Shifts of Antibiotic Resistomes in Soil Following Amendments of Antibiotics-Contained Dairy Manure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Sampling
2.2. DNA Extraction
2.3. High-Throughput qPCR (HT-qPCR) for ARGs Analysis
2.4. High-Throughput Sequencing for Bacterial 16S rRNA
2.5. Statistical and Network Analysis
3. Results
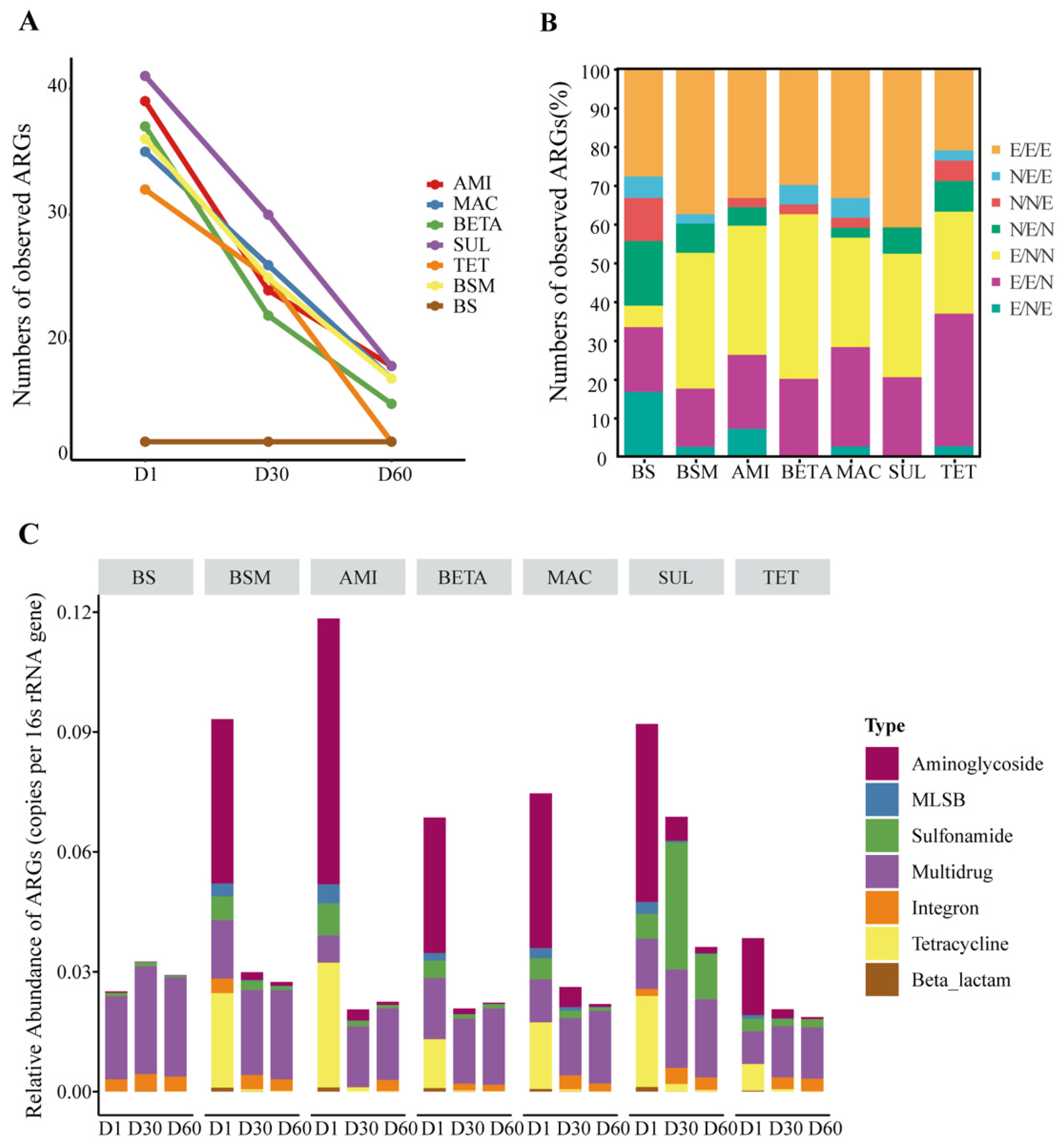
3.1. Temporal Shifts of ARG Diversity and Abundance
3.2. ARG Profiles and Detailed Composition
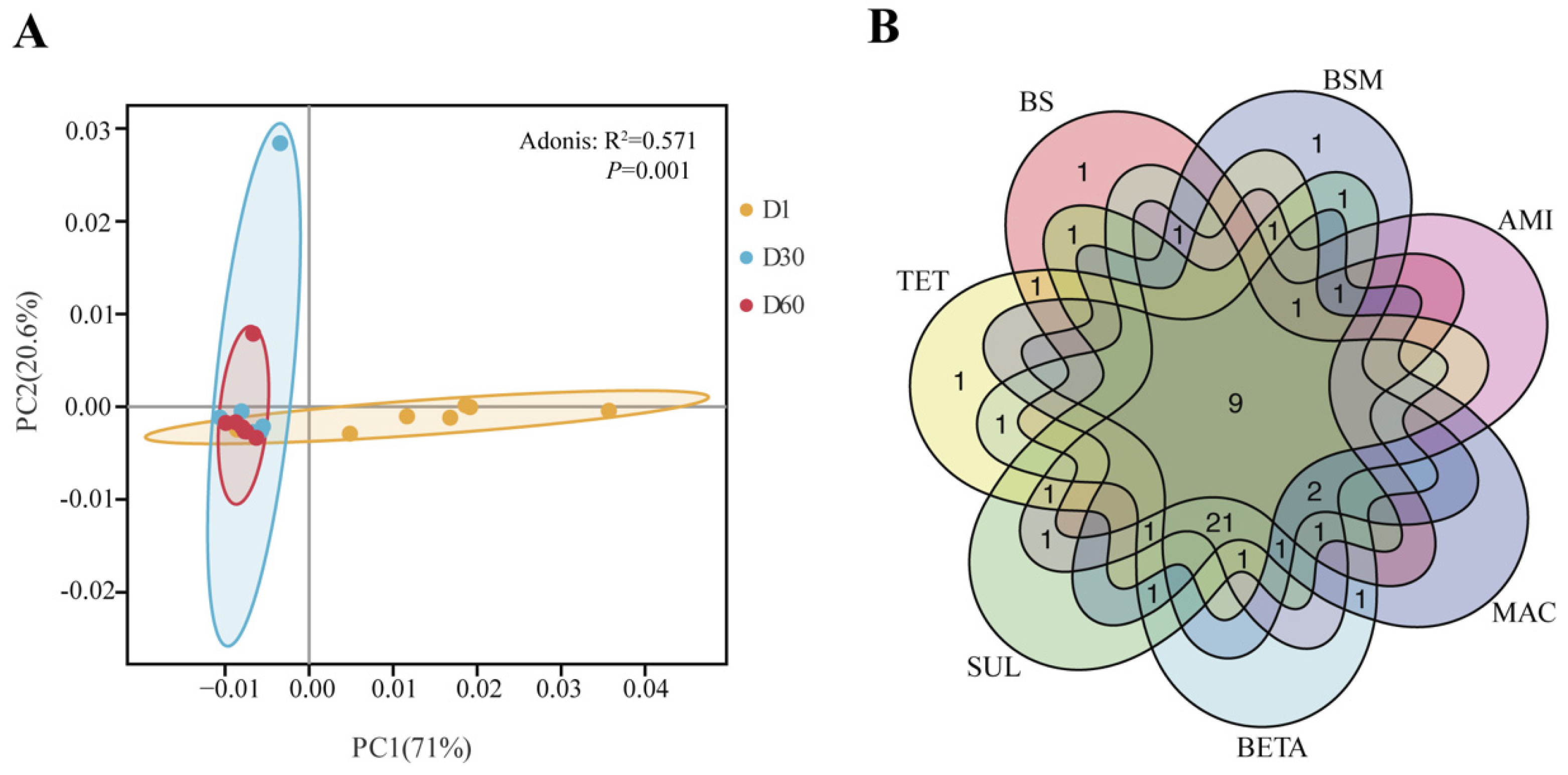
3.3. Dynamic Shifts of Bacterial Community
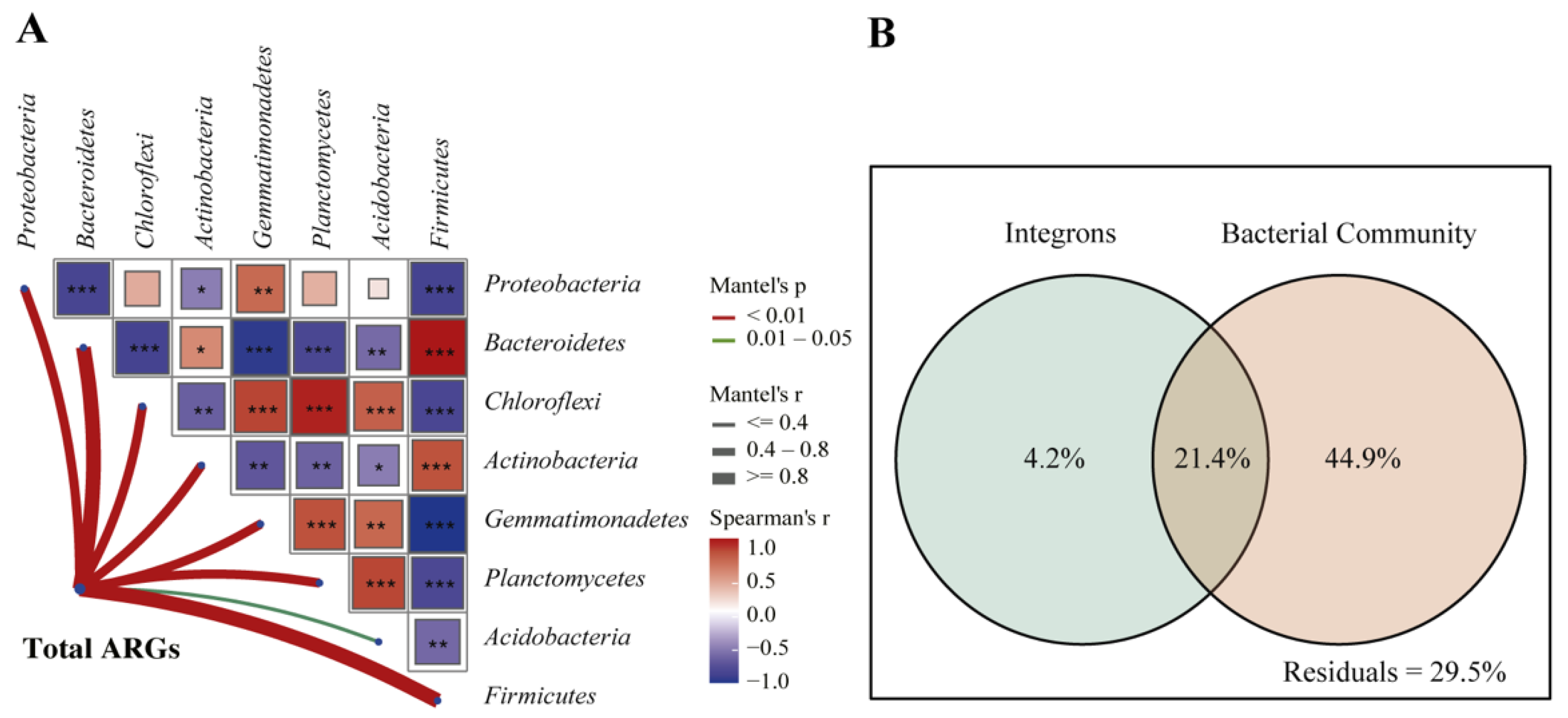
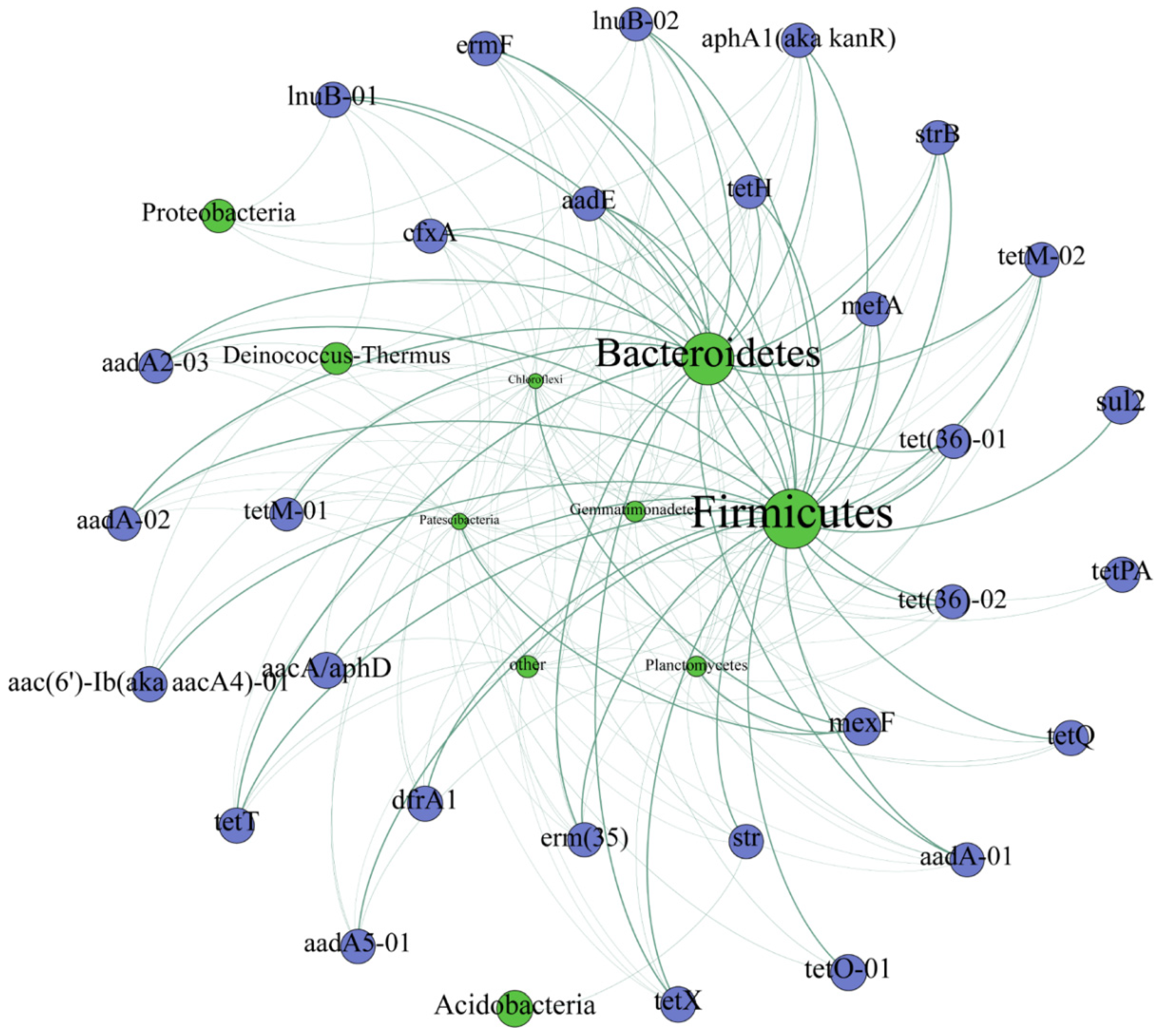
3.4. Correlations between ARGs and Bacteria Communities
4. Discussion
4.1. Dairy Manure Application Enriched ARGs in Soil
4.2. Various Impacts of Antibiotic Addition on ARGs in Soil
4.3. Shifts of Bacterial Communities were the Primary Driver for the Changes of ARGs in Soil
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, Y.; Cheng, H.; Tao, S. Environmental and human health challenges of industrial livestock and poultry farming in China and their mitigation. Environ. Int. 2017, 107, 111–130. [Google Scholar] [CrossRef] [PubMed]
- Tiseo, K.; Huber, L.; Gilbert, M.; Robinson, T.P.; Van Boeckel, T.P. Global trends in antimicrobial use in food animals from 2017 to 2030. Antibiotics 2020, 9, 918. [Google Scholar] [CrossRef] [PubMed]
- Wepking, C.; Avera, B.; Badgley, B.; Barrett, J.E.; Franklin, J.; Knowlton, K.F.; Ray, P.P.; Smitherman, C.; Strickland, M.S. Exposure to dairy manure leads to greater antibiotic resistance and increased mass-specific respiration in soil microbial communities. Proc. Biol. Sci. 2017, 284, 20162233. [Google Scholar] [CrossRef]
- China, M.O. Action of Reduction of Antimicrobial Agents Used in Veterinary Practice (2018–2021). Available online: http://www.moa.gov.cn/govpublic/SYJ/201804/t20180420_6140711.htm (accessed on 20 April 2018).
- Knafl, D.; Winhofer, Y.; Lotsch, F.; Weisshaar, S.; Steininger, C.; Burgmann, H.; Thalhammer, F. Tigecycline as last resort in severe refractory Clostridium difficile infection: A case report. J. Hosp. Infect. 2016, 92, 296–298. [Google Scholar] [CrossRef]
- Li, R.; Peng, K.; Li, Y.; Liu, Y.; Wang, Z. Exploring tet(X)-bearing tigecycline-resistant bacteria of swine farming environments. Sci. Total Environ. 2020, 733, 139306. [Google Scholar] [CrossRef]
- Umar, Z.; Chen, Q.; Tang, B.; Xu, Y.; Wang, J.; Zhang, H.; Ji, K.; Jia, X.; Feng, Y. The poultry pathogen Riemerella anatipestifer appears as a reservoir for Tet(X) tigecycline resistance. Environ. Microbiol. 2021, 23, 7465–7482. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Cui, C.Y.; Yu, J.J.; He, Q.; Wu, X.T.; He, Y.Z.; Cui, Z.H.; Li, C.; Jia, Q.L.; Shen, X.G.; et al. Genetic diversity and characteristics of high-level tigecycline resistance Tet(X) in Acinetobacter species. Genome Med. 2020, 12, 111. [Google Scholar] [CrossRef]
- Vinayamohan, P.G.; Pellissery, A.J.; Venkitanarayanan, K. Role of Horizontal Gene Transfer in the dissemination of antimicrobial resistance in food animal production. Curr. Opin. Food Sci. 2022, 47, 100882. [Google Scholar] [CrossRef]
- Mehrtens, A.; Licha, T.; Broers, H.P.; Burke, V. Tracing veterinary antibiotics in the subsurface—A long-term field experiment with spiked manure. Environ. Pollut. 2020, 265, 114930. [Google Scholar] [CrossRef]
- Gu, Y.; Shen, S.; Han, B.; Tian, X.; Yang, F.; Zhang, K. Family livestock waste: An ignored pollutant resource of antibiotic resistance genes. Ecotoxicol. Environ. Saf. 2020, 197, 110567. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Hu, H.W.; Chen, Q.L.; Singh, B.K.; Yan, H.; Chen, D.; He, J.Z. Transfer of antibiotic resistance from manure-amended soils to vegetable microbiomes. Environ. Int. 2019, 130, 104912. [Google Scholar] [CrossRef] [PubMed]
- Grout, L.; Baker, M.G.; French, N.; Hales, S. A review of potential public health impacts associated with the global dairy sector. Geohealth 2020, 4, e2019GH000213. [Google Scholar] [CrossRef] [PubMed]
- Maculewicz, J.; Kowalska, D.; Swiacka, K.; Tonski, M.; Stepnowski, P.; Bialk-Bielinska, A.; Dolzonek, J. Transformation products of pharmaceuticals in the environment: Their fate, (eco)toxicity and bioaccumulation potential. Sci. Total Environ. 2022, 802, 149916. [Google Scholar] [CrossRef]
- Jeon, S.J.; Lima, F.S.; Vieira-Neto, A.; Machado, V.S.; Lima, S.F.; Bicalho, R.C.; Santos, J.E.P.; Galvao, K.N. Shift of uterine microbiota associated with antibiotic treatment and cure of metritis in dairy cows. Vet. Microbiol. 2018, 214, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.P.; Gooch, C.A.; Lansing, S.; Schueler, J.; Hurst, J.J.; Sassoubre, L.; Crossette, E.M.; Aga, D.S. Invited review: Fate of antibiotic residues, antibiotic-resistant bacteria, and antibiotic resistance genes in US dairy manure management systems. J. Dairy Sci. 2020, 103, 1051–1071. [Google Scholar] [CrossRef]
- Shinozuka, Y.; Kawai, K.; Takeda, A.; Yamada, M.; Kayasaki, F.; Kondo, N.; Sasaki, Y.; Kanai, N.; Mukai, T.; Sawaguchi, M.; et al. Influence of oxytetracycline susceptibility as a first-line antibiotic on the clinical outcome in dairy cattle with acute Escherichia coli mastitis. J. Vet. Med. Sci. 2019, 81, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Holman, D.B.; Yang, W.; Alexander, T.W. Antibiotic treatment in feedlot cattle: A longitudinal study of the effect of oxytetracycline and tulathromycin on the fecal and nasopharyngeal microbiota. Microbiome 2019, 7, 86. [Google Scholar] [CrossRef]
- Chen, C.; Pankow, C.A.; Oh, M.; Heath, L.S.; Zhang, L.; Du, P.; Xia, K.; Pruden, A. Effect of antibiotic use and composting on antibiotic resistance gene abundance and resistome risks of soils receiving manure-derived amendments. Environ. Int. 2019, 128, 233–243. [Google Scholar] [CrossRef]
- Shawver, S.; Wepking, C.; Ishii, S.; Strickland, M.S.; Badgley, B.D. Application of manure from cattle administered antibiotics has sustained multi-year impacts on soil resistome and microbial community structure. Soil Biol. Biochem. 2021, 157, 108252. [Google Scholar] [CrossRef]
- Heuer, H.; Solehati, Q.; Zimmerling, U.; Kleineidam, K.; Schloter, M.; Muller, T.; Focks, A.; Thiele-Bruhn, S.; Smalla, K. Accumulation of sulfonamide resistance genes in arable soils due to repeated application of manure containing sulfadiazine. Appl. Environ. Microbiol. 2011, 77, 2527–2530. [Google Scholar] [CrossRef] [Green Version]
- Xiong, W.; Wang, M.; Dai, J.; Sun, Y.; Zeng, Z. Application of manure containing tetracyclines slowed down the dissipation of tet resistance genes and caused changes in the composition of soil bacteria. Ecotoxicol. Environ. Saf. 2018, 147, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Hong, J.K.; Jho, E.H.; Nam, K. Interaction among soil physicochemical properties, bacterial community structure, and arsenic contamination: Clay-induced change in long-term arsenic contaminated soils. J. Hazard Mater. 2019, 378, 120729. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, W.; Yang, L.; Stedtfeld, R.D.; Peng, A.; Gu, C.; Boyd, S.A.; Li, H. Antibiotic resistance genes and bacterial communities in cornfield and pasture soils receiving swine and dairy manures. Environ. Pollut. 2019, 248, 947–957. [Google Scholar] [CrossRef]
- Moreira Junior, R.E.; de Carvalho, L.M.; Pedersen, A.S.B.; Damasceno, S.; Maioli, T.U.; de Faria, A.M.C.; Godard, A.L.B. Interaction between high-fat diet and ethanol intake leads to changes on the fecal microbiome. J. Nutr. Biochem. 2019, 72, 108215. [Google Scholar] [CrossRef] [PubMed]
- Bushnell, B. BBmap Files. Available online: https://sourceforge.net/projects/bbmap/files/ (accessed on 8 December 2017).
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahe, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glockner, F.O. The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res. 2014, 42, D643–D648. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Chen, T.; Liu, Y.X.; Huang, L. ImageGP: An easy-to-use data visualization web server for scientific researchers. iMeta 2022, 1, e5. [Google Scholar] [CrossRef]
- Liu, W.; Cheng, Y.; Guo, J.; Duan, Y.; Wang, S.; Xu, Q.; Liu, M.; Xue, C.; Guo, S.; Shen, Q.; et al. Long-term manure inputs induce a deep selection on agroecosystem soil antibiotic resistome. J. Hazard. Mater. 2022, 436, 129163. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson-Palme, J.; Kristiansson, E.; Larsson, D.G.J. Environmental factors influencing the development and spread of antibiotic resistance. FEMS Microbiol. Rev. 2018, 42, fux053. [Google Scholar] [CrossRef]
- Cooray, T.; Zhang, J.; Zhong, H.; Zheng, L.; Wei, Y.; Weragoda, S.K.; Jinadasa, K.; Weerasooriya, R. Profiles of antibiotic resistome and microbial community in groundwater of CKDu prevalence zones in Sri Lanka. J. Hazard. Mater. 2021, 403, 123816. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Singh, A.; Chowdhary, P.; Pandey, A.; Gupta, P. Occurrence of emerging sulfonamide resistance (sul1 and sul2) associated with mobile integrons-integrase (intI1 and intI2) in riverine systems. Sci. Total Environ. 2021, 751, 142217. [Google Scholar] [CrossRef] [PubMed]
- Subirats, J.; Timoner, X.; Sanchez-Melsio, A.; Balcazar, J.L.; Acuna, V.; Sabater, S.; Borrego, C.M. Emerging contaminants and nutrients synergistically affect the spread of class 1 integron-integrase (intI1) and sul1 genes within stable streambed bacterial communities. Water Res. 2018, 138, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.; Morra, M.J.; Stalder, T.; Jechalke, S.; Top, E.; Pollard, A.T.; Popova, I. Dairy manure as a potential source of crop nutrients and environmental contaminants. J. Environ. Sci. 2021, 100, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Mu, M.; Yang, F.; Han, B.; Tian, X.; Zhang, K. Manure application: A trigger for vertical accumulation of antibiotic resistance genes in cropland soils. Ecotoxicol. Environ. Saf. 2022, 237, 113555. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, F.; Udikovic-Kolic, N.; Andrew, S.; Handelsman, J. Diverse antibiotic resistance genes in dairy cow manure. MBio 2014, 5, e01017. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Hu, H.W.; Gou, M.; Wang, J.T.; Chen, D.; He, J.Z. Temporal succession of soil antibiotic resistance genes following application of swine, cattle and poultry manures spiked with or without antibiotics. Environ. Pollut. 2017, 231, 1621–1632. [Google Scholar] [CrossRef]
- Wang, H.; Su, X.; Su, J.; Zhu, Y.; Ding, K. Profiling the antibiotic resistome in soils between pristine and human-affected sites on the Tibetan Plateau. J. Environ. Sci. 2022, 111, 442–451. [Google Scholar] [CrossRef]
- Chen, B.; Lin, L.; Fang, L.; Yang, Y.; Chen, E.; Yuan, K.; Zou, S.; Wang, X.; Luan, T. Complex pollution of antibiotic resistance genes due to beta-lactam and aminoglycoside use in aquaculture farming. Water Res. 2018, 134, 200–208. [Google Scholar] [CrossRef]
- Song, J.; Rensing, C.; Holm, P.E.; Virta, M.; Brandt, K.K. Comparison of metals and tetracycline as selective agents for development of tetracycline resistant bacterial communities in agricultural soil. Environ. Sci. Technol. 2017, 51, 3040–3047. [Google Scholar] [CrossRef] [PubMed]
- Kyselková, M.; Kotrbová, L.; Bhumibhamon, G.; Chroňáková, A.; Jirout, J.; Vrchotová, N.; Schmitt, H.; Elhottová, D. Tetracycline resistance genes persist in soil amended with cattle feces independently from chlortetracycline selection pressure. Soil. Biol. Biochem. 2015, 81, 259–265. [Google Scholar] [CrossRef]
- Macedo, G.; van Veelen, H.P.J.; Hernandez-Leal, L.; van der Maas, P.; Heederik, D.; Mevius, D.; Bossers, A.; Schmitt, H. Targeted metagenomics reveals inferior resilience of farm soil resistome compared to soil microbiome after manure application. Sci. Total Environ. 2021, 770, 145399. [Google Scholar] [CrossRef] [PubMed]
- Rath, S.; Fostier, A.H.; Pereira, L.A.; Dioniso, A.C.; de Oliveira Ferreira, F.; Doretto, K.M.; Maniero Peruchi, L.; Viera, A.; de Oliveira Neto, O.F.; Dal Bosco, S.M.; et al. Sorption behaviors of antimicrobial and antiparasitic veterinary drugs on subtropical soils. Chemosphere 2019, 214, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Mehrtens, A.; Freund, W.; Ludeke, P.; Licha, T.; Burke, V. Understanding flow patterns from the field—Controlled laboratory experiments on the transport behavior of veterinary antibiotics in the presence of liquid manure. Sci. Total Environ. 2022, 821, 153415. [Google Scholar] [CrossRef] [PubMed]
- Vieuble Gonod, L.; Dellouh, L.P.Y.; Andriamalala, A.; Dumeny, V.; Bergheaud, V.; Cambier, P. Fate of sulfamethoxazole in compost, manure and soil amended with previously stored organic wastes. Sci. Total Environ. 2022, 803, 150023. [Google Scholar] [CrossRef]
- Deng, Y.; Li, B.; Zhang, T. Bacteria that make a meal of sulfonamide antibiotics: Blind spots and emerging opportunities. Environ Sci. Technol. 2018, 52, 3854–3868. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, D.; Xue, J.; Feng, Y.; Wakelin, S.A.; Weaver, L.; Shehata, E.; Li, Z. Fate of bacterial community, antibiotic resistance genes and gentamicin residues in soil after three-year amendment using gentamicin fermentation waste. Chemosphere 2022, 291, 132734. [Google Scholar] [CrossRef]
- Tang, Z.; Zhang, L.; He, N.; Gong, D.; Gao, H.; Ma, Z.; Fu, L.; Zhao, M.; Wang, H.; Wang, C.; et al. Soil bacterial community as impacted by addition of rice straw and biochar. Sci. Rep. 2021, 11, 22185. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Valera, E.; de Melo Rangel, W.; Elhottová, D. Cattle manure application triggers short-term dominance of Acinetobacter in soil microbial communities. Appl. Soil Ecol. 2022, 176, 104466. [Google Scholar] [CrossRef]
- Zaheer, R.; Lakin, S.M.; Polo, R.O.; Cook, S.R.; Larney, F.J.; Morley, P.S.; Booker, C.W.; Hannon, S.J.; Van Domselaar, G.; Read, R.R.; et al. Comparative diversity of microbiomes and Resistomes in beef feedlots, downstream environments and urban sewage influent. BMC Microbiol. 2019, 19, 197. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ling, N.; Guo, J.; Ruan, Y.; Wang, M.; Shen, Q.; Guo, S. Dynamics of the antibiotic resistome in agricultural soils amended with different sources of animal manures over three consecutive years. J. Hazard. Mater. 2021, 401, 123399. [Google Scholar] [CrossRef]
- Zhang, R.; Gu, J.; Wang, X.; Li, Y.; Zhang, K.; Yin, Y.; Zhang, X. Contributions of the microbial community and environmental variables to antibiotic resistance genes during co-composting with swine manure and cotton stalks. J. Hazard. Mater. 2018, 358, 82–91. [Google Scholar] [CrossRef]
- Chen, Q.; An, X.; Li, H.; Su, J.; Ma, Y.; Zhu, Y.G. Long-term field application of sewage sludge increases the abundance of antibiotic resistance genes in soil. Environ. Int. 2016, 92, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.; Xiao, D.; Xie, L.; Yang, J.; Zhao, R.; Hao, J.; Huo, Z.; Zeng, Z.; Xiong, W. Swine manure facilitates the spread of antibiotic resistome including tigecycline-resistant tet(X) variants to farm workers and receiving environment. Sci. Total Environ. 2022, 808, 152157. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ma, W.; Zhou, K.; An, B.; Huo, M.; Lin, X.; Wang, L.; Wang, H.; Liu, Z.; Cheng, G.; et al. Effects of composting on the fate of doxycycline, microbial community, and antibiotic resistance genes in swine manure and broiler manure. Sci. Total Environ. 2022, 832, 155039. [Google Scholar] [CrossRef] [PubMed]
- Subirats, J.; Murray, R.; Scott, A.; Lau, C.H.; Topp, E. Composting of chicken litter from commercial broiler farms reduces the abundance of viable enteric bacteria, Firmicutes, and selected antibiotic resistance genes. Sci. Total Environ. 2020, 746, 141113. [Google Scholar] [CrossRef]
- Liu, B.; Yu, K.; Ahmed, I.; Gin, K.; Xi, B.; Wei, Z.; He, Y.; Zhang, B. Key factors driving the fate of antibiotic resistance genes and controlling strategies during aerobic composting of animal manure: A review. Sci. Total Environ. 2021, 791, 148372. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.; Liu, Y.; Chen, X.; Xu, F.; Xiong, W.; Li, X. Shifts of Antibiotic Resistomes in Soil Following Amendments of Antibiotics-Contained Dairy Manure. Int. J. Environ. Res. Public Health 2022, 19, 10804. https://doi.org/10.3390/ijerph191710804
Kang J, Liu Y, Chen X, Xu F, Xiong W, Li X. Shifts of Antibiotic Resistomes in Soil Following Amendments of Antibiotics-Contained Dairy Manure. International Journal of Environmental Research and Public Health. 2022; 19(17):10804. https://doi.org/10.3390/ijerph191710804
Chicago/Turabian StyleKang, Jijun, Yiming Liu, Xiaojie Chen, Fei Xu, Wenguang Xiong, and Xiubo Li. 2022. "Shifts of Antibiotic Resistomes in Soil Following Amendments of Antibiotics-Contained Dairy Manure" International Journal of Environmental Research and Public Health 19, no. 17: 10804. https://doi.org/10.3390/ijerph191710804
APA StyleKang, J., Liu, Y., Chen, X., Xu, F., Xiong, W., & Li, X. (2022). Shifts of Antibiotic Resistomes in Soil Following Amendments of Antibiotics-Contained Dairy Manure. International Journal of Environmental Research and Public Health, 19(17), 10804. https://doi.org/10.3390/ijerph191710804






