Acid Mine Drainage Effects in the Hydrobiology of Freshwater Streams from Three Mining Areas (SW Portugal): A Statistical Approach
Abstract
:1. Introduction
2. Study Area
2.1. Geology
2.2. Mineralisation
2.3. Exploitation
3. Methods
3.1. Sample Collection and Processing
3.2. Analytical Methods of Waters, Salt Efflorescences and Diatoms
3.3. Data Analysis
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grande, J.A. Drenaje Ácido de Mina en la Faja Pirítica Ibérica: Técnicas de Estudio e Inventario de Explotaciones; Servicio de Publicaciones de la Universidad de Huelva: Huelva, Spain, 2016; p. 345. [Google Scholar]
- Sarmiento, A.M.; Grande, J.A.; Luís, A.T.; Dávila, J.M.; Fortes, J.C.; Santisteban, M.; Curiel, J.; de la Torre, M.L.; Ferreira da Silva, E. Negative pH values in an open-air radical environment affected by acid mine drainage. Characterization and proposal of a hydrogeochemical model. Sci. Total Environ. 2018, 644, 1244–1253. [Google Scholar] [CrossRef] [PubMed]
- Nordstrom, D.K.; Alpers, C.N. Negative pH, efflorescent mineralogy, and consequences for environmental restoration at the Iron Mountain Superfund site, California. Proc. Natl. Acad. Sci. USA 1999, 96, 3455–3462. [Google Scholar] [CrossRef] [PubMed]
- Grande, J.A.; Borrego, J.; de la Torre, M.L.; Sáinz, A. Application of cluster analysis to the geochemistry zonation of the estuary waters in the Tinto and Odiel Rivers (Huelva, Spain). Environ. Geochem. Health 2003, 25, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Grande, J.A.; Jiménez, A.; Borrego, J.; de la Torre, M.L.; Gómez, T. Relationships Between Conductivity and pH in Channels Exposed to Acid Mine Drainage Processes: Study of a Large Mass of Data Using Classical Statistics. Water Resour. Manag. 2010, 24, 4579–4587. [Google Scholar] [CrossRef]
- Gomes, P.; Valente, T.; Braga, M.A.S.; Grande, J.A.; de la Torre, M.L. Enrichment of trace elements in the clay size fraction of mining soils. Environ. Sci. Pollut. Res. 2016, 23, 6039–6045. [Google Scholar] [CrossRef]
- Soyol-Erdene, T.O.; Valente, T.; Grande, J.A.; de la Torre, M.L. Mineralogical controls on mobility of rare earth elements. Chemosphere 2018, 205, 317–327. [Google Scholar] [CrossRef]
- Grande, J.A.; Luís, A.T.; Santisteban, M.; Dávila, J.M.; Sarmiento, A.M.; Fortes, J.C.; Ferreira da Silva, E.; Córdoba, F. A common paragenesis and two A.M.D. pollution sources in the Iberian Pyrite Belt (SW Spain): Proposal of a natural attenuation model in the affected fluvial network. J. Iber. Geol. 2022, 29, 31749–31760. [Google Scholar] [CrossRef]
- Valente, T.; Grande, J.A.; de la Torre, M.L.; Gomes, P.; Santisteban, M.; Borrego, J.; Sequeira Braga, M.J. Mineralogy and geochemistry of a clogged mining reservoir affected by historical acid mine drainage in an abandoned mining area. J. Geochemical. Explor. 2015, 157, 66–76. [Google Scholar] [CrossRef]
- Sainz, A.; Grande, J.A.; de la Torre, M.L. Application of a systemic approach to the study of pollution of the Tinto and Odiel rivers (Spain). Environ. Monit. Assess 2005, 102, 435–445. [Google Scholar] [CrossRef]
- Mulholland, P.J.; Elwood, J.W.; Palumbo, A.V.; Stevenson, R.J. Effects of stream acidification on periphyton composition, chlorophyll and productivity. Can. J. Fish. Aquat. Sci. 1986, 43, 1846–1858. [Google Scholar] [CrossRef]
- Verb, R.G.; Vis, M.L. Comparison of benthic diatom assemblages from streams draining abandoned and reclaimed coal mines and nonimpacted sites. J. N. Am. Benthol. Soc. 2000, 19, 274–288. [Google Scholar] [CrossRef]
- Kwandrans, J. Diversity and Ecology of Benthic Diatom Communities in Relation to Acidity, Acidification and Recovery of Lakes and Rivers; Diatom Monographs, A.R., Gantner, G., Liechtenstein, K.G., Eds.; Gantner Verlag: Ruggell, Liechtenstein, 2007; Volume 9, pp. 1–169. [Google Scholar]
- Gustavson, K.; Wängberg, S.-Å. Tolerance induction and succession in microalgae communities exposed to copper and atrazine. Aquat. Toxicol. 1995, 32, 283–302. [Google Scholar] [CrossRef]
- Luís, A.T.; Teixeira, P.; Almeida, S.F.P.; Ector, L.; Matos, J.X.; Ferreira da Silva, E.A. Impact of acid mine drainage (AMD) on water quality, stream sediments and periphytic diatom communities in the surrounding streams of Aljustrel mining area (Portugal). Water Air Soil Pollut. 2009, 200, 147–167. [Google Scholar] [CrossRef]
- Luís, A.T.; Durães, N.; Almeida, S.F.P.; Ferreira da Silva, E. Integrating geochemical (surface waters, stream sediments) and biological (diatoms) approaches to assess environmental impact in a pyritic mining area: Aljustrel (Alentejo, Portugal). J. Environ. Sci. 2016, 42, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Luís, A.T.; Grande, J.A.; Durães, N.; Dávila, J.M.; Santisteban, M.; Almeida, S.F.P.; Sarmiento, A.M.; de la Torre, M.L.; Fortes, J.C.; Ferreira da Silva, E. Biogeochemical characterization of surface waters in the Aljustrel mining area (South Portugal). Environ. Geochem. Health 2019, 211, 736–744. [Google Scholar] [CrossRef]
- Leland, H.V.; Carter, J.L. Effects of copper on species composition of periphyton in a Sierra Nevada, California stream. Freshw. Biol. 1984, 14, 281–296. [Google Scholar] [CrossRef]
- Schermerhorn, L.J.G.; Zbyszewski, G.; Ferreira, O.V. Carta Geológica de Portugal 1:50,000, Notícia Explicativa da Folha 42 D: Aljustrel; Serviços Geológicos de Portugal: Lisboa, Portugal, 1987; p. 55. [Google Scholar]
- Barriga, F.J.A.S.; Carvalho, D.; Ribeiro, A. Introduction to the Iberian Pyrite Belt. SEG Neves Corvo Field Conference 1997; Guidebook Series; Society of Economic Geologists: Neves Corvo, Portugal, 1997; Volume 27, pp. 1–20. [Google Scholar]
- Barriga, F.J.A.S.; Fyfe, W.S. Multi-phase water-rhyolite interaction and ore fluid generation at Aljustrel, Portugal. Mineral. Deposita. 1997, 33, 88–207. [Google Scholar] [CrossRef]
- Strauss, G.K. Sobre la Geologia de la Provincia Piritífera del SW de la Península Ibérica y de sus Yacimientos, en Especial Sobre la Mina de Pirita de Lousal (Portugal); Memoria del Instituto Geológico y Minero de Spain: Madrid, Spain, 1970; Volume 77, p. 266. [Google Scholar]
- Ferreira da Silva, E.; Patinha, C.; Reis, P.; Cardoso Fonseca, E.; Matos, J.X.; Barrosinho, J.; Santos Oliveira, J.M. Interaction of acid mine drainage with waters and sediments at the Corona stream, Lousal mine (Iberian Pyrite Belt, Southern Portugal). Environ. Geol. 2006, 50, 1001–1013. [Google Scholar] [CrossRef]
- Pinedo Vara, I. Piritas de Huelva. Su Historia, Minería y Aprovechmiento; Summa: Madrid, Spain, 1963; pp. 1–1003. [Google Scholar]
- Carvalho, D. Mina de S. Domingos. Livro-Guia nº4. I Congresso Hispano-Luso- Americano de Geologia Economica; Direccao-Geral de Minas e Servicos Geologicos: Lisboa, Portugal, 1971; pp. 59–64. [Google Scholar]
- Luís, A.T.; Novais, M.H.; Van de Vijver, B.; Almeida, S.F.P.; Ferreira da Silva, E.A.; Hoffmann, L.; Ector, L. Pinnularia aljustrelica sp. nov. (Bacillariophyceae), a new diatom species found in acidic waters in the Aljustrel mining area (Portugal), and further observations on the taxonomy, morphology and ecology of P. acidophila HOFMANN et KRAMMER and P. acoricola HUSTEDT. Fottea 2012, 12, 27–40. [Google Scholar]
- Álvarez-Valero, A.M.; Perez-Lopez, R.; Matos, J.X.; Capitan, M.A.; Nieto, J.M.; Saez, R.; Delgado, J.; Caraballo, M. Potential environmental impact at S. Domingos mining district (Iberian Pyrite Belt, SW Iberian Peninsula): Evidence from a chemical and mineralogical characterization. Environ. Geol. 2008, 55, 1797–1809. [Google Scholar] [CrossRef]
- Prygiel, J.; Coste, M. Guide Méthodologique Pour la Mise en Oeuvre de l’Indice Biologique Diatomées NF T 90-354; Agence de l’eau Artois Picardie: Douai, France, 2000; pp. 1–134. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. Naviculaceae. In Süßwasserflora von Mitteleuropa; Gustav Fischer: Stuttgart, Germany, 1986; Volume 1. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. Bacillariaceae, Epithemiaceae, Surirellacea. In Süßwasserflora von Mitteleuropa; Gustav Fischer: Stuttgart, Germany, 1988; Volume 2. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. Centrales, Fragilariaceae, Eunoticeae. In Süßwasserflora von Mitteleuropa; Gustav Fischer: Stuttgart, Germany, 1991; Volume 3. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. Achnanthaceae, Kristische Ergänzungen zu Navicula (Lineolatae) und Gomphonema Gesamtliteraturverzeichnis. In Süßwasserflora von Mitteleuropa; Gustav Fischer: Stuttgart, Germany, 1991; Volume 4. [Google Scholar]
- Bisquerra, R. Introducción Conceptual al Análisis Multivariable; Promociones y Publicaciones Universitarias: Barcelona, Spain, 1989; pp. 20–40. [Google Scholar]
- Luís, A.T.; Teixeira, P.; Almeida, S.F.P.; Matos, J.X.; Ferreira da Silva, E. Environmental impact of mining activities in the Lousal area (Portugal): Chemical and diatom characterization of metal-contaminated stream sediments and surface water of Corona stream. Sci. Total Environ. 2011, 409, 4312–4325. [Google Scholar] [CrossRef]
- Durães, N.; Bobos, I.; da Silva, E.F. Speciation and precipitation of heavy metals in high-metal and high-acid mine waters from the Iberian Pyrite Belt (Portugal). Environ. Sci. Pollut. Res. 2017, 24, 4562–4576. [Google Scholar] [CrossRef]
- Valente, T.; Grande, J.A.; de la Torre, M.L. Magnesium and aluminium sulfates in salt efflorescences from acid mine drainage in the Iberian Pyrite Belt (SW Spain). In Mining Meets Water-Conflicts and Solutions; Carsten, D., Michael, P., Eds.; Proceedings IMWA: Freiberg, Germany, 2016. [Google Scholar]
- Nordstrom, D.K.; Alpers, C.N. Geochemistry of acid mine waters. In The Environmental Geochemistry of Mineral Deposits, Part A: Processes, Methods, and Health Issues; Plumlee, G.S., Logsdon, M.J., Eds.; Rev Econ Geol Littleton, Society of Economic Geology: Littleton, CO, USA, 1999; Volume 6A, pp. 133–160. [Google Scholar]
- Luís, A.T.; Alexander, A.C.; Almeida, S.F.P.; Ferreira da Silva, E.; Culp, J.M. Benthic diatom communities in streams from zinc mining areas in continental (Canada) and Mediterranean climates (Portugal). Water Qual. Res. J. Can. 2013, 48, 180–191. [Google Scholar] [CrossRef]
- Rivera, M.J.; Luís, A.T.; Grande, J.A.; Sarmiento, A.M.; Dávila, J.M.; Fortes, J.C.; Córdoba, F.; Díaz-Curiel, J.; Santisteban, M. Physico-Chemical Influence of Surface Water Contaminated by Acid Mine Drainage on the Populations of Diatoms in Dams (Iberian Pyrite Belt, SW Spain). Int. J. Environ. Res. Public Health 2019, 16, 4516. [Google Scholar] [CrossRef]
- Rivera, M.J.; Santisteban, M.; Aroba, J.; Grande, J.A.; Dávila, J.M.; Sarmiento, A.M.; Fortes, J.C.; Diaz-Curiel, J.; Luís, A.T. Application of Fuzzy Logic Techniques for Biogeochemical Characterization of Dams Affected by Acid Mine Drainage (AMD) Processes in the Iberian Pyrite Belt (IPB), Spain. Water Air Soil Pollut. 2020, 231, 142. [Google Scholar] [CrossRef]
- Chacon-Baca, E.; Santos, A.; Sarmiento, A.M.; Luís, A.T.; Santisteban, M.; Fortes, J.C.; Dávila, J.M.; Curiel, J.; Grande, J.A. Acid Mine Drainage as energizing microbial niches for the formation of clastic iron stromatolites: The Tintillo river in SW Spain. Astrobiology 2021, 21, 443–463. [Google Scholar] [CrossRef]
- Grande, J.A.; Luís, A.T.; Córdoba, F.; Leiva, M.; Dávila, J.M.; Fortes, J.C.; Santisteban, M.; Ferreira da Silva, E.; Sarmiento, A.M. Odiel River (SW Spain), a Singular Scenario Affected by Acid Mine Drainage (AMD): Graphical and Statistical Models to Assess Diatoms and Water Hydrogeochemistry Interactions. Int. J. Environ. Res. Public Health 2021, 18, 8454. [Google Scholar] [CrossRef]
- Córdoba, F.; Luís, A.T.; Leiva, M.; Sarmiento, A.M.; Santisteban, M.; Fortes, J.C.; Dávila, J.M.; Álvarez-Bajo, O.; Grande, J.A. Biogeochemical indicators (waters/diatoms) of acid mine drainage pollution in the Odiel river (Iberian Pyritic Belt, SW Spain). Environ. Sci. Pollut. Res. 2022, 29, 31749–31760. [Google Scholar] [CrossRef]
- Luís, A.T.; Córdoba, F.; Antunes, C.; Loayza-Muro, R.; Grande, J.A.; Silva, B.; Diaz-Curiel, J.; Ferreira da Silva, E. Extremely Acidic Eukaryotic (Micro) Organisms: Life in Acid Mine Drainage Polluted Environments—Mini-Review. Int. J. Environ. Res. Public Health 2022, 19, 376. [Google Scholar] [CrossRef]

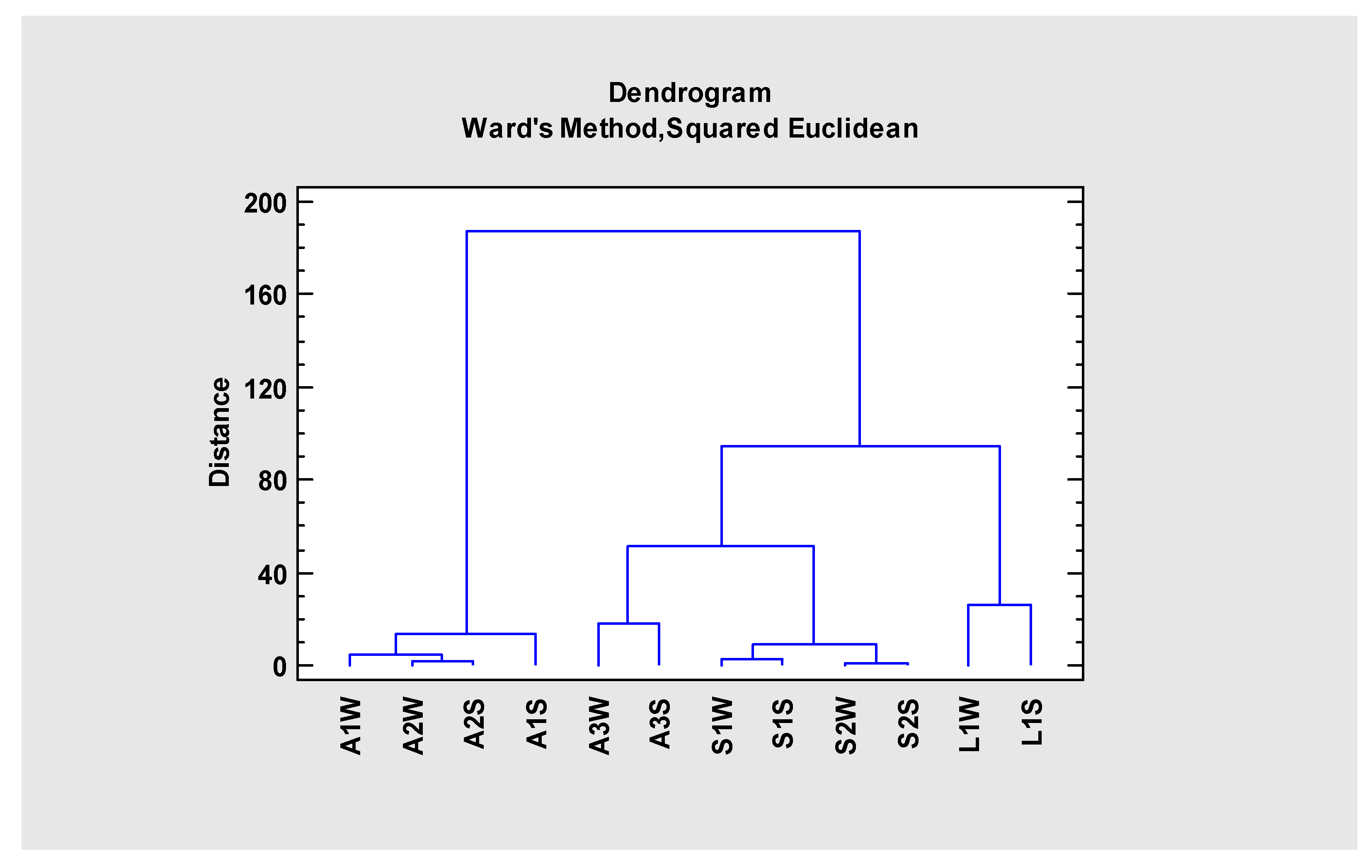
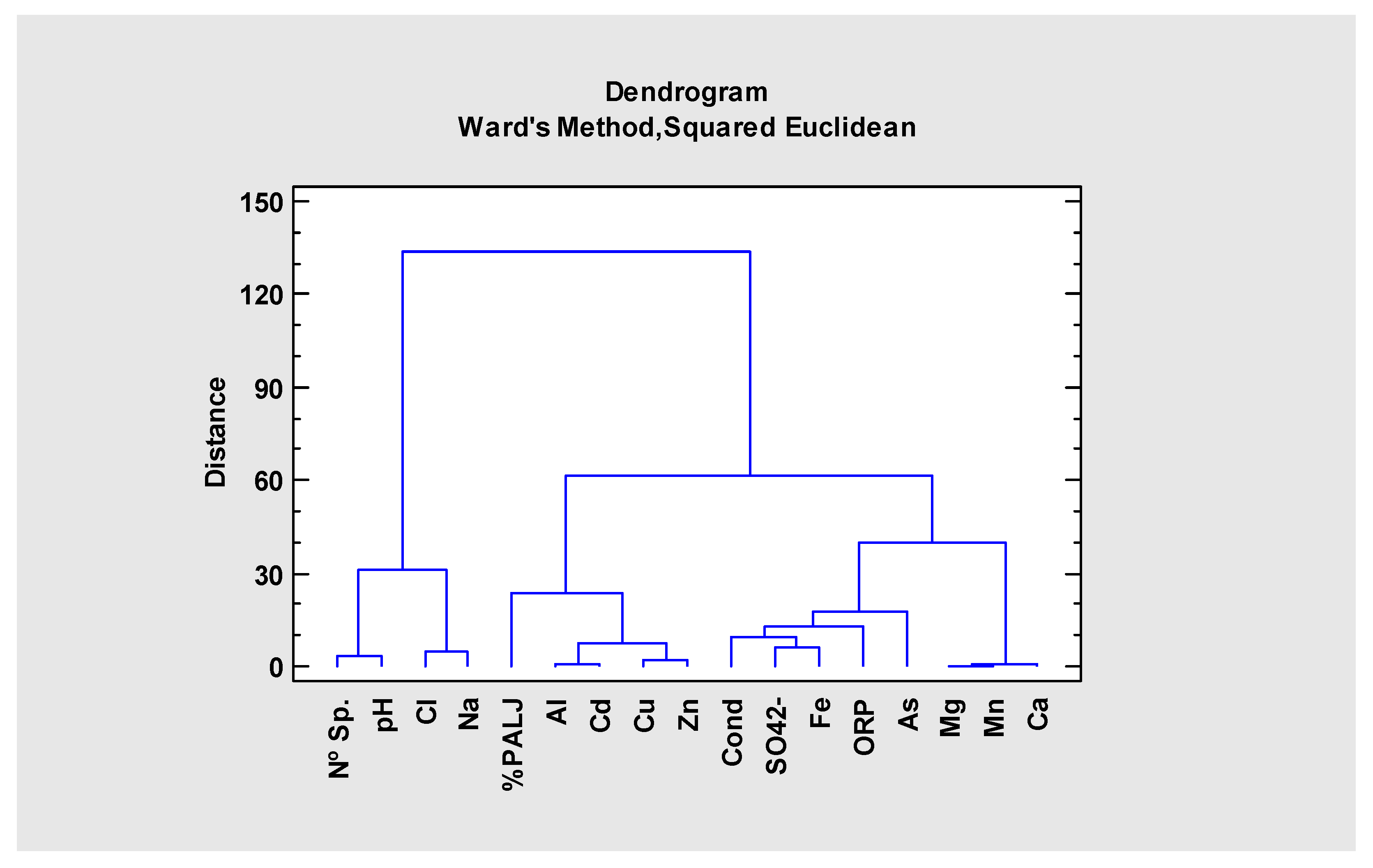

| Sites | Nº Sp | %PALJ | pH | Cond | ORP | SO42− | Cl | Na | Mg | Ca | Al | As | Cd | Cu | Fe | Mn | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1W | 19 | N.D. | 8.70 | 2184 | 99 | 117 | 516 | 257 | 106 | 153 | 0.01 | N.D. | N.D. | N.D. | 0.15 | 0.05 | N.D. |
| A2W | 19 | N.D. | 8.22 | 1518 | 120 | 55 | 313 | 172 | 65 | 104 | 0.01 | N.D. | N.D. | 0.01 | 0.10 | 0.05 | N.D. |
| A3W | 9 | 41.39 | 2.59 | 5200 | 498 | 3901 | 170 | 94 | 221 | 208 | 275.57 | 0.54 | 0.29 | 53.77 | 4583.94 | 30.96 | 128.62 |
| S1W | 1 | 100 | 2.56 | 3451 | 522 | 1867 | 98 | 63 | 95 | 96 | 146.18 | 0.36 | 0.15 | 16.04 | 956.49 | 10.60 | 25.14 |
| S2W | 4 | 30.67 | 2.55 | 5064 | 498 | 1469 | 96 | 71 | 79 | 81 | 94.09 | 0.15 | 0.8 | 10.49 | 921.93 | 6.74 | 15.52 |
| L1W | 3 | 87.28 | 2.51 | 8300 | 502 | 5192 | 288 | 260 | 902 | 585 | 167.20 | 1.36 | 0.11 | 11.46 | 3897.71 | 117.63 | 60.69 |
| A1S | 34 | N.D. | 8.11 | 3153 | 303 | 217 | 698 | 275 | 130 | 238 | 0.01 | 0.01 | N.D. | N.D. | 0.15 | N.D. | 0.03 |
| A2S | 36 | N.D. | 8.02 | 1488 | 161 | 51 | 370 | 152 | 58 | 111 | 0.02 | N.D. | N.D. | N.D. | 0.51 | 0.029 | 0.03 |
| A3S | 1 | 100 | 2.23 | 6426 | 460 | 3739 | 403 | 158 | 233 | 237 | 199.20 | 0.09 | 0.25 | 58.24 | 2038.37 | 27.38 | 117.36 |
| S1S | 1 | 100 | 2.41 | 3656 | 300 | 2229 | 109 | 58 | 96 | 112 | 161.94 | 0.35 | 0.16 | 20.70 | 1153.48 | 11.45 | 34.67 |
| S2S | 4 | 30.75 | 2.19 | 4413 | 381 | 2006 | 121 | 66 | 93 | 118 | 130.53 | 0.02 | 0.10 | 16.17 | 1189.42 | 9.65 | 27.04 |
| L1S | 2 | 23.41 | 2.54 | 7782 | 475 | 2007 | 390 | 218 | 726 | 514 | 115.81 | 0.23 | 0.10 | 12.27 | 3463.86 | 104.95 | 81.02 |
| Sites | L1 | A3 | S1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Summer (2004) | Winter (2004) | Summer (2016) | Winter (2016) | Summer (2008) | Winter (2008) | Summer (2016) | Winter (2016) | Summer (2014) | Winter (2014) | Summer (2016) | Winter (2016) | |
| pH | 2.4 | 2.8 | 2.5 | 2.5 | 2.3 | 2.0 | 2.2 | 2.6 | 2.6 | 2.4 | 2.4 | 2.6 |
| Cond | 8970 | 6650 | 8300 | 7782 | 14,200 | 14,100 | 6426 | 5200 | 3806 | 3690 | 3656 | 3451 |
| SO42− | 8109 | 6069 | 2007 | 5192 | 16,995 | 36,900 | 3739 | 3901 | 2323 | 1960 | 2229 | 1867 |
| Cl | 539 | 432 | 390 | 288 | 99 | 1 | 403 | 170 | 234 | 108 | 109 | 98 |
| Ca | 568 | 453 | 514 | 586 | 250 | 434 | 237 | 208 | 61 | 81 | 112 | 96 |
| Mg | 1061 | 743 | 726 | 902 | 438 | 365 | 233 | 221 | 58 | 69 | 96 | 95 |
| Na | 297 | 227 | 218 | 261 | 88 | 40 | 158 | 94 | 37 | 35 | 58 | 63 |
| Al | 63.0 | 35.0 | 115.8 | 167.2 | 757.0 | 482.0 | 199.2 | 275.6 | 211.0 | 295.0 | 161.9 | 146.2 |
| As | 0.0 | 0.0 | 0.2 | 1.4 | 29.8 | 52.0 | 0.1 | 0.5 | 0.3 | 0.6 | 0.3 | 0.4 |
| Cd | 0.3 | 0.2 | 0.1 | 0.1 | 1.7 | 2.1 | 0.3 | 0.3 | 0.1 | 0.1 | 0.2 | 0.2 |
| Cu | 3.7 | 2.5 | 11.5 | 11.5 | 130.0 | 216.0 | 58.2 | 53.8 | 21.0 | 10.7 | 20.7 | 16.0 |
| Fe | 562.0 | 504.0 | 3463.9 | 3897.7 | 3688.0 | 4841.0 | 2038.4 | 4583.9 | 178.0 | 81.0 | 1153.5 | 956.5 |
| Mn | 157.0 | 110.0 | 105.0 | 117.6 | 89.0 | 66.0 | 27.4 | 31.0 | 11.3 | 5.6 | 11.5 | 10.6 |
| Zn | 115.0 | 77.0 | 81.0 | 60.7 | 970.0 | 1112.0 | 117.4 | 128.6 | 38.0 | 16.8 | 34.7 | 25.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luís, A.T.; Grande, J.A.; Durães, N.; Santisteban, M.; Rodríguez-Pérez, Á.M.; Ferreira da Silva, E. Acid Mine Drainage Effects in the Hydrobiology of Freshwater Streams from Three Mining Areas (SW Portugal): A Statistical Approach. Int. J. Environ. Res. Public Health 2022, 19, 10810. https://doi.org/10.3390/ijerph191710810
Luís AT, Grande JA, Durães N, Santisteban M, Rodríguez-Pérez ÁM, Ferreira da Silva E. Acid Mine Drainage Effects in the Hydrobiology of Freshwater Streams from Three Mining Areas (SW Portugal): A Statistical Approach. International Journal of Environmental Research and Public Health. 2022; 19(17):10810. https://doi.org/10.3390/ijerph191710810
Chicago/Turabian StyleLuís, Ana Teresa, José Antonio Grande, Nuno Durães, María Santisteban, Ángel Mariano Rodríguez-Pérez, and Eduardo Ferreira da Silva. 2022. "Acid Mine Drainage Effects in the Hydrobiology of Freshwater Streams from Three Mining Areas (SW Portugal): A Statistical Approach" International Journal of Environmental Research and Public Health 19, no. 17: 10810. https://doi.org/10.3390/ijerph191710810
APA StyleLuís, A. T., Grande, J. A., Durães, N., Santisteban, M., Rodríguez-Pérez, Á. M., & Ferreira da Silva, E. (2022). Acid Mine Drainage Effects in the Hydrobiology of Freshwater Streams from Three Mining Areas (SW Portugal): A Statistical Approach. International Journal of Environmental Research and Public Health, 19(17), 10810. https://doi.org/10.3390/ijerph191710810











