Geriatric Ward Patients: A Cross-Sectional Study in Poland. Diabetes Metab. Syndr. Obes. 2020, 13, 4599–4607. [Google Scholar] [CrossRef] [PubMed]
- Kalache, A.F.; Fu, D.; Yoshida, S.; Al-Faisal, W.; Beattie, L.; Chodzko-Zajko, W.; Fu, H.; James, K.; Kalula, S.; Krishnaswamy, B.; et al. World Health Organisation Global Report on Falls Prevention in Older Age; World Health Organization: Geneva, Switzerland, 2007.
- Levy, H.B. Polypharmacy Reduction Strategies: Tips on Incorporating American Geriatrics Society Beers and Screening Tool of Older People‘s Prescriptions Criteria. Clin. Geriatr. Med. 2017, 33, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, F.I. Functional evaluation: The Barthel index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Fillenbaum, G.G.; Smyer, M.A. The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. J. Gerontol. 1981, 36, 428–434. [Google Scholar] [CrossRef]
- Norton, D.; McLaren, R.; Exton-Smith, A.N. An Investigation of Geriatric Nursing Problems in Hospital; Churchill Livingstone: London, UK, 1975. [Google Scholar]
- Hodkinson, H.M. Evaluation of a mental test score for assessment of mental impairment in the elderly. Age Ageing 1972, 1, 233–238. [Google Scholar] [CrossRef]
- Yesavage, J.A. Geriatric Depression Scale. Psychopharmacol. Bull. 1988, 24, 709–711. [Google Scholar]
- Tinetti, M.E. Performance-oriented assessment of mobility problems in elderly patients. J. Am. Geriatr. Soc. 1986, 34, 119–126. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go“: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Cheung, A.; Haas, B.; Ringer, T.J.; McFarlan, A.; Wong, C.L. Canadian Study of Health and Aging Clinical Frailty Scale: Does It Predict Adverse Outcomes among Geriatric Trauma Patients? J. Am. Coll. Surg. 2017, 225, 658–665. [Google Scholar] [CrossRef]
- Kaiser, M.J.; Bauer, J.M.; Ramsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.R.; Anthony, P.; Charlton, K.E.; Maggio, M.; et al. Validation of the Mini Nutritional Assessment short-form (MNA-SF): A practical tool for identification of nutritional status. J. Nutr. Health Aging 2009, 13, 782–788. [Google Scholar] [CrossRef]
- White, A.M.; Tooth, L.R.; Peeters, G. Fall Risk Factors in Mid-Age Women: The Australian Longitudinal Study on Women‘s Health. Am. J. Prev. Med. 2018, 54, 51–63. [Google Scholar] [CrossRef]
- Canning, C.G.; Paul, S.S.; Nieuwboer, A. Prevention of falls in Parkinson‘s disease: A review of fall risk factors and the role of physical interventions. Neurodegener. Dis. Manag. 2014, 4, 203–221. [Google Scholar] [CrossRef] [Green Version]
- Schwendimann, R.; Bühler, H.; De Geest, S.; Milisen, K. Falls and consequent injuries in hospitalized patients: Effects of an interdisciplinary falls prevention program. BMC Health Serv. Res. 2006, 6, 69. [Google Scholar] [CrossRef]
- Milisen, K.; Staelens, N.; Schwendimann, R.; de Paepe, L.; Verhaeghe, J.; Braes, T.; Boonen, S.; Pelemans, W.; Kressig, R.W.; Dejaeger, E. Fall prediction in inpatients by bedside nurses using the St. Thomas‘s Risk Assessment Tool in Falling Elderly Inpatients (STRATIFY) instrument: A multicenter study. J. Am. Geriatr. Soc. 2007, 55, 725–733. [Google Scholar] [CrossRef]
- Bernet, N.S.; Everink, I.H.; Schols, J.M.; Halfens, R.J.; Richter, D.; Hahn, S. Hospital performance comparison of inpatient fall rates; the impact of risk adjusting for patient-related factors: A multicentre cross-sectional survey. BMC Health Serv. Res. 2022, 22, 225. [Google Scholar] [CrossRef]
- Struble-Fitzsimmons, D.; Oswald, A.; DiPersia, E. Patient Location and Mobility Factors Associated with Falls on an Inpatient Geriatric Psychiatry Unit. Act. Adapt. Aging 2019, 43, 276–283. [Google Scholar] [CrossRef]
- Draper, B.; Busetto, G.; Cullen, B. Risk factors for and prediction of falls in an acute aged care psychiatry unit. Australas. J. Ageing 2004, 23, 48–51. [Google Scholar] [CrossRef]
- Wojszel, Z.B.; Kasiukiewicz, A.; Magnuszewski, L. Health and Functional Determinants of Orthostatic Hypotension in Geriatric Ward Patients: A Retrospective Cross Sectional Cohort Study. J. Nutr. Health Aging 2019, 23, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Menant, J.C.; Wong, A.K.W.; Trollor, J.N.; Close, J.C.T.; Lord, S.R. Depressive Symptoms and Orthostatic Hypotension Are Risk Factors for Unexplained Falls in Community-Living Older People. J. Am. Geriatr. Soc. 2016, 64, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- de Carle, A.J.; Kohn, R. Risk factors for falling in a psychogeriatric unit. Int. J. Geriatr. Psychiatry 2001, 16, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-M.; Yuan, L.; Quo, N.; Bo, H.-X.; Jiao, J.; Wu, X.; Xu, T. Cognitive Frailty and Falls in a National Cohort of Older Chinese Inpatients. J. Nutr. Health Aging 2021, 25, 993–998. [Google Scholar] [CrossRef]
- O’Neil, C.A.; Krauss, M.J.; Bettale, J.; Kessels, A.; Costantinou, E.; Dunagan, W.C.; Fraser, V.J. Medications and Patient Characteristics Associated with Falling in the Hospital. J. Patient Saf. 2018, 14, 27–33. [Google Scholar] [CrossRef]
- Schoene, D.; Kiesswetter, E.; Sieber, C.C.; Freiberger, E. Musculoskeletal factors, sarcopenia and falls in old age. Z Gerontol. Geriatr. 2019, 52, 37–44. [Google Scholar] [CrossRef]
- Yuan, L.; Chang, M.; Wang, J. Abdominal obesity, body mass index and the risk of frailty in community-dwelling older adults: A systematic review and meta-analysis. Age Ageing 2021, 50, 1118–1128. [Google Scholar] [CrossRef]
- Scott, V.; Votova, K.; Scanlan, A.; Close, J. Multifactorial and functional mobility assessment tools for fall risk among older adults in community, home-support, long-term and acute care settings. Age Ageing 2007, 36, 130–139. [Google Scholar] [CrossRef]
- Large, J.; Gan, N.; Basic, D.; Jennings, N. Using the timed up and go test to stratify elderly inpatients at risk of falls. Clin. Rehabil. 2006, 20, 421–428. [Google Scholar] [CrossRef]
- Köpke, S.; Meyer, G. The Tinetti test: Babylon in geriatric assessment. Z Gerontol. Geriatr. 2006, 39, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Peel, N.M.; Jones, L.V.; Berg, K.; Gray, L.C. Validation of a Falls Risk Screening Tool Derived from InterRAI Acute Care Assessment. J. Patient Saf. 2021, 17, E1152–E1156. [Google Scholar] [CrossRef] [PubMed]
- Vance, R.C.; Healy, D.G.; Galvin, R.; French, H.P. Dual tasking with the timed “up & go” test improves detection of risk of falls in people with Parkinson disease. Phys. Ther. 2015, 95, 95–102. [Google Scholar]
- Kasiukiewicz, A.; Magnuszewski, L.; Swietek, M.; Wojszel, Z.B. The Performance of Dual-Task Tests Can Be a Combined Neuro-Psychological and Motor Marker of Mild Cognitive Impairment, Depression and Dementia in Geriatric Patients—A Cross-Sectional Study. J. Clin. Med. 2021, 10, 5358. [Google Scholar] [CrossRef] [PubMed]
- Fanciulli, A.; Campese, N.; Goebel, G.; Ndayisaba, J.P.; Eschlboeck, S.; Kaindlstorfer, C.; Raccagni, C.; Granata, R.; Bonuccelli, U.; Ceravolo, R.; et al. Association of transient orthostatic hypotension with falls and syncope in patients with Parkinson disease. Neurology 2020, 95, e2854–e2865. [Google Scholar] [CrossRef]

| Characteristic | All | In-Patient Non-Fallers | In-Patient Fallers | p Value a | Missing Values |
|---|---|---|---|---|---|
| No. (%) of patients | 416 (100.0) | 402 (96.7) | 14 (3.3) | - | - |
| Age (y), Md (IQR) | 82 (77.0–86.0) | 82 (77.0–86.0) | 81 (76.0–86.0) | 0.78 | - |
| Age (75+), n (%) | 350 (84.1) | 337 (83.8) | 13 (92.9) | 0.32 | - |
| Sex (men), n (%) | 94 (22.6) | 88 (21.9) | 6 (42.9) | 0.07 | - |
| Residence (rural), n (%) | 87 (20.9) | 85 (21.1) | 2 (14.3) | 0.41 | - |
| Length of hospital stay, days, Md (IQR) | 8 (5.0–9.0) | 8 (5.0–9.0) | 13 (8.8–17) | <0.001 | - |
| Number of chronic diseases b, Md (IQR) | 5.0 (3.0–6.0) | 5.0 (3.0–6.0) | 6.5 (5.0–8.0) | 0.001 | - |
| Multimorbidity, n (%) | 239 (57.5) | 226 (56.2) | 13 (92.9) | 0.004 | - |
| Falls in the last 12 months, n (%) | 157 (43.9) | 147 (42.4) | 10 (90.9) | 0.001 | 58 |
| Hospitalization in the last 12 months, n (%) | 122 (29.5) | 116 (29.0) | 6 (42.9) | 0.20 | 3 |
| Parkinson’s disease, n (%) | 55 (13.2) | 50 (12.4) | 5 (35.7) | 0.01 | - |
| Dementia, n (%) | 133 (32.0) | 127 (31.6) | 6 (42.9) | 0.27 | - |
| Depression, n (%) | 181 (56.9) | 172 (56.2) | 9 (75.0) | 0.12 | 98 |
| Diabetes, n (%) | 126 (30.3) | 116 (28.9) | 10 (71.4) | 0.001 | - |
| Hypertension, n (%) | 327 (78.6) | 315 (78.4) | 12 (85.7) | 0.39 | - |
| Orthostatic hypotension, n (%) | 57 (16.2) | 51 (15.0) | 6 (42.9) | 0.01 | 63 |
| Ischemic heart diseases, n (%) | 223 (53.6) | 213 (53.0) | 10 (71.4) | 0.13 | - |
| Osteoporosis, n (%) | 74 (17.8) | 73 (18.2) | 1 (7.1) | 0.25 | - |
| Osteoarthritis, n (%) | 324 (77.9) | 313 (77.9) | 11 (78.6) | 0.62 | - |
| Chronic cardiac failure, n (%) | 378 (90.9) | 364 (90.6) | 14 (100.0) | 0.22 | - |
| Atrial fibrillation, n (%) | 98 (23.6) | 95 (23.6) | 3 (21.4) | 0.57 | - |
| Peripheral arterial disease, n (%) | 64 (15.4) | 61 (15.2) | 3 (21.4) | 0.36 | - |
| Stroke/ TIA, n (%) | 56 (13.5) | 53 (13.18) | 3 (21.4) | 0.28 | - |
| Rheumatoid arthritis, n (%) | 20 (4.81) | 20 (5.0) | 0 (0.0) | 0.39 | - |
| Cholesterol- total, mg/dL, Md (IQR) | 178 (146.0–212.0) | 179 (147.0–213.0) | 133.5 (112.0–165.0) | <0.001 | 27 |
| LDL, mg/dL, Md (IQR) | 118 (87.0–143.0) | 120 (88.0–144.5) | 76.0 (67.0–87.0) | <0.001 | 118 |
| HDL, mg/dL, Md (IQR) | 48 (39.0–57.0) | 49 (40.0–58.0) | 40.5 (32.0–42.0) | <0.001 | 101 |
| Creatinin, mg/dL Md (IQR) | 0.98 (0.84–1.19) | 0.97 (0.84–1.18) | 1.3 (0.9–1.41) | 0.04 | 11 |
| eGFR, mL/min/1.73 m2, M (SD) | 57.9 (46.0–71.6) | 58.1 (46.1–71.9) | 49.4 (40.5–66.6) | 0.13 | 11 |
| TSH, mU/L, Md (IQR) | 1.4 (0.82–2.1) | 1.4 (0.82–2.1) | 1.8 (1.2–2.3) | 0.24 | 35 |
| Vitamin B12, pg/mL, Md (IQR) | 334.5 (242.6–430.6) | 336.2 (242.8–430.6) | 299.7 (221.8–343.8) | 0.46 | 38 |
| Na, mmol/L, Md (IQR) | 140.0 (138.0–141.0) | 140.0 (138.0–141.0) | 142.0 (134.0–142.0) | 0.60 | 10 |
| Ca, mmol/L, Md (IQR) | 4.6 (4.4–4.7) | 4.6 (4.4–4.7) | 4.5 (4.3–4.6) | 0.17 | 102 |
| Hemoglobin, g/dL, Md (IQR) | 12.6 (11.5–13.7) | 12.6 (11.5–13.7) | 12.6 (11.3–13.2) | 0.70 | 12 |
| Albumin ≥ 35 mg/mL, n (%) | 58 (14.9) | 54 (14.4) | 4 (28.6) | 0.14 | 27 |
| Systolic BP at admittance, mmHg, Md (IQR) | 130.0 (120.0–140.0) | 130.0 (120.0–140.0) | 131.0 (120–140.0) | 0.84 | 7 |
| Diastolic BP at admittance, mmHg, Md (IQR) | 70.0 (60.0–80.0) | 70.0 (60.0–80.0) | 70.0 (60.0–80.0) | 0.41 | 7 |
| CHA2DS2-VASc, Md (IQR) | 4.0 (3.0–5.0) | 4.0 (3.0–5.0) | 5.5 (4.0–6.0) | 0.005 | 2 |
| Parameter | All | In-Patient Non-Fallers | In-Patient Fallers | p Value a | Missing Values |
|---|---|---|---|---|---|
| No. (%) of patients | 416 (100.0) | 402 (96.7) | 14 (3.3) | - | - |
| Number of drugs at admittance, Md (IQR) | 6.0 (5.0–9.0) | 7.0 (5.0–9.0) | 10.5 (6.0–12.0) | 0.01 | 9 |
| Polypharmacy, n (%) | 322 (79.1) | 309 (78.6) | 13 (92.9) | 0.17 | 9 |
| α1-blockers, n (%) | 25 (6.1) | 23 (5.9) | 2 (14.3) | 0.19 | 10 |
| ß-blockers, n (%) | 258 (63.6) | 250 (63.8) | 8 (57.1) | 0.40 | 10 |
| Calcium channel blockers, n (%) | 144 (28.1) | 110 (28.1) | 4 (28.6) | 0.58 | 10 |
| ACEI/ ARB, n (%) | 222 (54.3) | 212 (53.7) | 10 (71.4) | 0.18 | 7 |
| Thiazide/ loop diuretics, n (%) | 196 (42.3) | 188 (48.0) | 8 (57.1) | 0.34 | 10 |
| Aldosterone- receptor antagonists (spironolactone, eplerenone), n (%) | 71 (17.5) | 66 (16.8) | 5 (35.7) | 0.07 | 10 |
| Statins, n (%) | 142 (35.0) | 133 (33.4) | 9 (64.3) | 0.01 | 10 |
| Digoxin, n (%) | 30 (7.4) | 28 (7.1) | 2 (14.3) | 0.27 | 10 |
| Antiarrhythmics, n (%) | 9 (2.2) | 9 (2.3) | 0 (0.0) | 0.56 | 10 |
| Insulin, n (%) | 31 (7.6) | 26 (6.6) | 5 (35.7) | <0.001 | 10 |
| Metformin, n (%) | 65 (16.0) | 58 (14.8) | 7 (50.0) | <0.001 | 10 |
| Oral anti-diabetic drugs (SM, metformin), n (%) | 90 (22.2) | 82 (20.1) | 8 (57.1) | 0.001 | 10 |
| Anti-diabetic drugs (SM, metformin, insulin), n (%) | 105 (25.9) | 96 (24.5) | 9 (64.3) | <0.001 | 10 |
| Thyroid hormones, n (%) | 30 (7.4) | 28 (7.1) | 2 (14.3) | 0.28 | 10 |
| Cholecalciferol, n (%) | 88 (21.7) | 86 (22.0) | 2 (14.3) | 0.49 | 10 |
| BDA, n (%) | 45 (11.1) | 41 (10.4) | 4 (28.6) | 0.03 | 9 |
| SSRI, n (%) | 115 (28.3) | 108 (27.5) | 7 (50.0) | 0.07 | 10 |
| Antidepressants (SSRI, mianserin), n (%) | 133 (32.8) | 126 (32.1) | 7 (50.0) | 0.13 | 10 |
| Quetiapine, n (%) | 49 (12.0) | 45 (11.5) | 4 (28.6) | 0.05 | 10 |
| Neuroleptics (quetiapine, haloperidol), n (%) | 54 (13.3) | 50 (12.8) | 4 (28.6) | 0.08 | 10 |
| AChE-I (donepezil, rivastigmine), n (%) | 41 (10.10) | 38 (9.7) | 3 (21.4) | 0.15 | 10 |
| Procognitive medications (AChEIs, memantine), n (%) | 46 (11.3) | 43 (11.0) | 3 (21.4) | 0.20 | 10 |
| Characteristic | All | In-Patient Non-Fallers | In-Patient Fallers | p Value a | Missing Values |
|---|---|---|---|---|---|
| No. (%) of patients | 416 (100.0) | 402 (96.7) | 14 (3.3) | - | - |
| AMTS, Md (IQR) | 8.0 (6.0–9.0) | 8.0 (6.0–9.0) | 7.0 (6.0–8.0) | 0.32 | 35 |
| MMSE, Md (IQR) | 21.0 (16.0–24.0) | 21.0 (16.0–25.0) | 19.0 (16.5–21.5) | 0.40 | 231 |
| GDS, Md (IQR) | 7.0 (3.0–10.0) | 6.5 (4.0–10.0) | 8.5 (3.0–11.0) | 0.36 | 52 |
| POMA, Md (IQR) | 23.0 (17.0–28.0) | 23.0 (18.0–28.0) | 17.0 (14.0–23.5) | 0.03 | 94 |
| POMA < 26, n (%) | 199 (61.8) | 188 (60.7) | 11 (91.7) | 0.02 | 94 |
| POMA < 19, n (I%) | 95 (29.5) | 88 (28.4) | 7 (58.3) | 0.02 | 94 |
| TUG, s, Md (IQR) | 17.4 (11.9–28.0) | 17.2 (11.7–27.7) | 25.3 (20.7–29.1) | 0.11 | 115 |
| Gait speed, m/s, Md (IQR) | 0.65 (0.4–0.96) | 0.66 (0.4–0.59) | 0.43 (0.32–0.63) | 0.10 | 102 |
| Gait speed ≤ 0.8 m/s, n (%) | 166 (52.9) | 158 (52.0) | 8.0 (80.0) | 0.08 | 102 |
| Handgrip strength, kg, Md (IQR) | 18.2 (13.7–22.8) | 18.1 (13.8–22.8) | 19.4 (13.2–22.5) | 0.92 | 66 |
| Dynapenia, n (%) | 233.0 (66.6) | 223.0 (66.2) | 10.0 (76.9) | 0.32 | 66 |
| Norton scale, Md (IQR) | 17.0 (15.0–19.0) | 17.0 (15.0–19.0) | 16.0 (15.0–18.0) | 0.25 | 6 |
| Clinical Frailty Scale, Md (IQR) | 5.0 (4.0–5.0) | 5.0 (4.0–5.0) | 5.5 (5.0–6.0) | 0.01 | - |
| Clinical Frailty Scale classification | 0.06 | - | |||
| Robust, n (%) | 62 (14.9) | 62 (15.4) | 0 (0) | ||
| Pre-frail, n (%) | 124 (29.8) | 122 (30.3) | 2 (14.3) | ||
| Frail, n (%) | 230 (55.3) | 218 (54.2) | 12 (85.7) | ||
| Severe frailty (CFS = 6 or 7), n (%) | 102 (24.5) | 95 (23.6) | 7 (50.0) | 0.02 | - |
| MNA-SF, Md (IQR) | 12.0 (9.0–13.0) | 12.0 (9.0–13.0) | 12.0 (7.0–13.0) | 0.54 | 12 |
| MNA-SF score < 8, n (%) | 198 (49.0) | 192 (49.2) | 6 (42.9) | 0.42 | 12 |
| BMI, kg/m2, M (SD) | 28.5 (25.0–33.6) | 28.5 (24.9–33.7) | 30.3 (27.0–31.6) | 0.55 | 62 |
| BMI < 24 kg/m2, n (%) | 66 (18.6) | 65 (19.0) | 1 (8.3) | 0.31 | 62 |
| BMI > 30 kg/m2, n (%) | 148 (41.8) | 141 (41.2) | 7 (58.3) | 0.18 | 62 |
| WHR, Md (IQR) | 0.90 (0.87–0.95) | 0.90 (0.87–0.96) | 0.93 (0.87–0.96) | 0.45 | 63 |
| MAC, cm, Md (IQR) | 28.0 (26.0–30.0) | 28.0 (26.0–30.0) | 28.5 (26.0–30.0) | 0.70 | 49 |
| MAC ≤ 22 cm, n (%) | 89 (24.3) | 87 (24.7) | 2 (14.3) | 0.29 | 49 |
| CC, cm, Md (IQR) | 34.0 (31.5–37.0) | 34.0 (31.0–37.0) | 35.5 (33.0–40.0) | 0.12 | 51 |
| CC < 31 cm, n (%) | 74 (20.3) | 72 (20.5) | 2 (14.3) | 0.43 | 51 |
| Characteristic | All | In-Patient Non-Fallers | In-Patient Fallers | p Value a | Missing Values |
|---|---|---|---|---|---|
| Barthel Index, Md (IQR) | 90.0 (70.0–100.0) | 90.0 (70.0–100.0) | 77.5 (55.0–85.0) | 0.06 | 6 |
| Independent in: | |||||
| Bowel control, n (%) | 360 (87.4) | 346 (86.9) | 14 (100.0) | 0.15 | 4 |
| Feeding, n (%) | 343 (83.0) | 332 (83.2) | 11 (78.6) | 0.65 | 3 |
| Grooming, n (%) | 333 (80.6) | 332 (80.7) | 11 (78.6) | 0.84 | 3 |
| Transferring (bed to chair and back), n (%) | 297 (72.1) | 289 (72.6) | 8 (57.1) | 0.20 | 4 |
| Mobility on level surfaces, n (%) | 292 (70.4) | 284 (70.8) | 8 (57.1) | 0.27 | 1 |
| Toilet use, n (%) | 280 (67.8) | 276 (69.2) | 4 (28.6) | <0.001 | 3 |
| Dressing, n (%) | 276 (66.8) | 270 (67.7) | 6 (42.9) | 0.05 | 3 |
| Stairs managing, n (%) | 227 (55.2) | 219 (55.2) | 8 (57.1) | 0.88 | 5 |
| Bladder control, n (%) | 218 (53.0) | 214 (53.9) | 4 (28.6) | 0.05 | 5 |
| Bathing, n (%) | 209 (21.4) | 206 (51.9) | 3 (21.4) | 0.02 | 5 |
| IADL, Md (IQR) | 7.0 (3.0–11.0) | 7.0 (3.0–11.0) | 4.5 (2.0–8.0) | 0.07 | 10 |
| Independent in: | |||||
| Phone talk, n (%) | 273 (66.8) | 267 (67.6) | 6 (42.9) | 0.05 | 7 |
| Taking medicines, n (%) | 235 (57.5) | 229 (58.0) | 6 (42.9) | 0.26 | 7 |
| Disposing of money, n (%) | 230 (56.4) | 226 (57.4) | 4 (28.6) | 0.03 | 9 |
| Making meals, n (%) | 177 (43.7) | 174 (44.5) | 3 (21.4) | 0.07 | 11 |
| Going shopping, n (%) | 126 (31.1) | 125 (32.0) | 1 (7.1) | 0.03 | 11 |
| Doing housework, n (%) | 101 (25.0) | 101 (25.8) | 0 (0.0) | 0.02 | 11 |
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Falls in the last 12 months | 10.89 | 1.28–92.56 | 0.03 | 50.92 | 1.84–1412.6 | 0.02 |
| Orthostatic hypotension | 4.76 | 1.13–19.97 | 0.03 | 14.53 | 1.12–189.27 | 0.04 |
| CFS | 2.63 | 1.08–6.42 | 0.03 | 2.84 | 0.96–8.47 | 0.06 |
| Parkinson’s disease | 37.64 | 2.71–522.02 | 0.007 | |||
| Statins | 20.91 | 1.23–355.31 | 0.04 | |||
| Insulin | 56.79 | 3.66–880.59 | 0.004 | |||
| Benzodiazepines | 31.11 | 2.41–540.72 | 0.009 | |||
| Negelkerk’s R2 | 0.208 | 0.579 | ||||
| % correctly predicted | 96.7% | 98.2% | ||||
| sensitivity | 0% | 44.4% | ||||
| specificity | 100% | 100% | ||||
| Hosmer–Lemeshow goodness of fit | 0.877 | 0.939 | ||||
| Block 1 | Block 2 | |||||
| Variables in block 1 and block 2 | Falls in the last 12 months, orthostatic hypotension, POMA, CFS | Barthel index, IADL, sex, DM, Parkinson’s disease, number of diseases, statins, insulin, SM, metformin, quetiapine, neuroleptics, BDA, SSRI, cholesterol, creatinine | ||||
| Model 3 | |||
|---|---|---|---|
| OR | 95% CI | p Value | |
| Falls in the last 12 months | 41.74 | 2.47–705.95 | 0.010 |
| Orthostatic hypotension | 21.53 | 2.25–206.28 | 0.008 |
| Parkinson’s disease | 40.89 | 3.28–510.16 | 0.004 |
| Statins | 43.96 | 2.97–651.72 | 0.006 |
| Insulin | 34.76 | 3.33–362.75 | 0.003 |
| Benzodiazepines | 34.93 | 2.82–432.16 | 0.006 |
| Negelkerk’s R2 | 0.541 | ||
| % correctly predicted | 97.5% | ||
| sensitivity | 27.3% | ||
| specificity | 100% | ||
| Hosmer–Lemeshow goodness of fit | 0.703 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magnuszewski, L.; Wojszel, A.; Kasiukiewicz, A.; Wojszel, Z.B. Falls at the Geriatric Hospital Ward in the Context of Risk Factors of Falling Detected in a Comprehensive Geriatric Assessment. Int. J. Environ. Res. Public Health 2022, 19, 10789. https://doi.org/10.3390/ijerph191710789
Magnuszewski L, Wojszel A, Kasiukiewicz A, Wojszel ZB. Falls at the Geriatric Hospital Ward in the Context of Risk Factors of Falling Detected in a Comprehensive Geriatric Assessment. International Journal of Environmental Research and Public Health. 2022; 19(17):10789. https://doi.org/10.3390/ijerph191710789
Chicago/Turabian StyleMagnuszewski, Lukasz, Aleksandra Wojszel, Agnieszka Kasiukiewicz, and Zyta Beata Wojszel. 2022. "Falls at the Geriatric Hospital Ward in the Context of Risk Factors of Falling Detected in a Comprehensive Geriatric Assessment" International Journal of Environmental Research and Public Health 19, no. 17: 10789. https://doi.org/10.3390/ijerph191710789
APA StyleMagnuszewski, L., Wojszel, A., Kasiukiewicz, A., & Wojszel, Z. B. (2022). Falls at the Geriatric Hospital Ward in the Context of Risk Factors of Falling Detected in a Comprehensive Geriatric Assessment. International Journal of Environmental Research and Public Health, 19(17), 10789. https://doi.org/10.3390/ijerph191710789






