Preparation of Mn/Zn@PG Catalyst for Catalytic Oxidation Treatment of Coal Chemical Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Preparation of Mn/Zn@PG Catalyst
2.3. Characterization of Mn/Zn@PG Catalysts
2.4. Ozone Catalytic Oxidation Experiment
2.5. Water Quality Analysis Method
2.6. Hierarchical–Principal Component Comprehensive Evaluation Model Analysis
3. Results and Discussion
3.1. Catalyst Preparation and Optimization
3.2. Characterization of Catalysts
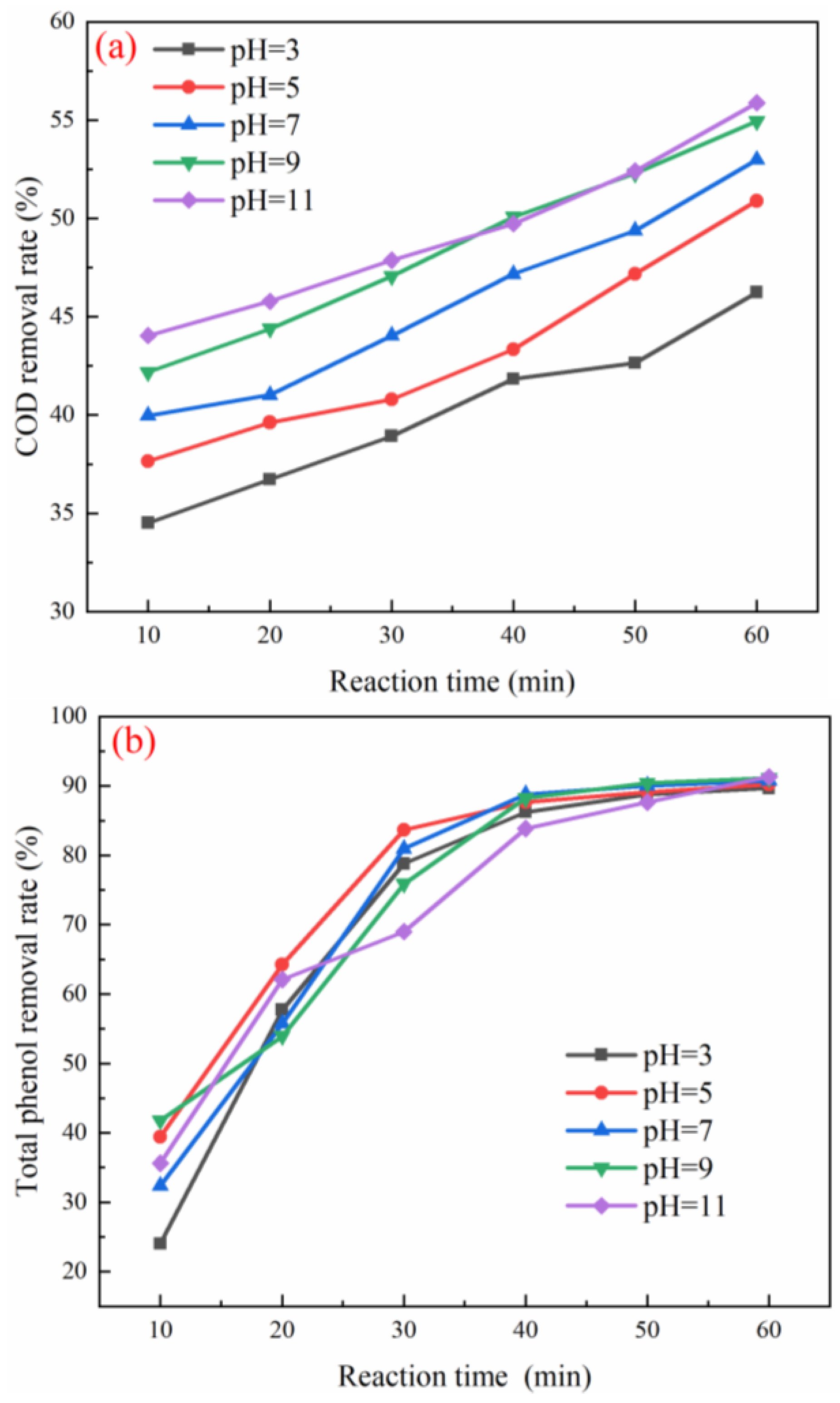
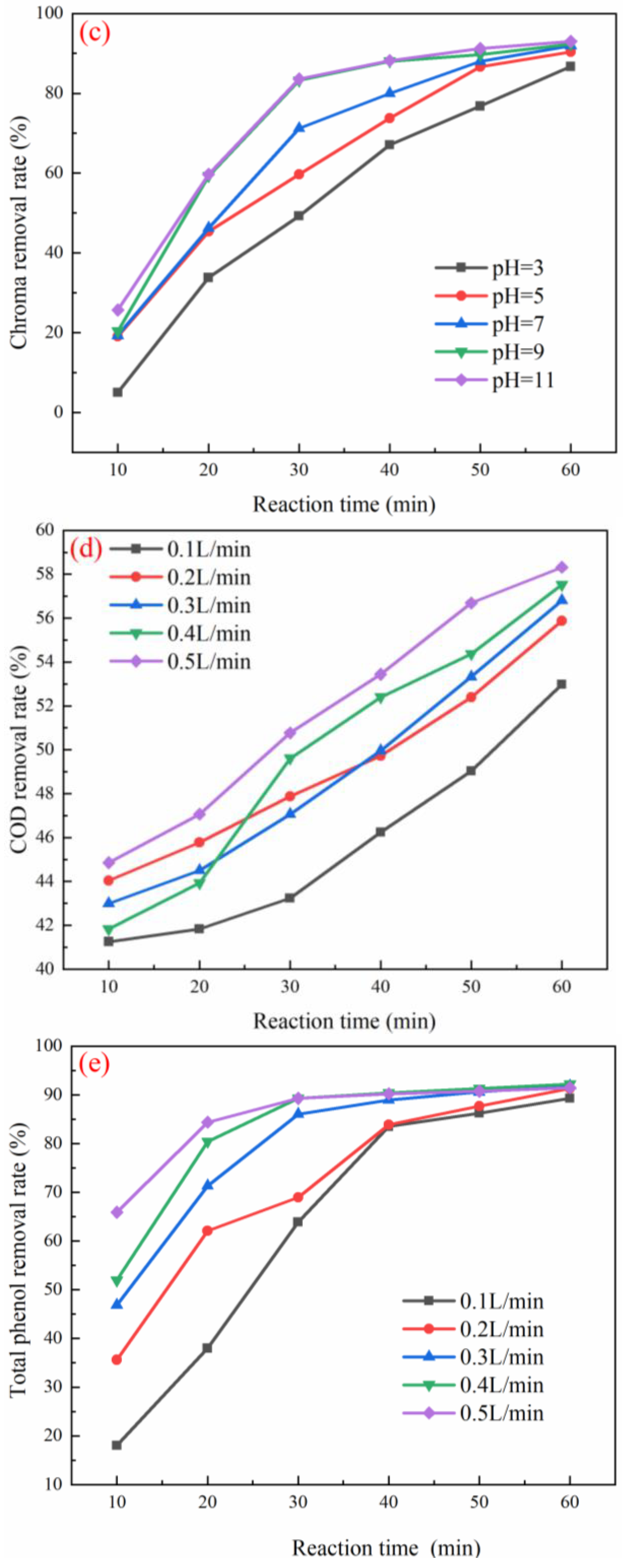
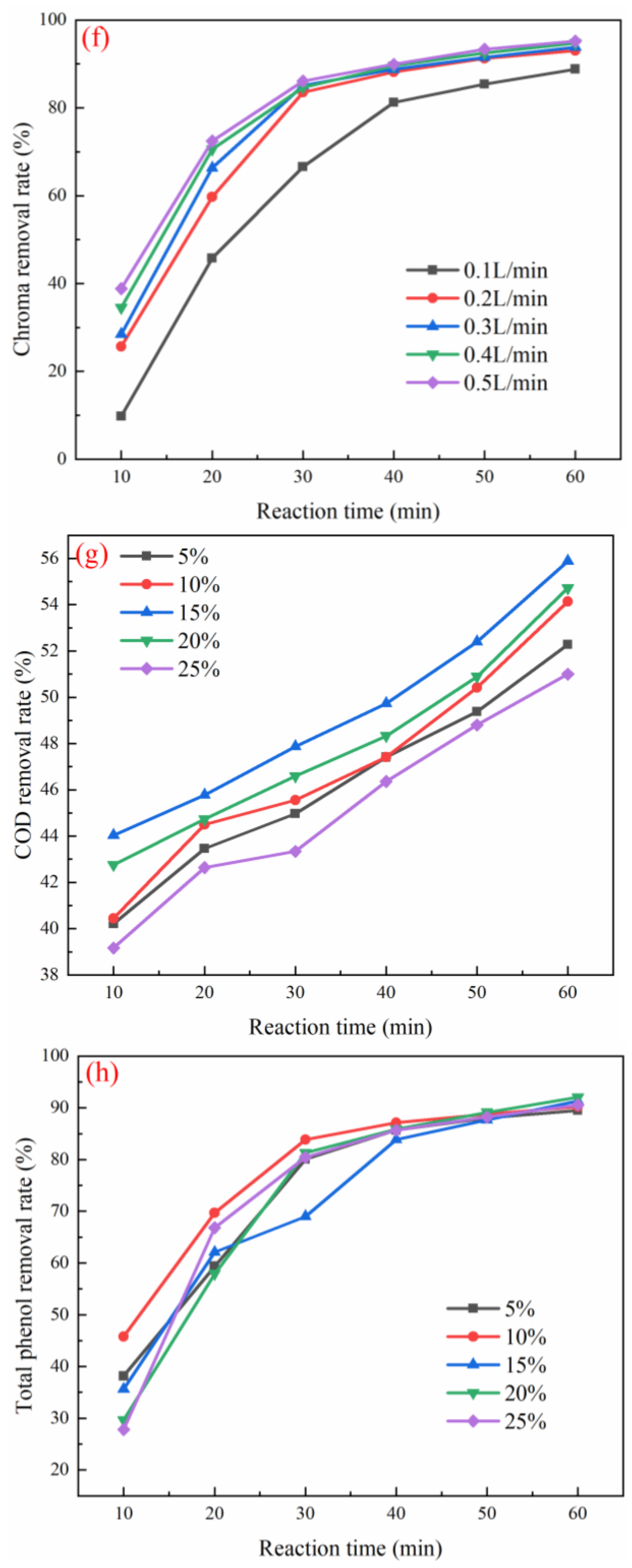
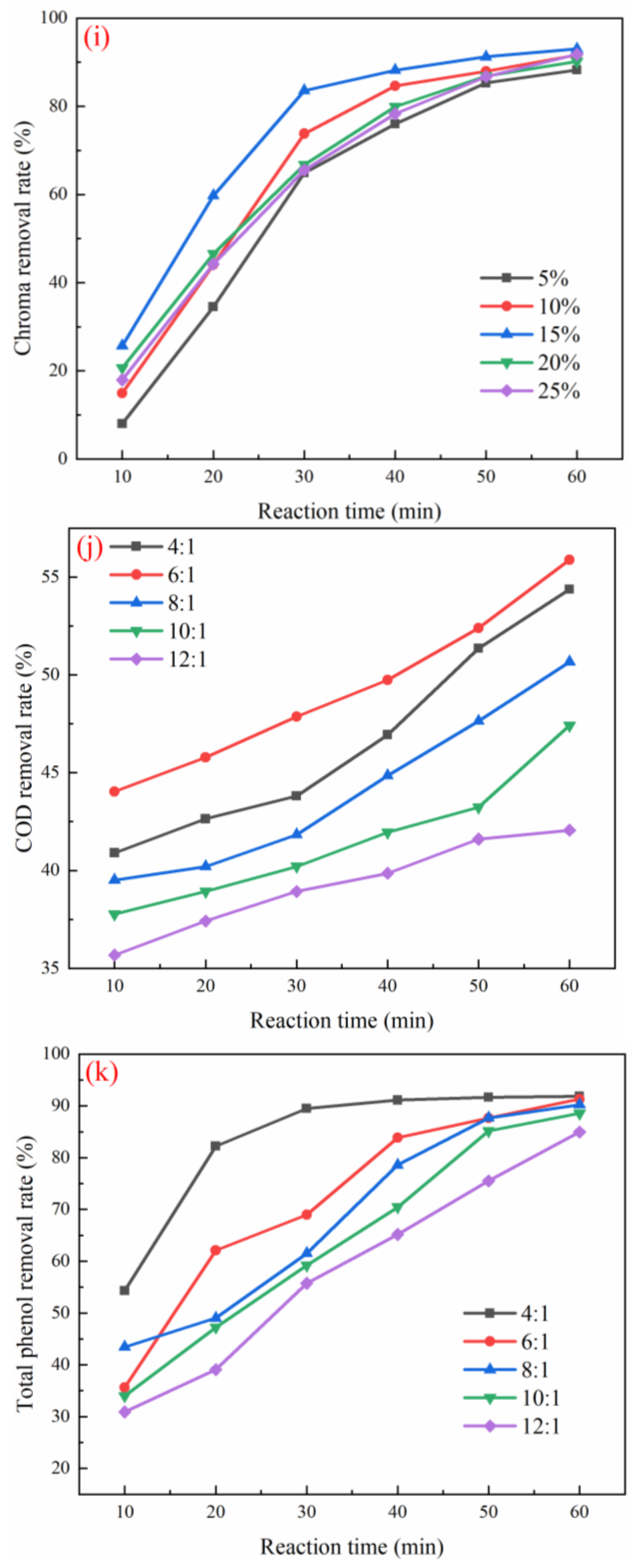
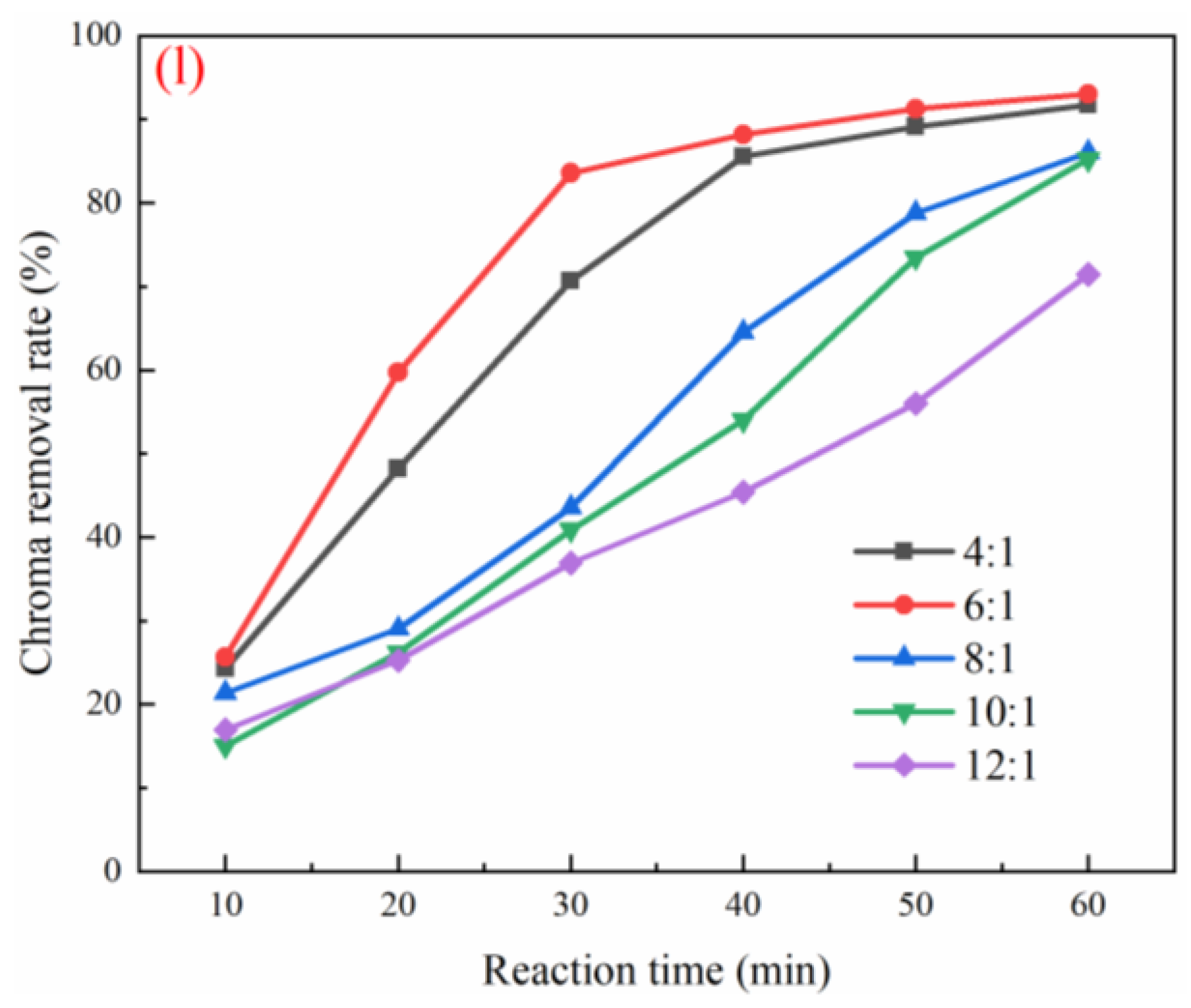
3.3. Optimization of Working Conditions for Catalyst Degradation of Coal Chemical Wastewater
3.4. Stability Analysis of Mn/Zn@PG Ozone Catalyst
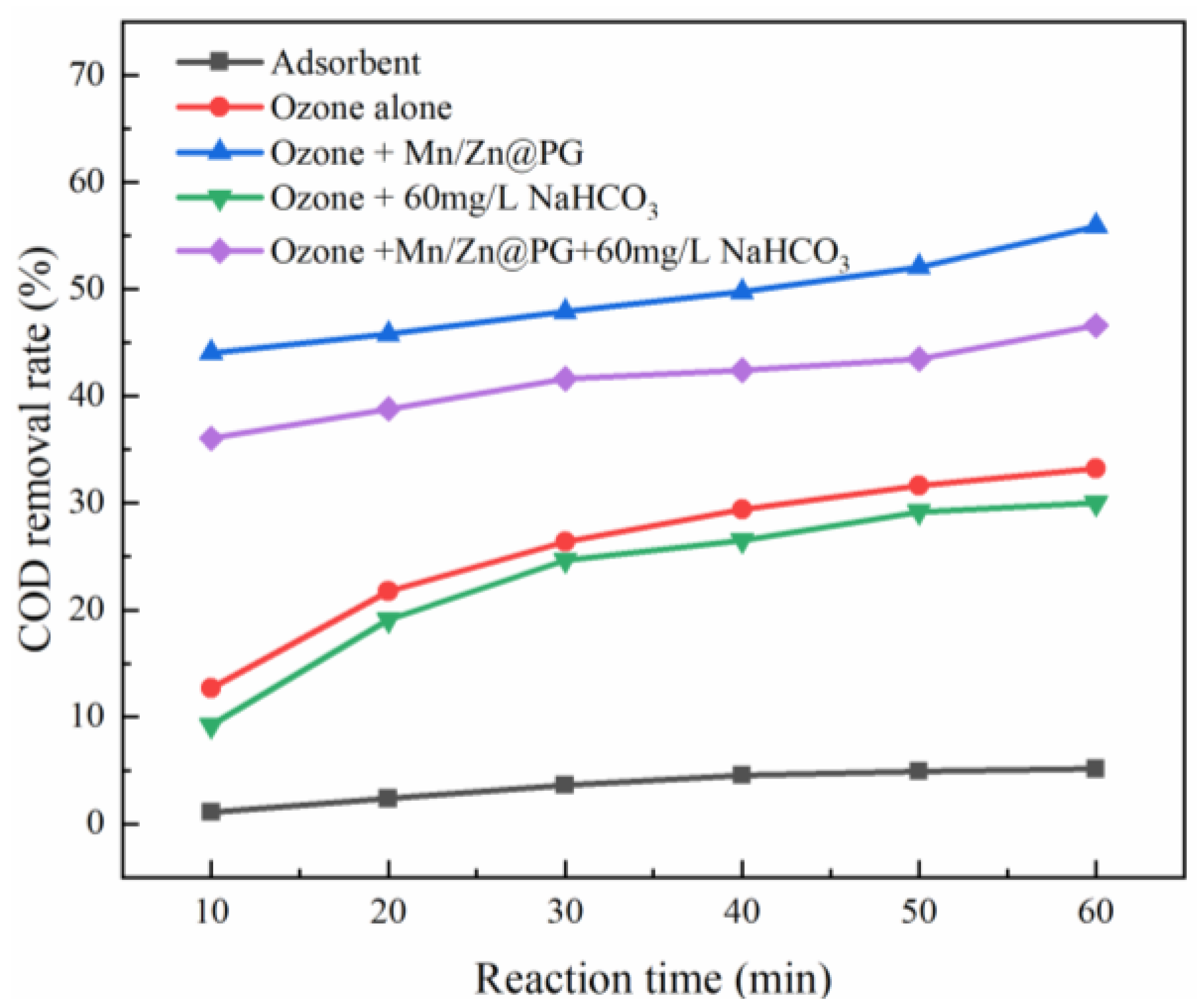
3.5. Effect of Dosage of Radical Quencher on Different Reaction Systems
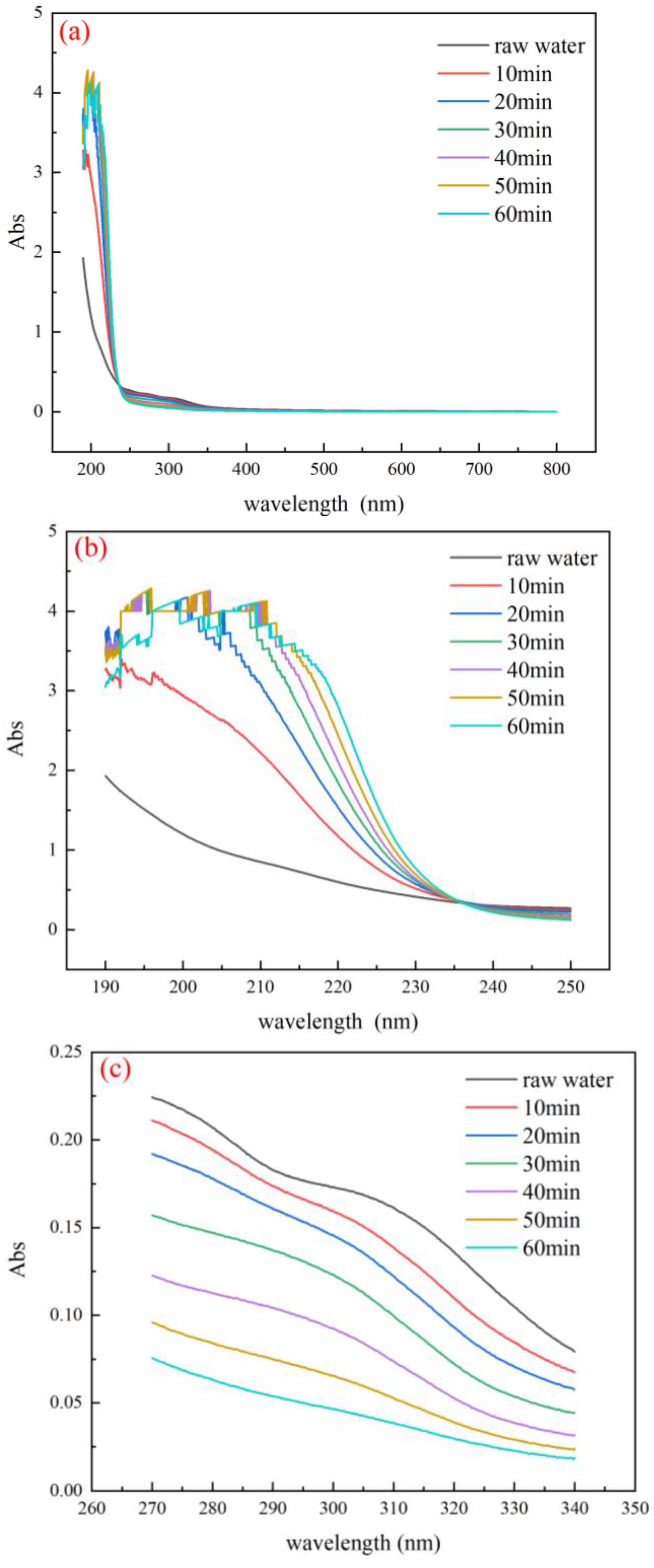
3.6. Analysis of UV Absorption Peaks of Wastewater before and after Reaction
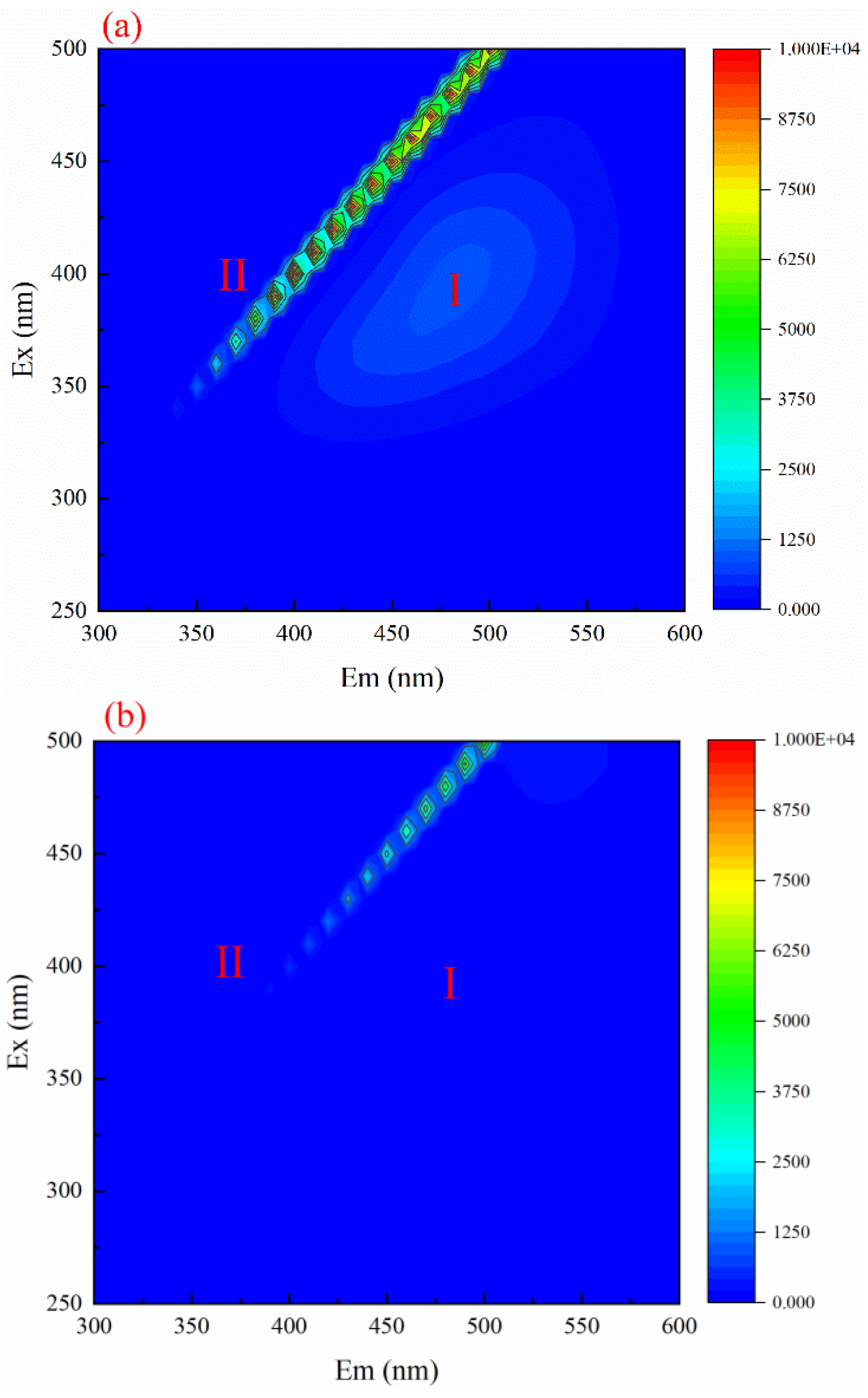
3.7. 3D Fluorescence Spectroscopic Analysis of Wastewater before and after Reaction
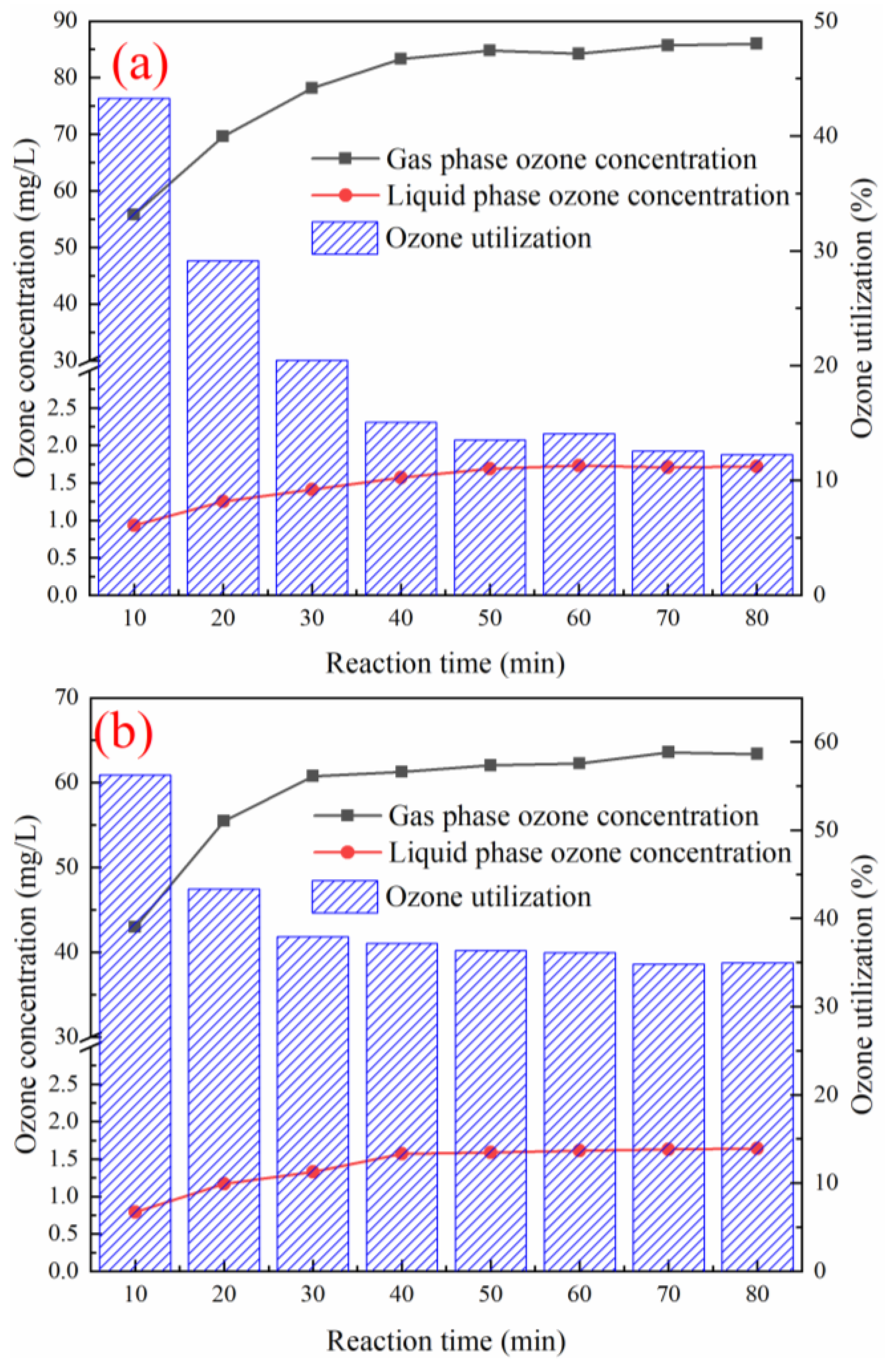
3.8. Effect of Various Reaction Systems on Ozone Utilization
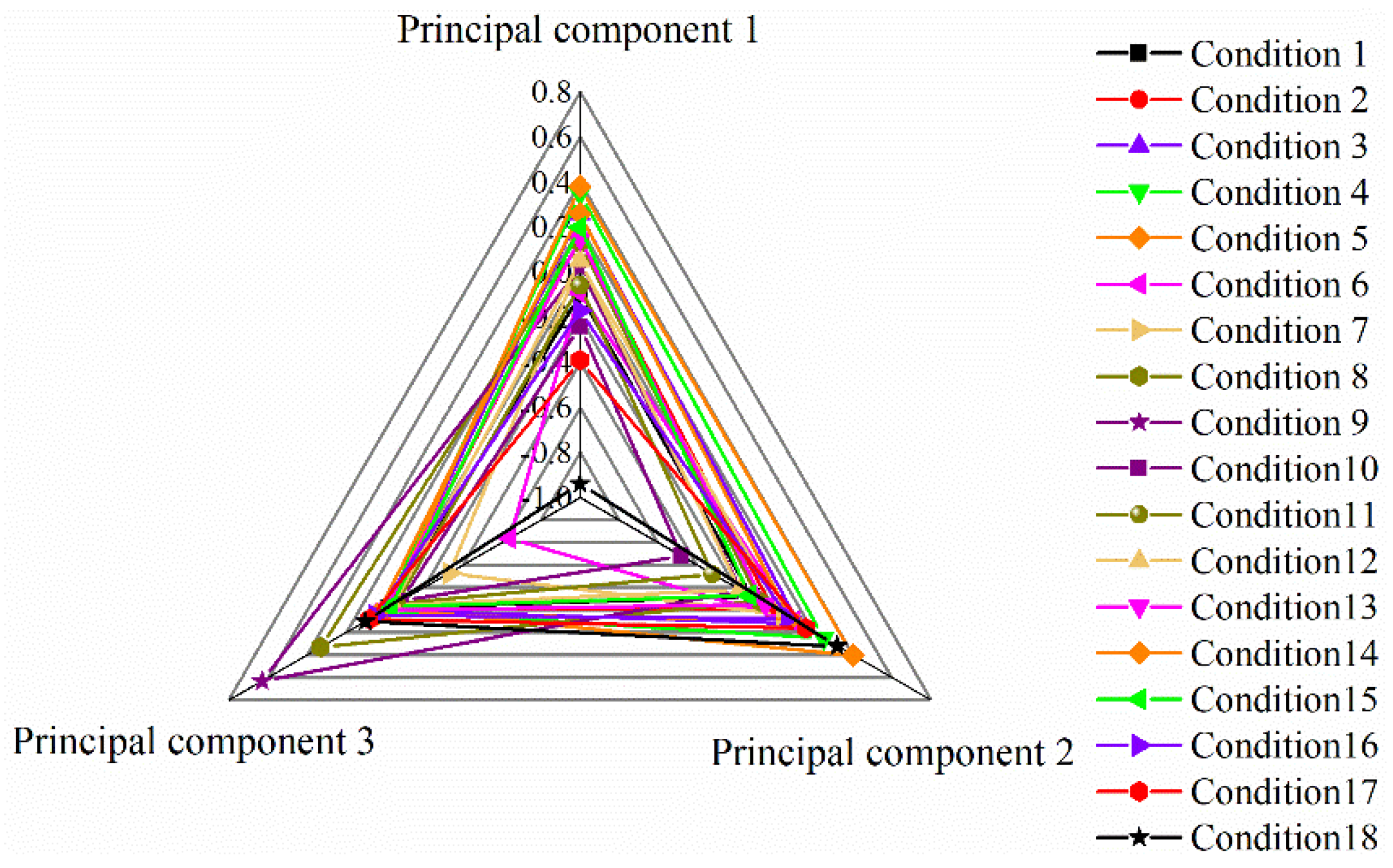
3.9. Analysis of Comprehensive Evaluation Model of Ozone Catalytic Oxidation Experimental Hierarchy–Principal Component
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, Q.; Zhang, B.; Chen, Q.; Pan, M.; Ren, J.; He, C. Optimal synthesis of water networks for addressing high-concentration wastewater in coal-based chemical plants. ACS Sustain. Chem. Eng. 2017, 5, 10792–10805. [Google Scholar] [CrossRef]
- Shi, J.; Xu, C.; Han, Y.; Han, H. Enhanced anaerobic biodegradation efficiency and mechanism of quinoline, pyridine, and indole in coal gasification wastewater. Chem. Eng. J. 2019, 361, 1019–1029. [Google Scholar] [CrossRef]
- Huang, L.; Wang, D.; He, C.; Pan, M.; Zhang, B.; Chen, Q.; Ren, J. Industrial wastewater desalination under uncertainty in coal-chemical eco-industrial parks. Resour. Conserv. Recycl. 2019, 145, 370–378. [Google Scholar] [CrossRef]
- Qin, Q.; Yang, H.; Xu, H.; Deng, J.; Zhao, R.; Huang, G.; Wang, P.; Wang, J. Experiment study on the separation of bituminous coal adsorption and the synergism of ultraviolet and electrochemistry in the pretreatment of coal chemical wastewater. Fuel 2021, 288, 119712. [Google Scholar] [CrossRef]
- Sheikhi, S.; Dehghanzadeh, R.; Maryamabadi, A.; Aslani, H. Chlorpyrifos removal from aqueous solution through sequential use of coagulation and advanced oxidation processes: By-products, degradation pathways, and toxicity assessment. Environ. Technol. Innov. 2021, 23, 101564. [Google Scholar] [CrossRef]
- Wu, M.; Kwok, Y.H.; Zhang, Y.; Szeto, W.; Huang, H.; Leung, D.Y.C. Synergetic effect of vacuum ultraviolet photolysis and ozone catalytic oxidation for toluene degradation over MnO2-rGO composite catalyst. Chem. Eng. Sci. 2021, 231, 116288. [Google Scholar] [CrossRef]
- Sun, W.; Sun, Y.; Zhu, H.; Zheng, H. Catalytic activity and evaluation of Fe-Mn@Bt for ozonizing coal chemical biochemical tail water. Sep. Purif. Technol. 2020, 239, 116524. [Google Scholar] [CrossRef]
- Chen, J.; He, Z.; Ji, Y.; Li, G.; An, T.; Choi, W. OH radicals determined photocatalytic degradation mechanisms of gaseous styrene in TiO2 system under 254 nm versus 185 nm irradiation: Combined experimental and theoretical studies. Appl. Catal. B Environ. 2019, 257, 117912. [Google Scholar] [CrossRef]
- Chávez, A.M.; Rey, A.; Beltrán, F.J.; Álvarez, P.M. Solar photo-ozonation: A novel treatment method for the degradation of water pollutants. J. Hazard. Mater. 2016, 317, 36–43. [Google Scholar] [CrossRef]
- Mehrjouei, M.; Müller, S.; Möller, D. A review on photocatalytic ozonation used for the treatment of water and wastewater. Chem. Eng. J. 2015, 263, 209–219. [Google Scholar] [CrossRef]
- Azhariyah, A.S.; Pradyasti, A.; Bismo, S. Preparation and characterization of copper oxide catalyst with activated carbon support for ozone decomposition in industrial environment. IOP Conf. Series. Earth Environ. Sci. 2018, 105, 12012. [Google Scholar] [CrossRef]
- Gopi, T.; Swetha, G.; Chandra Shekar, S.; Ramakrishna, C.; Saini, B.; Krishna, R.; Rao, P.V.L. Catalytic decomposition of ozone on nanostructured potassium and proton containing δ-MnO2 catalysts. Catal. Commun. 2017, 92, 51–55. [Google Scholar] [CrossRef]
- Uribe López, M.C.; Alvarez Lemus, M.A.; Hidalgo, M.C.; López González, R.; Quintana Owen, P.; Oros-Ruiz, S.; Uribe López, S.A.; Acosta, J.; Zhen, Y.; Yu, Z. Synthesis and characterization of ZnO-ZrO2 nanocomposites for photocatalytic degradation and mineralization of phenol. J. Nanomater. 2019, 2019, 1015876. [Google Scholar] [CrossRef]
- Dai, H.; Yu, P.; Liu, H.; Xiong, S.; Xiao, X.; Deng, J.; Huang, L. Ni-Based catalysts supported on natural clay of attapulgite applied in the dry reforming of methane reaction. New J. Chem. 2020, 44, 1611–1619. [Google Scholar] [CrossRef]
- Li, B.; Li, L.; Zhang, Q.; Weng, W.; Wan, H. Attapulgite as natural catalyst for glucose isomerization to fructose in water. Catal. Commun. 2017, 99, 20–24. [Google Scholar] [CrossRef]
- Kong, L.; Tian, Y.; Li, N.; Liu, Y.; Zhang, J.; Zhang, J.; Zuo, W. Highly-effective phosphate removal from aqueous solutions by calcined nano-porous palygorskite matrix with embedded lanthanum hydroxide. Appl. Clay Sci. 2018, 162, 507–517. [Google Scholar] [CrossRef]
- Dong, Y.Z.; Piao, S.H.; Choi, H.J. Fe3O4/sepiolite magnetic composite particles and their magneto- responsive characteristics. Colloid Polym. Sci. 2017, 296, 11–19. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Xu, P.; Jiang, Z.; Sun, J. The design of a novel and resistant Zn(PZDC)(ATZ) MOF catalyst for the chemical fixation of CO2 under solvent-free conditions. Inorg. Chem Front. 2019, 6, 317–325. [Google Scholar] [CrossRef]
- Huang, H.; Huang, H.; Zhan, Y.; Liu, G.; Wang, X.; Lu, H.; Xiao, L.; Feng, Q.; Leung, D.Y.C. Efficient degradation of gaseous benzene by VUV photolysis combined with ozone-assisted catalytic oxidation: Performance and mechanism. Appl. Catal. B Environ. 2016, 186, 62–68. [Google Scholar] [CrossRef]
- Zhu, H.; Ma, W.; Han, H.; Han, Y.; Ma, W. Catalytic ozonation of quinoline using nano-MgO: Efficacy, pathways, mechanisms and its application to real biologically pretreated coal gasification wastewater. Chem. Eng. J. 2017, 327, 91–99. [Google Scholar] [CrossRef]
- Xing, S.; Lu, X.; Liu, J.; Zhu, L.; Ma, Z.; Wu, Y. Catalytic ozonation of sulfosalicylic acid over manganese oxide supported on mesoporous ceria. Chemosphere 2016, 144, 7–12. [Google Scholar] [CrossRef]
- Zhang, L.; Jia, Z.; Lyu, F.; Liang, S.; Lu, J. A review of catalytic performance of metallic glasses in wastewater treatment: Recent progress and prospects. Prog. Mater. Sci. 2019, 105, 100576. [Google Scholar] [CrossRef]
- Xia, F.; Xu, X.; Li, X.; Zhang, L.; Zhang, L.; Qiu, H.; Wang, W.; Liu, Y.; Gao, J. Preparation of bismuth nanoparticles in aqueous solution and its catalytic performance for the reduction of 4-nitrophenol. Ind. Eng. Chem. Res. 2014, 53, 10576–10582. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Q.; Yang, Z.; Liu, Y. Research on treatment of oily sludge from the tank bottom by ball milling combined with ozone-catalyzed oxidation. ACS Omega. 2020, 5, 12259–12269. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, L.; Chen, C.; Xi, J.; Cao, H.; Duan, X.; Xie, Y.; Song, W.; Wang, S. Occurrence of both hydroxyl radical and surface oxidation pathways in N-doped layered nanocarbons for aqueous catalytic ozonation. Appl. Catal. B Environ. 2019, 254, 283–291. [Google Scholar] [CrossRef]
- Dai, C.; Zhang, A.; Luo, L.; Zhang, X.; Liu, M.; Wang, J.; Guo, X.; Song, C. Hollow zeolite-encapsulated Fe-Cu bimetallic catalysts for phenol degradation. Catal. Today. 2017, 297, 335–343. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, C.; Wang, T.; Kong, Z.; Zheng, L.; Ding, H.; Liu, Y.; Zheng, H. Simultaneously promoted reactive manganese species and hydroxyl radical generation by electro-permanganate with low additive ozone. Water Res. 2021, 189, 116623. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, Y.; Cao, H.; Zhao, H.; Xie, Y. Reactive oxygen species and catalytic active sites in heterogeneous catalytic ozonation for water purification. Environ. Sci. Technol. 2020, 54, 5931–5946. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, H.; Wang, L.; Cai, B.; Chen, H. Removal of high-concentration 4-Chlorophenol (4-CP) in wastewater using carbon-based heterogeneous catalytic oxidation: Performance and mechanism. J. Clean. Prod. 2022, 346, 131176. [Google Scholar] [CrossRef]
- Zheng, D.; Cao, J.; Wang, P.; Zhao, J.; Zhao, Y.; Zhang, T.; Li, C. Catalytic ozonation of dibutyl phthalate in the presence of Ag-doped NiFe2O4 and its mechanism. Environ. Technol. 2021, 42, 4528–4538. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, C.; Dong, X. Fluorescence excitation-emission matrix spectroscopy analysis of landfill leachate DOM in coagulation-flocculation process. Environ. Technol. 2017, 38, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Huang, T.; Zhang, S.; Zheng, J.; Zheng, H. Synthesis of an amphoteric chitosan-based flocculant and its flocculation performance in the treatment of dissolved organic matter from drinking water. Desalin. Water Treat. 2020, 174, 171–177. [Google Scholar] [CrossRef]
- Khataee, A.; Rad, T.S.; Fathinia, M. The role of clinoptilolite nanosheets in catalytic ozonation process: Insights into the degradation mechanism, kinetics and the toxicity. J. Taiwan Inst. Chem. Eng. 2017, 77, 205–215. [Google Scholar] [CrossRef]


| Index | Measurement Methods | Unit | Content |
|---|---|---|---|
| COD | Dichromate method | mg/L | 8613 |
| Total phosphorus (TP) | Ammonium molybdate spectrophotometry | mg/L | 895 |
| Total nitrogen (TN) | Alkaline potassium persulfate digestion UV spectrophotometry | mg/L | 400 |
| NH3-N | Nessler’s reagent colorimetry | mg/L | 234 |
| pH | Glass electrode method | / | 9.28 |
| Conductivity | Glass electrode method | μs/cm | 1709 |
| Turbidity | Turbidimeter method | NTU | 10.4 |
| Chroma | Dilution factor method | PCU | 6425 |
| Total phenols | 4-aminoantipyrine spectrophotometry | mg/L | 790 |
| Material | SiO2 | MgO | Al2O3 | Na2O | Fe2O3 | CaO | ZnO | MnO2 |
|---|---|---|---|---|---|---|---|---|
| Palygorskite blank sample | 58.9072 | 13.5673 | 10.2627 | 6.8231 | 5.6022 | 1.8756 | 0.0149 | 0.1201 |
| Mn/Zn@PG catalyst | 51.4735 | 12.2776 | 9.0871 | 2.0537 | 5.2602 | 1.1365 | 5.4701 | 10.6499 |
| Mn/Zn@PG catalyst reused 20 times | 48.3199 | 11.7355 | 8.4605 | 7.0514 | 5.1028 | 1.2170 | 5.3694 | 10.3719 |
| Sample | Palygorskite | Mn/Zn@PG | Mn/Zn@PG after Utilization 20 Times | |||
|---|---|---|---|---|---|---|
| Element | Weight percentage | Atomic percentage | Weight percentage | Atomic percentage | Weight percentage | Atomic percentage |
| OK | 55.89 | 68.95 | 48.97 | 64.93 | 57.62 | 70.95 |
| MgK | 9.37 | 7.61 | 8.00 | 6.98 | 9.72 | 7.88 |
| AlK | 5.01 | 3.67 | 5.52 | 4.34 | 3.74 | 2.73 |
| SiK | 26.57 | 18.68 | 25.69 | 19.40 | 23.81 | 16.70 |
| FeK | 2.68 | 0.95 | 2.94 | 1.12 | 1.73 | 0.61 |
| MnK | 0.11 | 0.04 | 5.65 | 2.18 | 1.86 | 0.67 |
| ZnK | 0.35 | 0.11 | 3.24 | 1.05 | 1.51 | 0.46 |
| Elements | Mn (mg/L) | Zn (mg/L) |
|---|---|---|
| Raw water | 0 | 0 |
| Water sample after oxidation treatment | 15.52 | 14.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, W.; Xie, S.; Sun, Y.; Qiu, X.; Zhou, J. Preparation of Mn/Zn@PG Catalyst for Catalytic Oxidation Treatment of Coal Chemical Wastewater. Int. J. Environ. Res. Public Health 2022, 19, 10812. https://doi.org/10.3390/ijerph191710812
Sun W, Xie S, Sun Y, Qiu X, Zhou J. Preparation of Mn/Zn@PG Catalyst for Catalytic Oxidation Treatment of Coal Chemical Wastewater. International Journal of Environmental Research and Public Health. 2022; 19(17):10812. https://doi.org/10.3390/ijerph191710812
Chicago/Turabian StyleSun, Wenquan, Shuqian Xie, Yongjun Sun, Xiangtao Qiu, and Jun Zhou. 2022. "Preparation of Mn/Zn@PG Catalyst for Catalytic Oxidation Treatment of Coal Chemical Wastewater" International Journal of Environmental Research and Public Health 19, no. 17: 10812. https://doi.org/10.3390/ijerph191710812





