Abstract
Efficient removal of arsenic in wastewater is of fundamental importance due to the increasingly severe arsenic pollution. In this study, a new composite adsorbent (Fe3O4@ZIF-8) for As(V) removal from wastewater was synthesized by encapsulating magnetic Fe3O4 nanoparticles into metal organic frameworks. In order to evaluate the feasibility of Fe3O4@ZIF-8 as an adsorbent for As(V) removal, the adsorption properties of Fe3O4@ZIF-8 were systematically explored by studying the effects of dosage, pH, adsorption isotherm, kinetics, and thermodynamics. Additionally, the characterization of Fe3O4@ZIF-8 before and after adsorption was analyzed thoroughly using various tests including SEM-EDS, XPS, BET, XRD, TG, FTIR, and the properties and arsenic removal mechanism of the Fe3O4@ZIF-8 were further studied. The results showed that the Fe3O4@ZIF-8 has a specific surface area of 316 m2/g and has excellent adsorption performance. At 25 °C, the initial concentration of arsenic was 46.916 mg/L, and pH 3 was the optimum condition for the Fe3O4@ZIF-8 to adsorb arsenic. When the dosage of the Fe3O4@ZIF-8 was 0.60 g/L, the adsorption of arsenic by the Fe3O4@ZIF-8 can reach 76 mg/g, and the removal rate can reach 97.20%. The adsorption process of arsenic to the Fe3O4@ZIF-8 can be well described by the Langmuir isotherm model and the second-order kinetic equation. At pH 3 and temperature 298 K, the maximum adsorption capacity of arsenic by the Fe3O4@ZIF-8 was 116.114 mg/g. Through the analysis of thermodynamic parameters, it is proved that the adsorption process of arsenic by the Fe3O4@ZIF-8 is a spontaneous endothermic reaction. The Fe3O4@ZIF-8 has broad prospects for removing As(V) pollution in wastewater, because of its strong adsorption capacity, good water stability, and easy preparation.
1. Introduction
Human survival and development are inseparable from water resources. With the continuous expansion of human productivity and the continuous development of the manufacturing industry, the demand for mineral resources continues to increase, and the generated pollutants enter the water body, resulting in more and more serious water pollution [1]. Arsenic is a non-metallic element, which widely exists in nature [2]. At present, hundreds of arsenic minerals have been found. Arsenic and its compounds are used in pesticides, herbicides, insecticides, and various alloys [3,4]. In 2017, the World Health Organization’s International Agency for Research on Cancer placed arsenic and inorganic arsenic compounds on the list of Class I carcinogens [5]. In 2019, arsenic and arsenic compounds were included in the “List of Toxic and Harmful Water Pollutants (First Batch)” by the Ministry of Ecology and Environment. The sources of arsenic pollution in water bodies are wastewater, waste gas and industrial waste discharged from industrial production, various organic arsenic pesticides used in agricultural production to avoid the impact of insect pests on crops, and the natural environment accompanied by volcanic eruptions and rock weathering and diffusion [6]. Arsenic-containing substances and arsenic can denature proteins and enzymes in cells, and cause cell damage by increasing reactive oxygen species in cells [7]. Studies have shown that the prevalence of arsenic poisoning is as high as 133‰ in groups who drink water with an arsenic content of 0.3 mg/L for a long time, and among patients with chronic arsenic poisoning, the cancer rate is as high as 15% [8]. In the World Health Organization’s “Water Quality Standards for Drinking Water”, the index value of arsenic is 0.01 mg/L. Arsenic pollution in water will not only cause serious harm to the human body, but also harm the normal growth of plants, affecting the operation of internal water and chlorophyll [9]. Therefore, arsenic pollution in water has become an urgent problem to be solved. The arsenic removal technologies reported in the literature include oxidation, coagulation/filtration, ion exchange, membrane method, biological method, electro-flocculation, and adsorption method [10,11]. The adsorption method has simple steps and is more feasible for practical application [12,13]. MOF (Metal-organic framework) materials can effectively provide tunable porosity [14], stable pore structure [15], geometry, chemical function [16], structural uniformity, and ultra–high surface area [17].
MOFs are adsorbents composed of porous crystalline materials with ultra−high surface area and highly ordered structures composed of organic ligands and metal ions linked in a three-dimensional lattice, which are promising in the fabrication of various attractive new compounds [18]. It was reported that the first MOF material for the removal of arsenic contamination from water was Fe-BTC. Fe-BTC, consisting of iron nodes and 1,3,5 trimesic acid groups, was synthesized by a facile solvothermal method and used as an adsorbent to adsorb As(V) in the pH range of 2–10. At pH 4; the adsorption capacity of Fe-BTC was 12.3 mg/g, which was more than six times that of Fe2O3 nanoparticles [19]. By infrared measurement analysis, a new Fe-OAs group in the infrared band appeared at 824 cm−1, which confirmed the adsorption of As(V). In addition, by transmission electron microscopy, it was confirmed that the adsorption of As(V) occurred at the interior of the MOFs and interacts with the Fe nodes of the framework. Although the adsorption capacity is not strong, this is a landmark discovery in the research on arsenic removal by MOFs, which indicates that MOFs materials have great potential for arsenic capture. Moreover, the researchers also conducted studies on the removal of arsenic by classical MOFs. The reported adsorption capacity of ZIF-8 for arsenic at low equilibrium concentration (9.8 µg/L) was 76.5 mg/g. The study of the adsorption mechanism showed that the adsorption was due to the dissociative adsorption of water, which produced a large number of external active sites (Zn-OH) and formed an inner spherical complex with arsenate [20]. Due to its potential for arsenic adsorption, the research on the adsorption capacity of arsenic in wastewater provides a data basis for the treatment of arsenic pollution in wastewater.
The main objectives of this study were to: (1) synthesize a new composite adsorbent (Fe3O4@ZIF-8) by encapsulating magnetic Fe3O4 nanoparticles into metal organic frameworks for As(V) removal from wastewater, (2) evaluate the feasibility of Fe3O4@ZIF-8 as an adsorbent for As(V) removal by studying the effects of dos-age, pH, adsorption isotherm, kinetics, and thermodynamics, (3) analyze the characterization of Fe3O4@ZIF-8 before and after adsorption thoroughly using various tests including SEM-EDS, XPS, BET, XRD, TG, FTIR, and (4) study the properties and arsenic removal mechanism of the Fe3O4@ZIF-8.
2. Experimental Section
2.1. Chemicals
Arsenic standard solution (Beijing, China) was purchased from Beijing Northern Weiye Metrology Technology Research Institute. NH4OH (Tianjin, China) was purchased from Tianjin Damao Chemical Reagent Factory. FeCl3·6H2O and CH3OH (Tianjin, China) were purchased from Tianjin Zhiyuan Chemical Reagent Co., Ltd. In addition, FeCl2·4H2O (Shanghai, China), 2-methy imidazole (C4H6N2,Shanghai, China) were purchased from Shanghai McLean Biochemical Technology Co., Ltd. Zn(NO3)2·6H2O, and HNO3 (Guangzhou, China) were purchased from Guangzhou Chemical Reagent Factory. Deionized water is used throughout this study. The conductivity of H2O was less than 2 μs/cm. As(V) stock solution was prepared with 1001 μg/mL As(V) standard solution (H3AsO4, Beijing). The inductively coupled plasma atomic emission spectroscope (ICP-AES) was used to measure the As(V) concentration in the aqueous solutions.
2.2. Synthesis of Adsorbents
2.2.1. Synthesis of Magnetic Fe3O4 Nanoparticles
Magnetic Fe3O4 nanoparticles were synthesized using a modified iron (II) and iron (III) co-precipitation method as described in the literature [21]. FeCl2·4H2O (10 mmol) and FeCl3·6H2O (10 mmol) were gradually poured into 30 mL deionized water and mixed by mechanical stirring, the solution was heated to 60 °C for 30 min in the ultrasonic cleaner. Then 40.00 mL of 3.50 mol/L ammonia solution was introduced into the mixed iron(II)/iron(III) solution with continuous stirring at 60 °C for 30 min. After the reaction was completed, the produced mixture was filtered and washed with deionized water until neutral and finally dried at 60 °C under the vacuum for 12 h to obtain Fe3O4 nanoparticles.
2.2.2. Synthesis of Fe3O4@ZIF-8
The preparation of Fe3O4@ZIF-8 was improved according to reference [22]. Weighed 3.30 g of 2-methy imidazole and 0.10 g of magnetic nanometer Fe3O4, dissolved in 70.00 mL of methanol. The mixture was ultrasonicated for 15 min, 1.50 g of Zn(NO3)2·6H2O was weighed and dissolved in 70.00 mL of methanol, which was slowly added dropwise to the above-mentioned sonicated solution, mechanically stirred for 24 h, and then centrifuged. The obtained solid was washed with methanol at least three times, and then vacuum-dried at 80 °C for 12 h.
3. Results and Discussion
3.1. Adsorption Experiments
3.1.1. Effect of Adsorbent Dosage
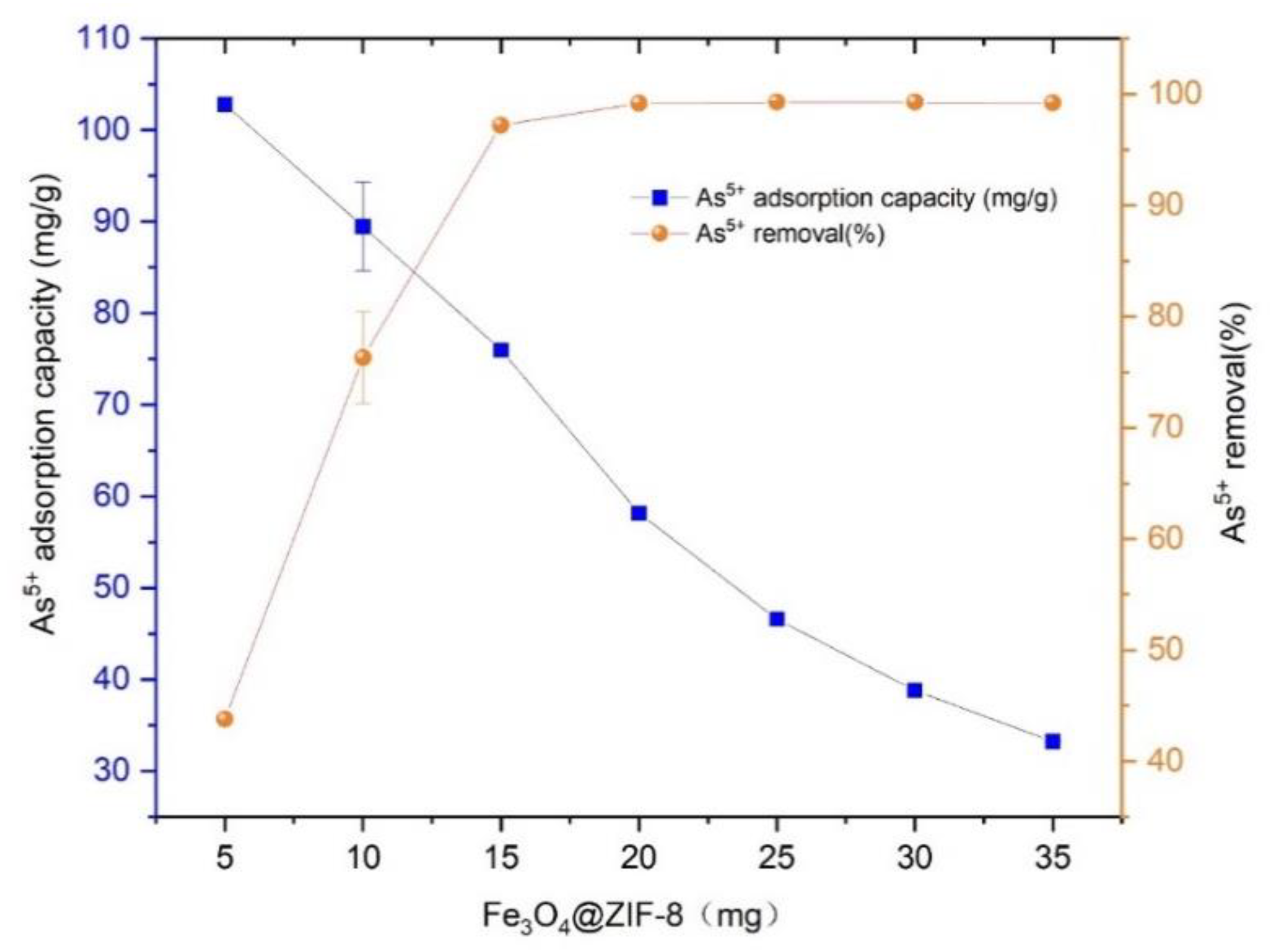
Prepared 46.916 mg/L As(V) solution, added 25 mL to several centrifuge tubes, and added Fe3O4@ZIF-8 adsorbent 5, 10, 15, 20, 25, 30, 35 mg respectively, at pH 3, temperature was 25 °C shook in a shaker for 24 h. The adsorption capacity and removal rate of As(V) by Fe3O4@ZIF-8 with the dosage of adsorbent are shown in Figure 1.
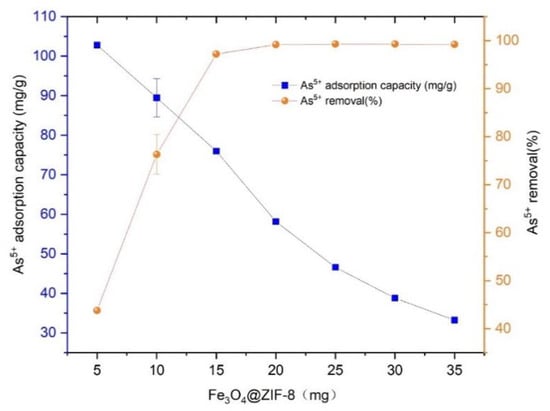
Figure 1.
The adsorption effect of adsorbent dose.
As illustrated in Figure 1, the equilibrium adsorption capacity of Fe3O4@ZIF-8 for As(V) shows decreasing trend with the dosage. It was because that the increase in the dosage of Fe3O4@ZIF-8, that the number of adsorption sites in water also increased, but the adsorption sites on the surface of Fe3O4@ZIF-8 had not reached saturation. Therefore, although the amount of adsorbed As(V) increased, the degree of saturation decreased, and Fe3O4@ZIF-8 the equilibrium adsorption capacity of As(V) decreased.
It can be seen in Figure 1 that when the dosage of Fe3O4@ZIF-8 was 10 mg, the removal rate of As(V) reached 76.29%. When the dosage of Fe3O4@ZIF-8 was increased to 15 mg, the removal rate of As(V) reached 97.20%. When the dosage of Fe3O4@ZIF-8 was 20–35 mg, the removal rate of As(V) reached more than 99%, and the removal was basically complete. Continue to increase the dosage of Fe3O4@ZIF-8 to As(V) the removal rate did not improve much. Therefore, from the perspective of economy and resource saving, the optimal dosage of Fe3O4@ZIF-8 in this experiment is 15 mg/25 mL, which is 0.6 g/L.
3.1.2. Effect of pH
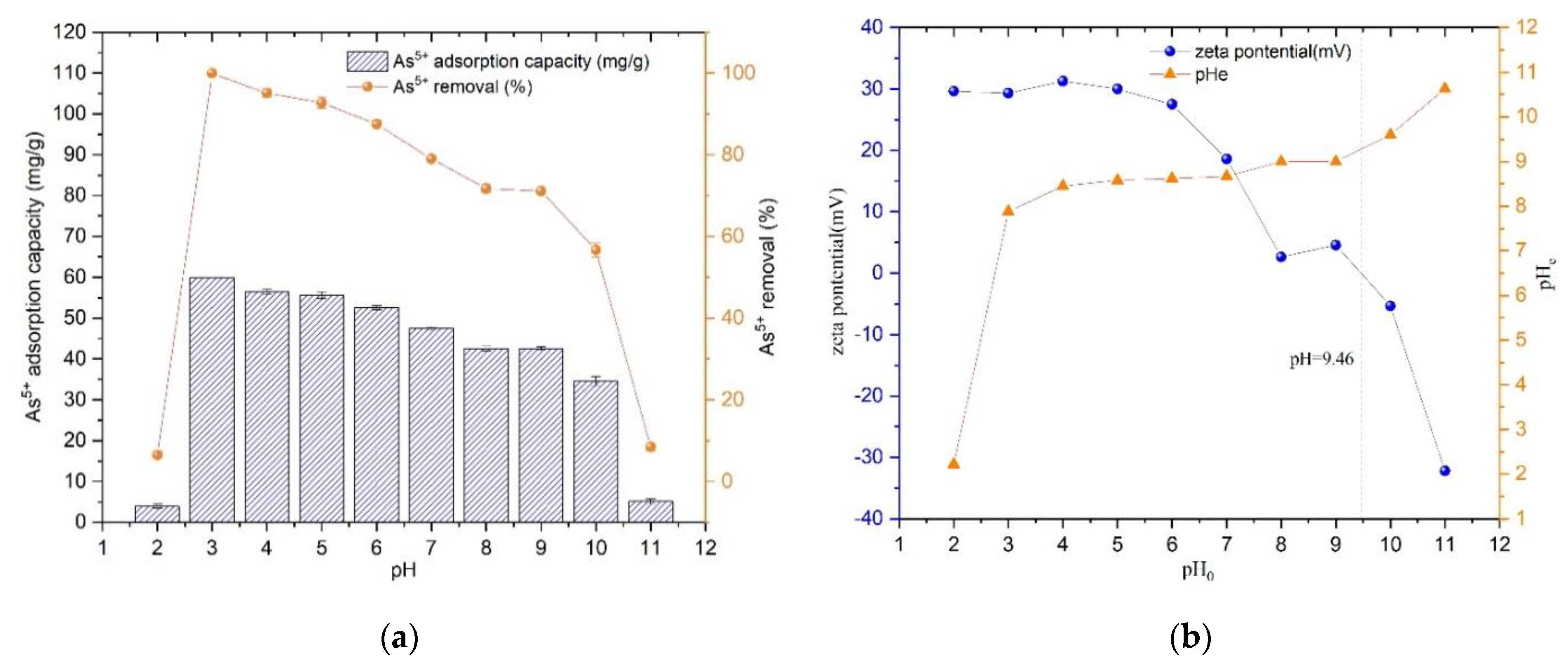
Prepared 25 mg/L As(V) solution, adjusted the pH from 2 to 11 by NaOH solution and HNO3 solution, pipetted 25 mL into several centrifuge tubes, and dosage of Fe3O4@ZIF-8 adsorbent was 10 mg. Shook on a shaker for 24 h at 25 °C. The adsorption capacity and removal rate of As(V) by Fe3O4@ZIF-8 are shown in Figure 2.
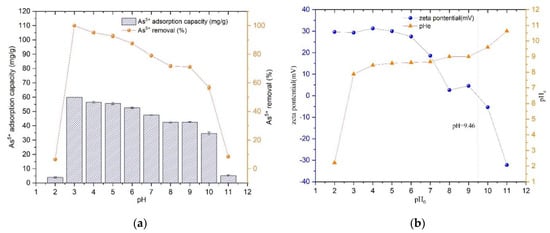
Figure 2.
(a) The adsorption effect of pH; (b) Isoelectric point of Fe3O4@ZIF-8.
From Figure 2a, it can be found that the adsorption amount of Fe3O4@ZIF-8 reached the maximum when pH = 3.0, and the removal rate of As(V) was the largest; when pH < 3.0, the adsorption of Fe3O4@ZIF-8 decreased rapidly. The amount of As(V) adsorption by Fe3O4@ZIF-8 decreased gradually with the increase of pH when pH > 3.0. When pH > 9.0, the adsorption capacity and removal rate of As(V) decreased rapidly. This phenomenon can be explained by Figure 2b. The experimentally measured isoelectric point of Fe3O4@ZIF-8 was 9.46. Therefore, when pH < 9.46, the surface of Fe3O4@ZIF-8 was positively charged. According to the literature, when pH < 2, As(V) mainly exists in the form of H3AsO4 in water. When pH is 2–7, As(V) mainly exists in the form of H2AsO4−. When pH > 7, As(V) It mainly exists in the form of H2AsO4− [23,24]. Combined with the experimental data, it can be seen that when pH = 2, the content of H3AsO4 was relatively high, which was not conducive to the adsorption of Fe3O4@ZIF-8. When pH = 3–9, the positive charge of Fe3O4@ZIF-8 decreased with the increase of pH and adsorption capacity for H2AsO4− and HAsO42− decreased. When pH > 9.46, the positive charge on the surface of Fe3O4@ZIF-8 changed to negative charge, which repelled each other with HAsO4, so the adsorption amount and removal rate of As(V) reduced rapidly.
3.1.3. Adsorption Isotherm
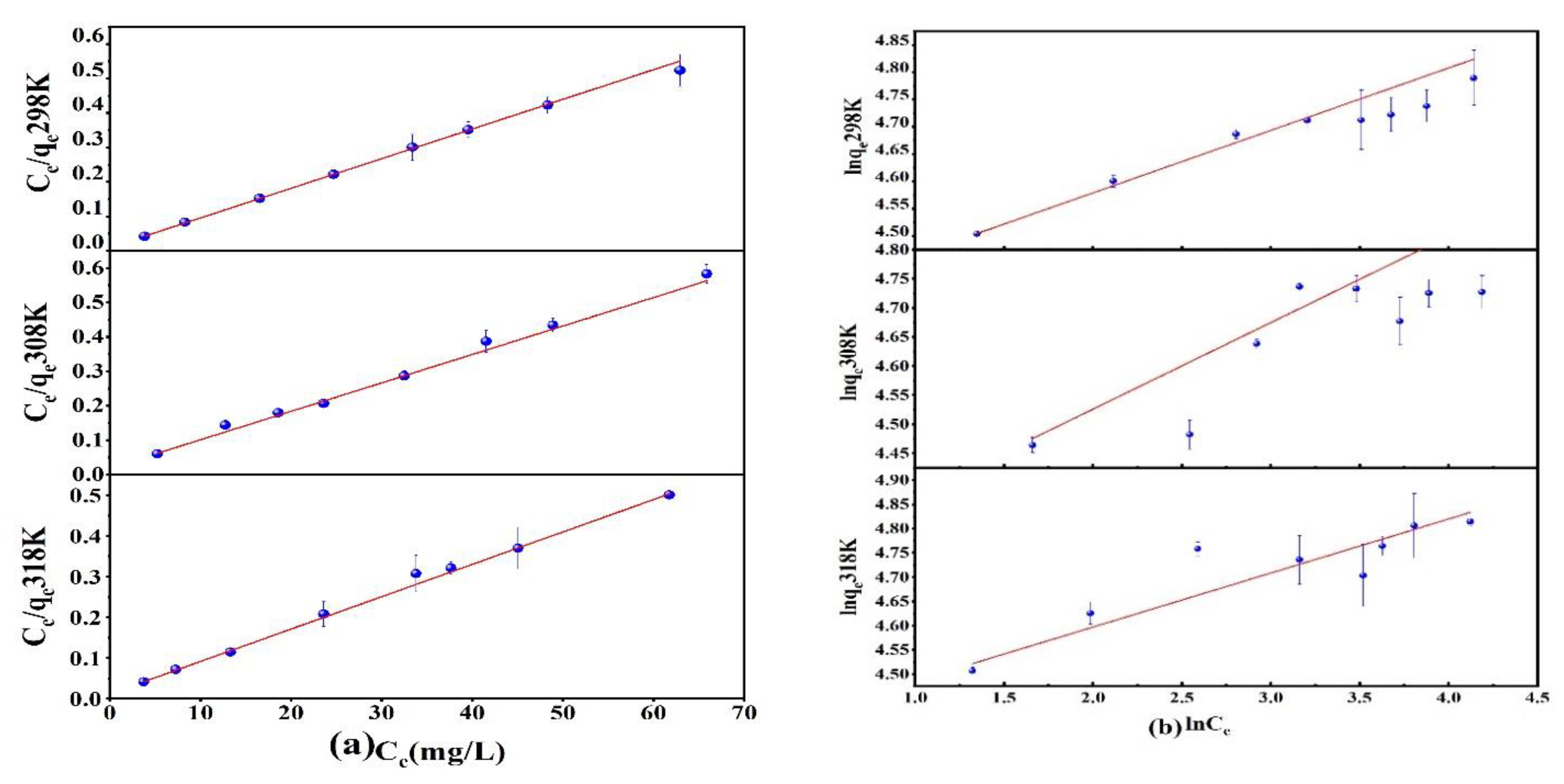
Prepared 25 mL of As(V) solution with an initial concentration of 40–120 mg/L and pH = 3 and added 10 mg of Fe3O4@ZIF-8 adsorbent. Shook for 24 h on a shaker at 25 °C, 35 °C and 45 °C. The obtained adsorption isotherms are shown in Figure 3. At different temperatures, the adsorption isotherms of Fe3O4@ZIF-8 for As(V) were fitted by Langmuir and Freundlich models as shown in Figure 3. From the data in Table 1, it can be seen that the Langmuir adsorption isotherm equation was more suitable for describing the adsorption process of As(V) by Fe3O4@ZIF-8, and the calculated correlation coefficient was higher than that of the Freundlich adsorption isotherm equation.
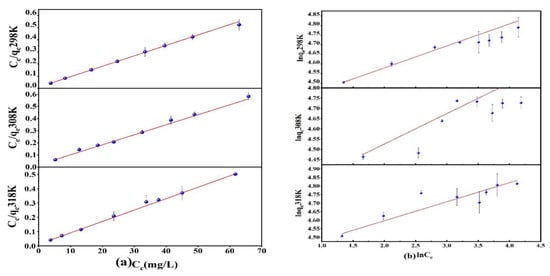
Figure 3.
(a) Langmuir adsorption isotherm model; (b) Freundlich adsorption isotherm model.

Table 1.
The kinetic parameters for adsorbing As(V) by Fe3O4@ZIF-8.
This indicated that the adsorption of As(V) by Fe3O4@ZIF-8 was monolayer adsorption. Moreover, the RL value was between 0 and 1, which meant that the adsorption was easy to proceed. When the reaction temperature was 318 K, the maximum adsorption capacity of Fe3O4@ZIF-8 simulated by the Langmuir adsorption isotherm model was 125.628 mg/g. It can be found that Fe3O4@ZIF-8 has a good adsorption effect on As(V).
3.1.4. Kinetic Study
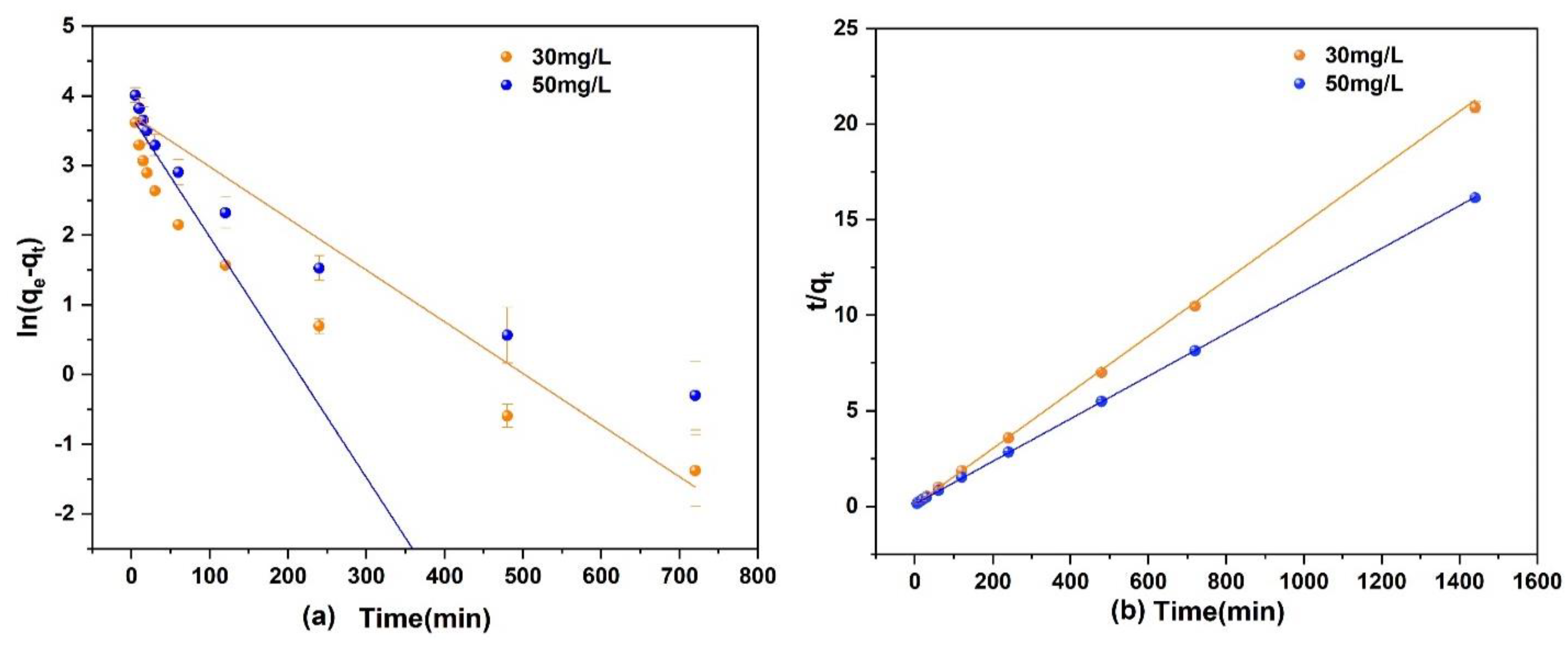
A 250 mL of As(V) solution with a concentration of 30 and 50 mg/L and a pH of 3 was prepared. Then, 0.1 g of Fe3O4@ZIF-8 adsorbent was added and shaken at 25 °C in a constant temperature shaker. The fitted kinetic equation of As(V) adsorption by Fe3O4@ZIF-8 is shown in Figure 4.
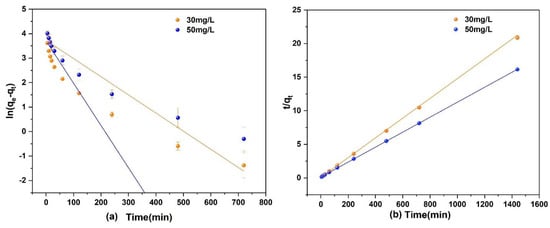
Figure 4.
(a) Pseudo-first order model; (b) Pseudo-second order model.
It can be seen in Figure 4 that the fitting effect of the second-order kinetic equation for the adsorption of As(V) to Fe3O4@ZIF-8 was obviously better than that of the first-order kinetic model. From the data in Table 2, the correlation coefficient R2 of the second-order kinetic model was much larger than that of the first-order kinetic model, and both were higher than 0.999. Under different concentrations of As(V) solution, the equilibrium adsorption capacity of Fe3O4@ZIF-8 calculated by the second-order kinetic model was basically consistent with actual adsorption capacity. Therefore, the adsorption process of As(V) by Fe3O4@ZIF-8 satisfies the second-order kinetic model. This means that the adsorption of As(V) by Fe3O4@ZIF-8 is mainly a chemical adsorption process.

Table 2.
Parameters of kinetic models for As(V) onto Fe3O4@ZIF-8.
3.1.5. Thermodynamic Studies
The thermodynamic parameters of Fe3O4@ZIF-8 adsorption of As(V) at temperatures of 298 K, 308 K, and 318 K were calculated by formula, as shown in Table 3.

Table 3.
Parameters of thermodynamic for the adsorption of As(V) by Fe3O4@ZIF-8.
It can be seen in Table 3 that the standard Gibbs free energy ΔG0 of Fe3O4@ZIF-8 adsorbing As(V) is less than 0 at different temperatures, which indicates that Fe3O4@ZIF-8 adsorbing As(V) is a spontaneous reaction, and with the increase of temperature, the absolute value of ΔG0 increases, which indicates that the increase of temperature plays a role in promoting the adsorption reaction. In addition, the standard enthalpy changes ΔH0 during the adsorption of As(V) by Fe3O4@ZIF-8 is greater than 0, indicating that the reaction is an endothermic reaction. The standard entropy change of Fe3O4@ZIF-8 adsorption of As(V) at different temperatures is ΔS0 > 0, which indicates that the system disorder becomes larger after the adsorption reaction, which is an entropy-driven process. The above data can prove that the adsorption process of As(V) by Fe3O4@ZIF-8 is a spontaneous endothermic reaction.
3.2. Characterization
3.2.1. Scanning Electron Microscopy (SEM)
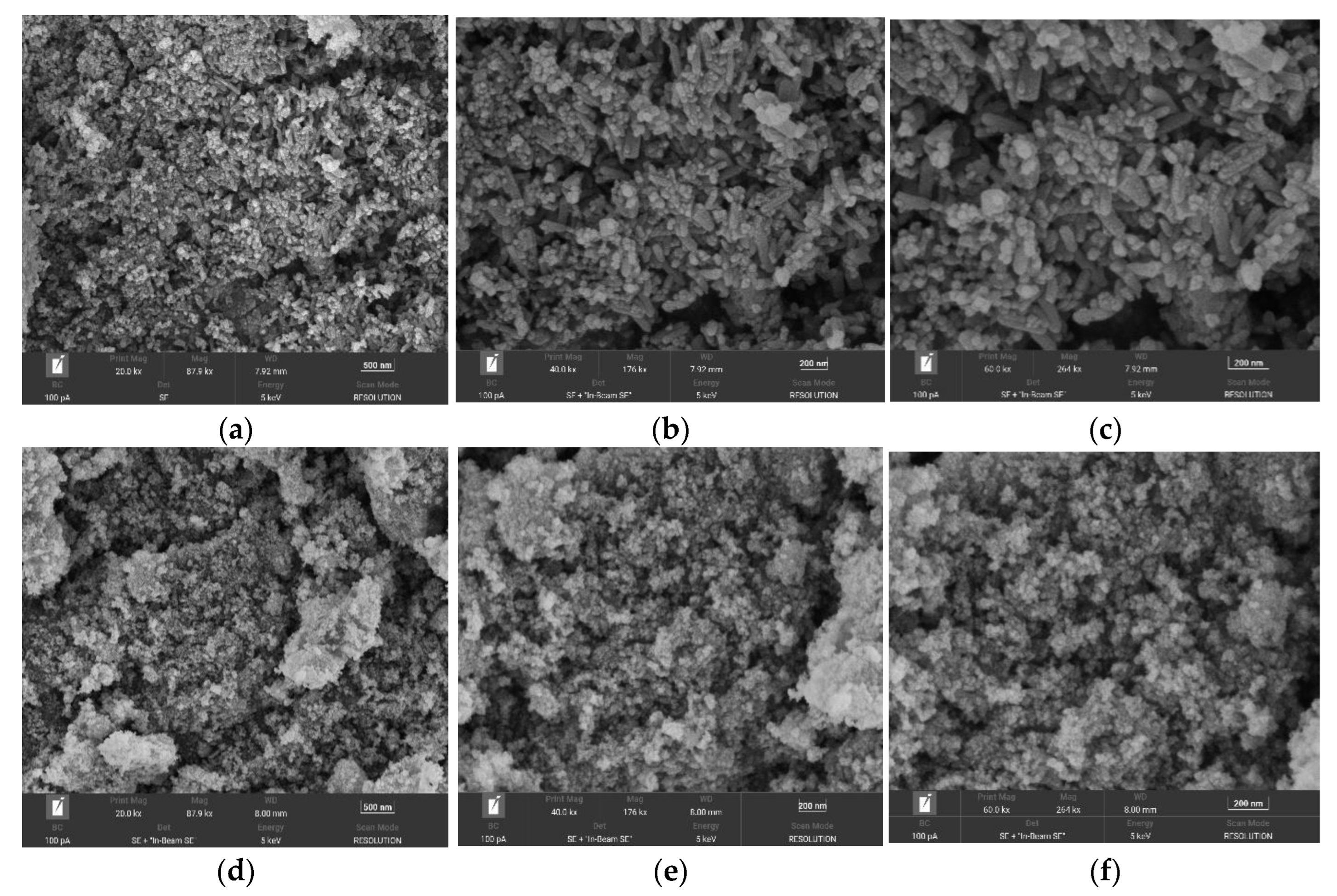
From a–c in Figure 5, it can be seen that the prepared Fe3O4@ZIF-8 has irregular columnar, spherical and cubic structure particles with rough surfaces. It can be seen from the figure that the particles were adhered to each other and stacked to form many channels, and this agglomeration phenomenon provided more adsorption sites for Fe3O4@ZIF-8 to adsorb arsenic. From the SEM image, it can be known that the particle size of the prepared Fe3O4@ZIF-8 was about 100 nm. It can be seen from pictures d–f in Figure 5 that the prepared Fe3O4 was a nanomaterial, which was approximately spherical particles, and the particles adhere to each other and agglomerate together. The particle size of the prepared magnetic nano Fe3O4 was much smaller than that of the Fe3O4@ZIF-8 material.

Figure 5.
(a–c) SEM of Fe3O4@ZIF-8; (d–f) SEM of Fe3O4.
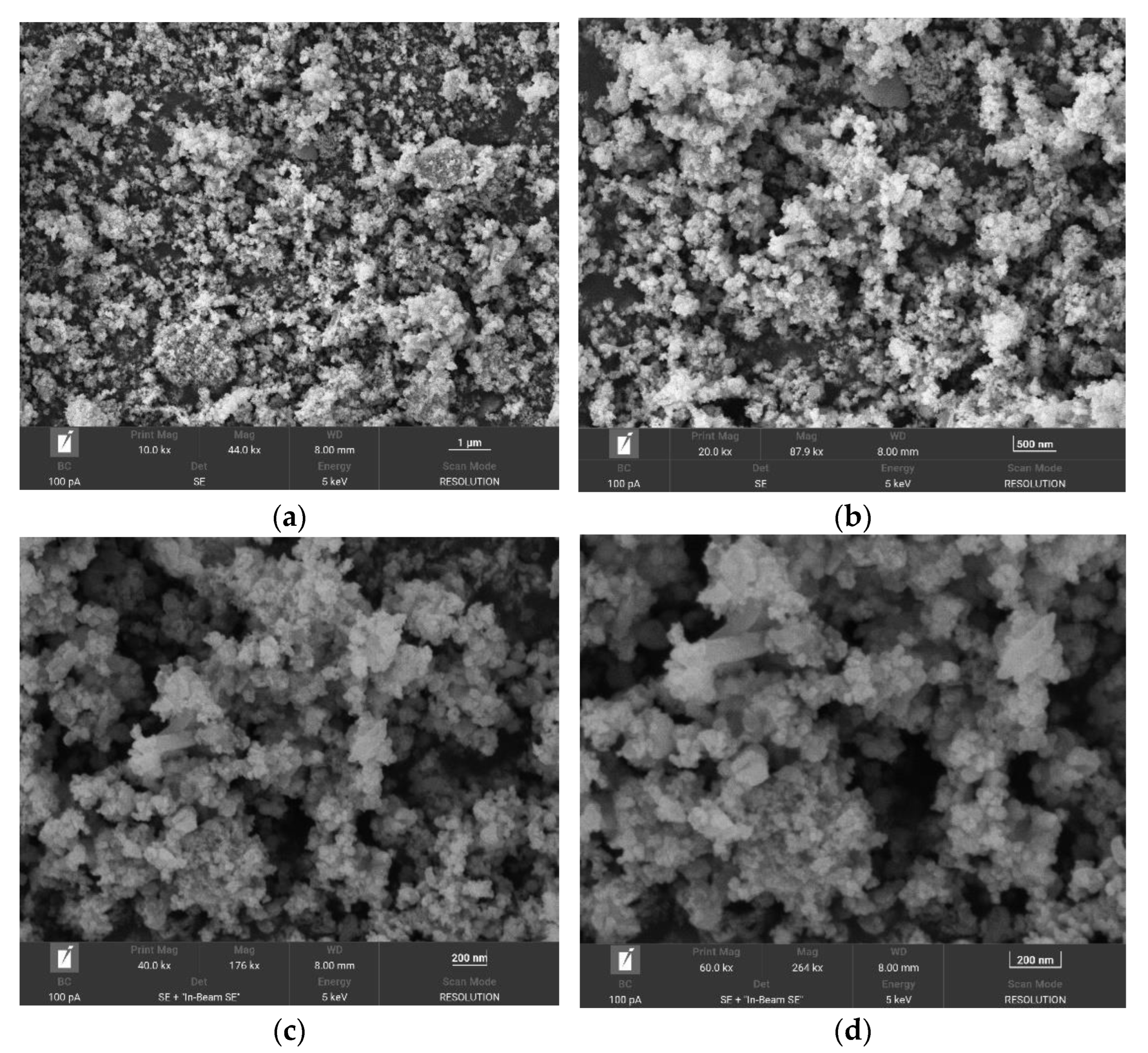
From Figure 6, it can be seen that the Fe3O4@ZIF-8 particle gap after adsorption of arsenic is reduced, which indicates that the arsenic in the water is adsorbed to the surface of Fe3O4@ZIF-8, thus filling the pores.
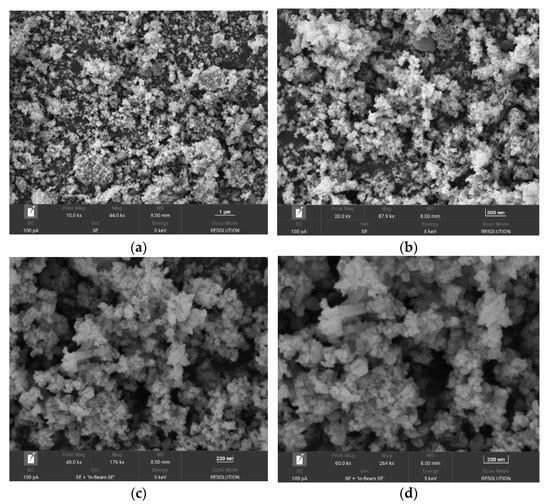
Figure 6.
SEM of Fe3O4@ZIF-8 after adsorption. (a–d) show the effects of 10,000 times, 20,000 times, 40,000 times and 60,000 times magnification respectively.
It can be seen from Figure 6 that the Fe3O4@ZIF-8 particle agglomeration phenomenon after adsorption is more obvious, which reflects the strong adsorption capacity of the material. The particle shape of Fe3O4@ZIF-8 after adsorption was changed and the particle size became smaller than that before adsorption.
3.2.2. EDS Analysis
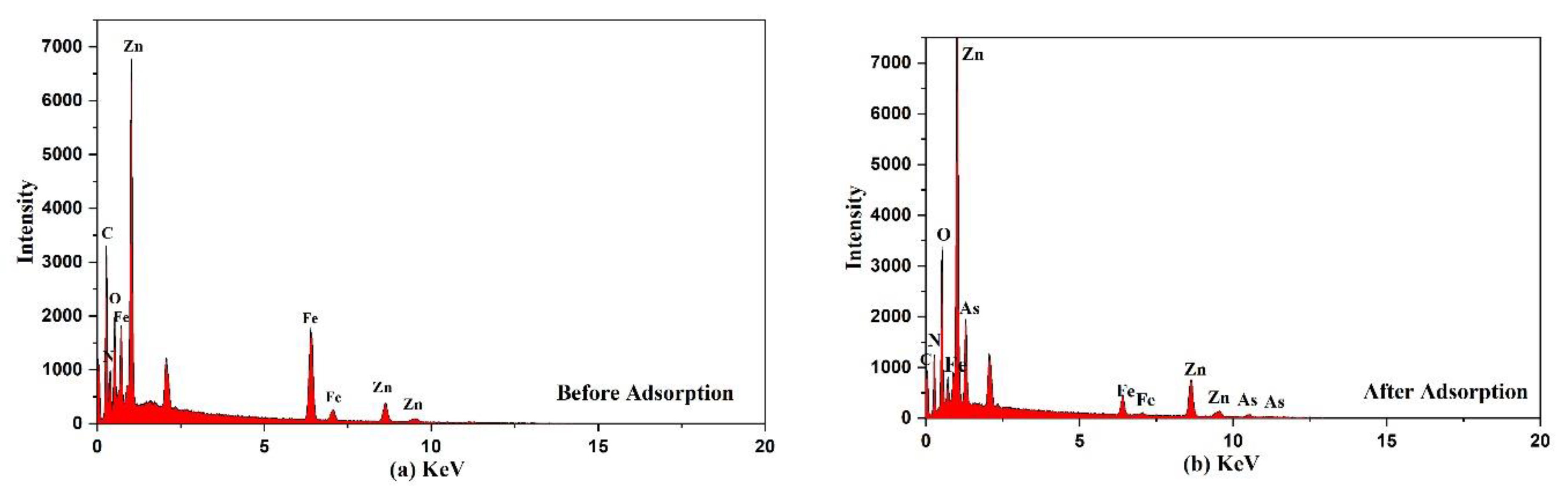
The EDS analysis of Fe3O4@ZIF-8 before and after arsenic adsorption are shown in Figure 7 and Table 4. Through EDS analysis of Fe3O4@ZIF-8, we can find that As element was not detected in Fe3O4@ZIF-8 before adsorption, while As element was detected in Fe3O4@ZIF-8 after adsorption. This proved that Fe3O4@ZIF-8 has adsorption capacity for arsenic. The Fe3O4@ZIF-8 before adsorption detected C, N, O, Fe and Zn elements, which were consistent with the elemental composition of the Fe3O4@ZIF-8 material, which proved that the metal-organic framework composite was successfully synthesized [25].
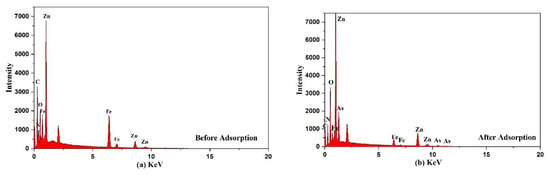
Figure 7.
EDS spectra of the adsorbent (a) before (b) after adsorption.

Table 4.
EDS analysis of Fe3O4@ZIF-8 before and after adsorption.
3.2.3. XRD Analysis
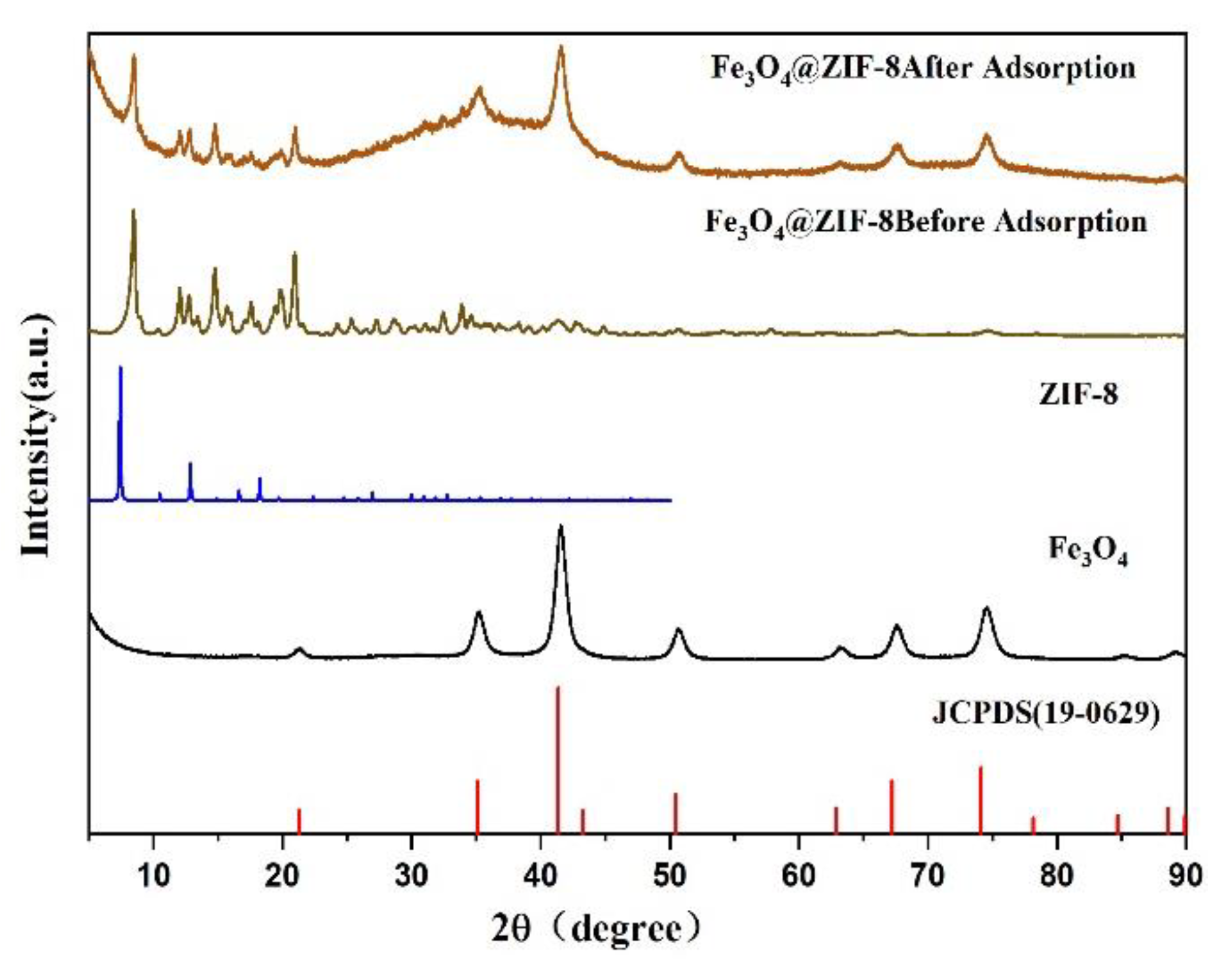
Figure 8 was the XRD pattern of Fe3O4 and Fe3O4@ZIF-8. These include the XRD patterns of Fe3O4@ZIF8 before and after adsorption, the XRD patterns of the prepared magnetic nano Fe3O4, the corresponding ZIF-8 standard pattern [26], and the Fe3O4 standard card (JCPDS 19-0629).
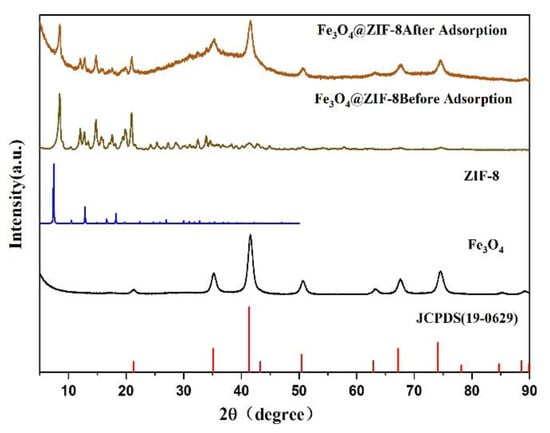
Figure 8.
XRD pattern of synthesized Fe3O4@ZIF-8, Fe3O4.
Comparing the prepared magnetic nano Fe3O4 and Fe3O4 standard cards, it is found that the characteristic peak positions of the standard cards were the same, which can prove the successful preparation of magnetic nano Fe3O4. By comparing the standard cards of Fe3O4@ZIF-8, ZIF-8, and Fe3O4, it can be found that the characteristic peaks are basically the same as those of Fe3O4 and standard cards, and there were diffraction peaks consistent with the standard pattern of ZIF-8, which can prove that Fe3O4@ZIF-8 synthesis was relatively successful. Comparing the XRD patterns of Fe3O4@ZIF-8 before and after adsorption, it can be found that the position of the diffraction peak of Fe3O4@ZIF-8 does not change after adsorption of arsenic, and the relative intensity of the peak increases, which indicates that Fe3O4@ZIF-8 is adsorbing arsenic. The arsenic process did not destroy the structure and properties. Therefore, the material can exist stably in an aqueous solution.
3.2.4. Thermogravimetric Analysis (TG)
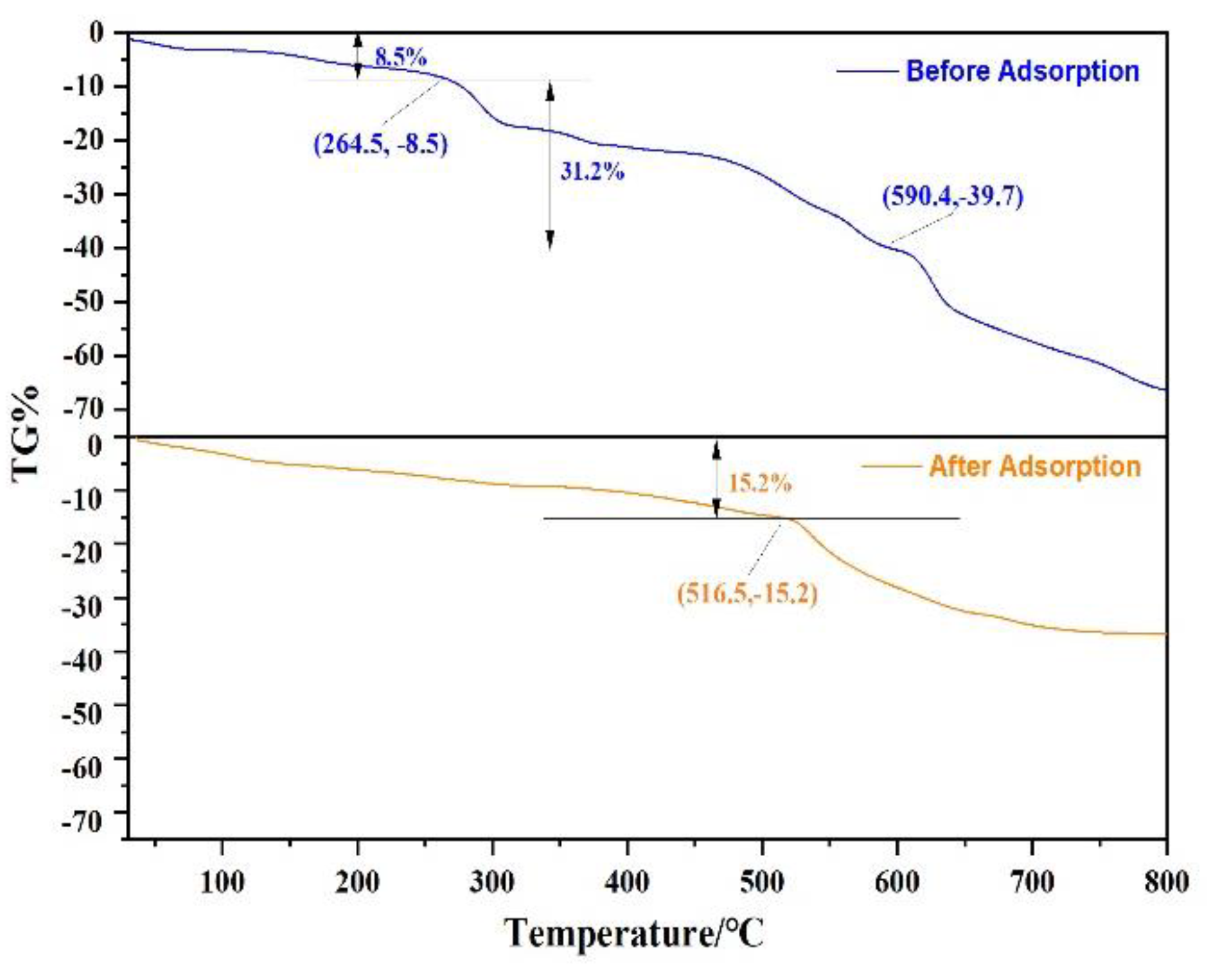
In order to evaluate the stability of Fe3O4@ZIF-8 material, Fe3O4@ZIF-8 was heated from 30 °C to 800 °C under N2, and the stage of material weight loss was analyzed. As shown in Figure 9, it can be observed that Fe3O4@ZIF-8 was in a state of gradual weight loss at 30–264.5 °C, with a weight loss of 8.5%. This can be attributed to desorption of adsorbed water or solvent. At 264.5–800 °C, the weight loss rate of Fe3O4@ZIF-8 increased and remained in a weightless state, which may be due to the decomposition of the ligand ZIF-8. Comparing the weight loss changes of Fe3O4@ZIF-8 before and after adsorption, Fe3O4@ZIF-8 after adsorption of arsenic was heated from 30 °C to 516.5 °C, and the weight loss was 15.2%. It was obvious from Figure 9 that Fe3O4 after the adsorption of arsenic, the weight loss rate of @ZIF-8 was significantly slowed down. At 800 °C, the weight loss of Fe3O4@ZIF-8 before adsorption reached 66.4%, and the weight loss of Fe3O4@ZIF-8 after adsorption was 36.8%. This shows that Fe3O4@ZIF-8 has good thermal stability after adsorbing arsenic, and arsenic is adsorbed to the surface of Fe3O4@ZIF-8, and it is not easy to desorb and decompose.
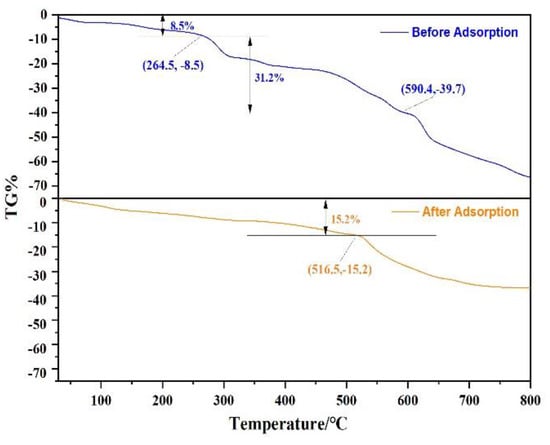
Figure 9.
The TGA curve of Fe3O4@ZIF-8.
3.2.5. FTIR Analysis
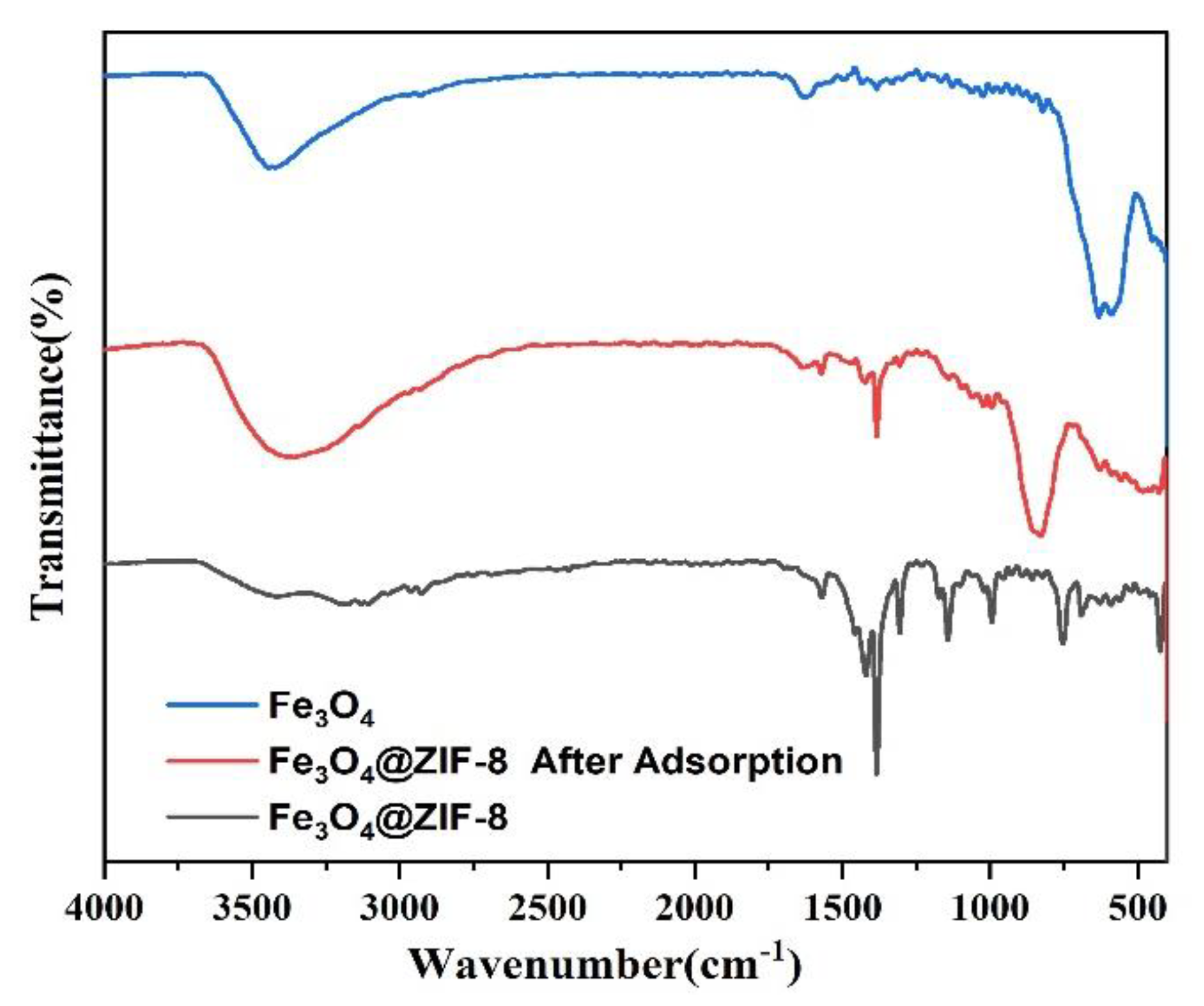
From the infrared spectrum of magnetic nano Fe3O4 in Figure 10, it can be seen that absorption bands corresponding to the carboxylate groups on the surface of Fe3O4 NPs appeared at 1384.25 cm−1 and 1628.70 cm−1 [27]. At 590.88 cm−1 and the peak at 631.52 cm−1 can be attributed to the Fe-O bond of Fe3O4 [28,29]. Analysis of the infrared spectrum of Fe3O4@ZIF-8 shows that the peaks at 3179.34 cm−1 and 2926.14 cm−1 can correspond to the characteristic peaks of =C−O bond and C−H bond with ZIF-8 structure, respectively [30,31]. The peak of C=N bond in the imidazole ring is 1569.76 cm−1. The absorption peak of CN bond appeared at 1143.10 cm−1 and 994.82 cm−1, and the vibration peak of functional group in Zn-N appeared at 423.37 cm−1. At 591.03 cm−1 and 628.44 cm−1 the peaks appearing at −1 can be assigned to Fe-O peaks [32,33,34]. The analysis of Fe3O4@ZIF-8 by FTIR can prove that the experiment successfully complexes Fe3O4 with ZIF-8. Comparing the infrared spectrum of Fe3O4@ZIF-8 after arsenic adsorption, 3359.15 cm−1 corresponds to the stretching vibration region of O−H and N−H. A new strong band is observed near 427.90 cm−1, which can be attributed to the Zn-O-As vibration, which means the formation of a new inner spherical complex [35,36,37]. A new peak appears at 828.21 cm−1, which may be due to the formation of As-O groups [38] showing that arsenic was bound to Fe3O4@ZIF-8.
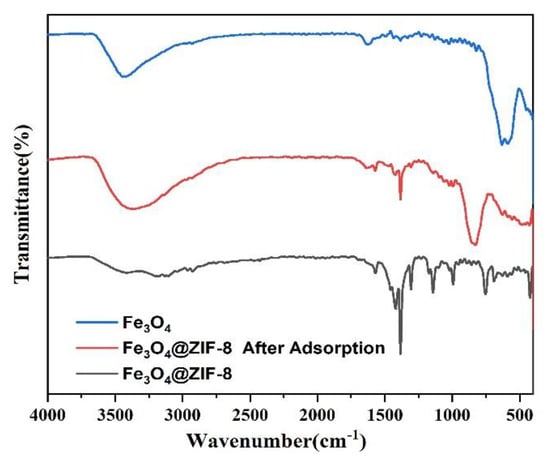
Figure 10.
FTIR pattern of synthesized Fe3O4, Fe3O4@ZIF-8.
3.2.6. XPS Analysis
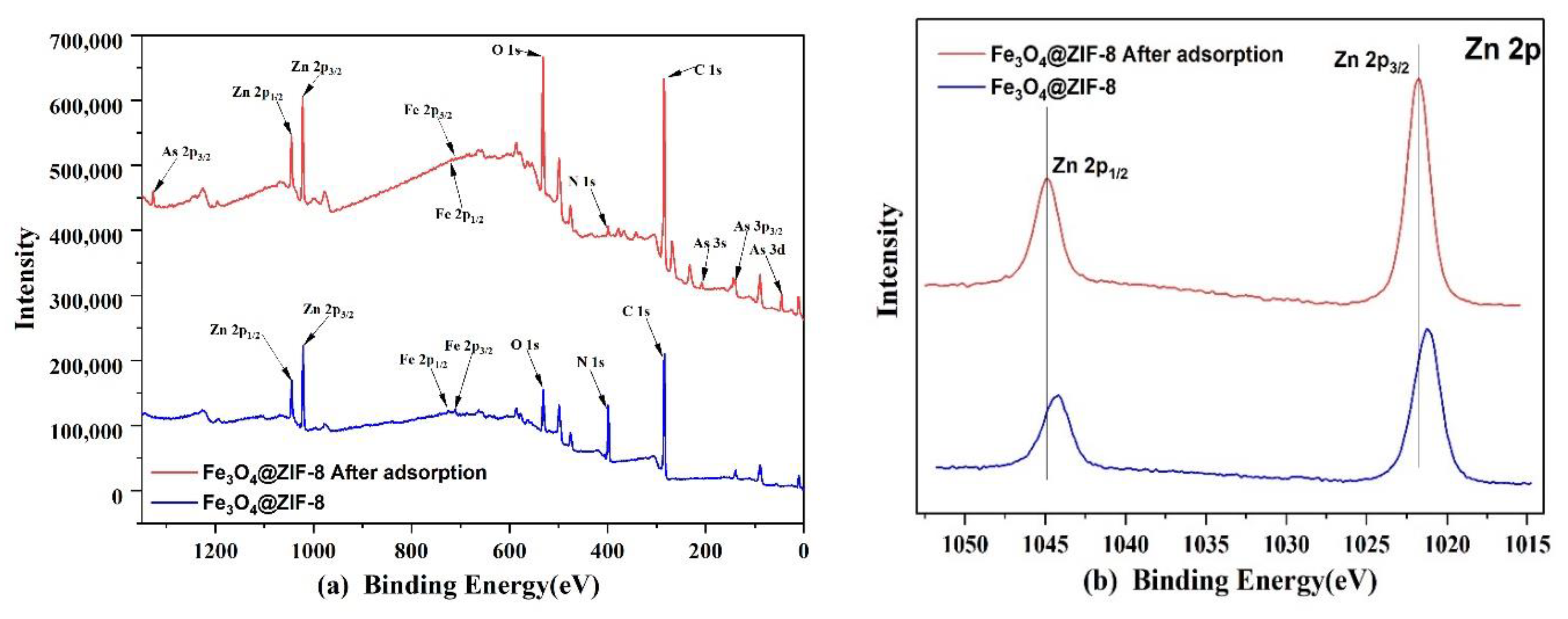
Through XPS analysis of Fe3O4@ZIF-8 before and after arsenic adsorption in Figure 11, it can be found that the full spectrum in Figure 11 of Fe3O4@ZIF-8 before adsorption can detect the peaks of Zn, Fe, O, N, C, which proves to be in line with the preset Chemical composition of Fe3O4@ZIF-8 material. Fe3O4@ZIF-8 after adsorption detected the characteristic peak of As, which proved that the material successfully combined with arsenic and had an adsorption effect.
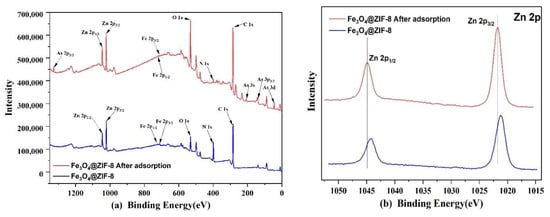
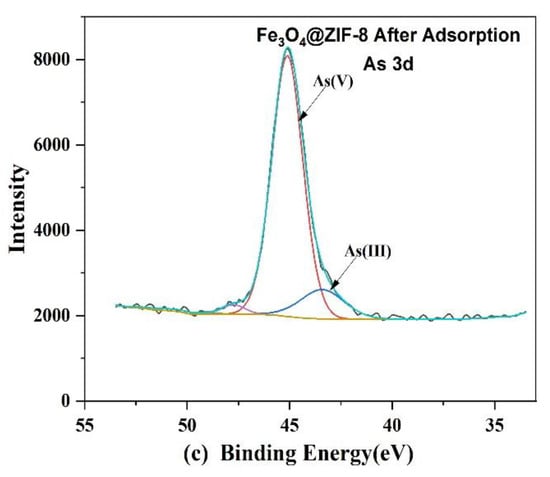
Figure 11.
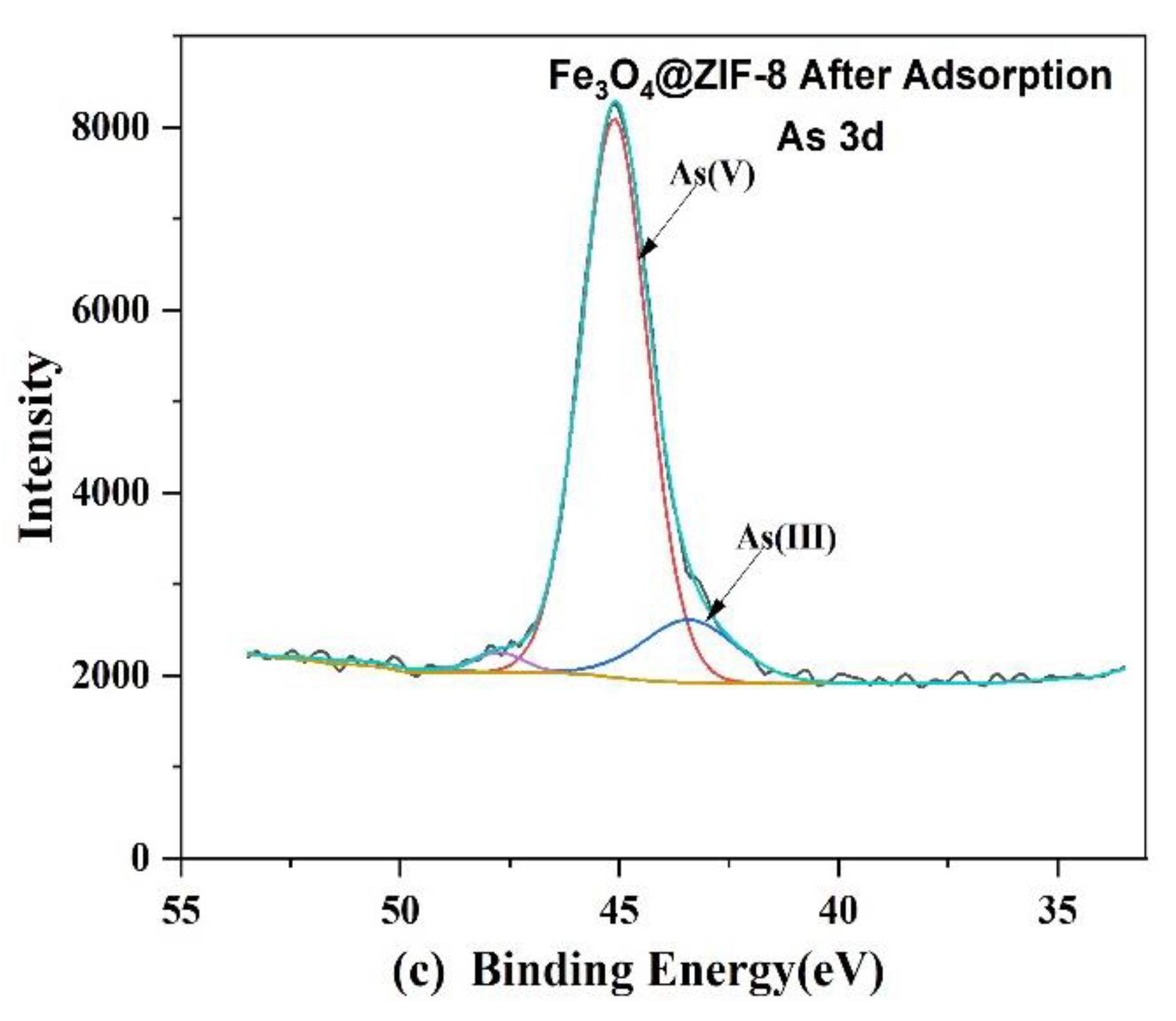
XPS spectra of Fe3O4@ZIF-8: (a) Wide scan; (b) Zn 2p core level. (c) As 3d core level after adsorption.
From Figure 11b, it can be observed that the positions of the peaks of Zn 2p1/2 and Zn 2p3/2, before and after adsorption, move to the position with higher binding energy, which means that the electronegativity of the surrounding atoms of Zn increases, resulting in Zn element. The binding energy also increases [39,40,41]. By fitting the peaks of As 3d after adsorption, it can be judged that after As(V) is adsorbed by Fe3O4@ZIF-8, a part of As(V) is reduced to As(III) by Fe3O4@ZIF-8.
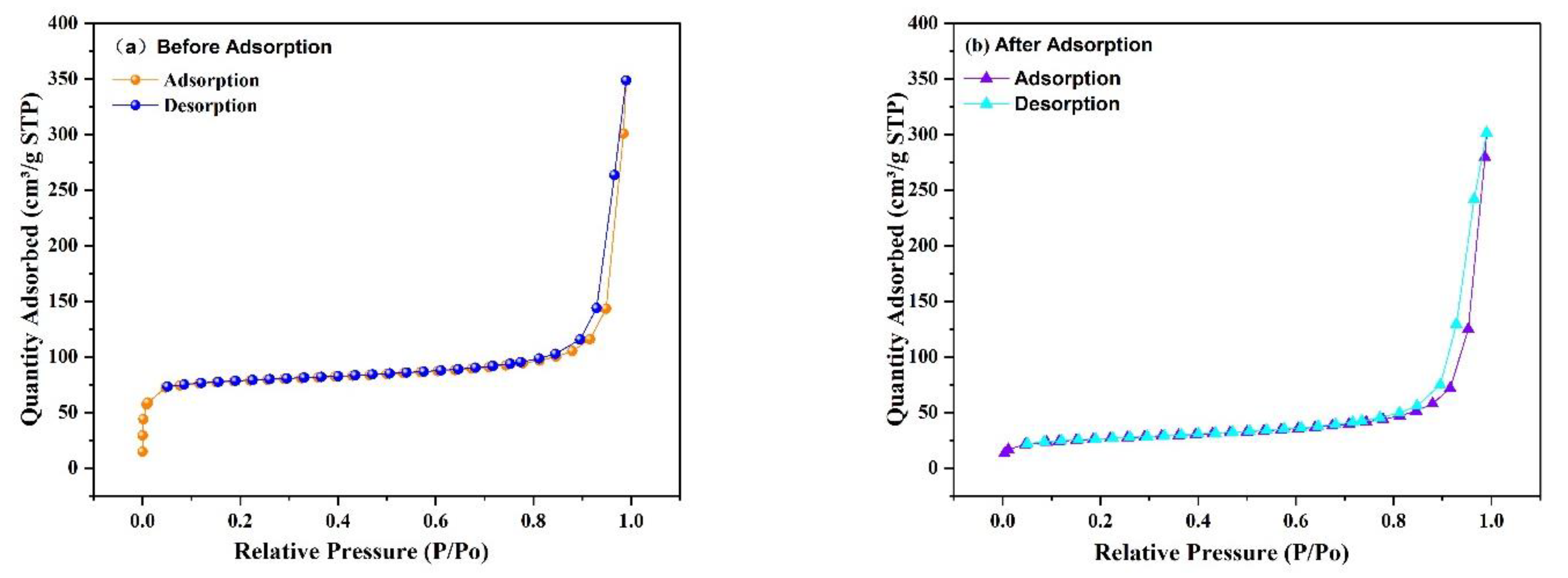
3.2.7. Surface Area Analysis (BET)
The porosity and specific surface area of the prepared Fe3O4@ZIF-8 composites were tested by N2 adsorption-desorption isotherms at the test temperature of 77 K. From Figure 12, it can be observed that the specific surface area of Fe3O4@ZIF-8 calculated by the BET method is 316.3593 m2/g, and the total pore volume measured by the single-point method is 0.224 cm3/g, and the micropore volume measured by the t-plot method is 0.097 cm3/g. The pore size is 2.83 nm, and the average mesopore size is 33.59 nm. The specific surface area of Fe3O4@ZIF-8 after adsorption of arsenic is 95.3942 m2/g, the measured total pore volume is 0.182 cm3/g, and the micropore volume is 0.017 cm3/g, the average pore size is 7.62 nm, and the average mesopore size is 34.35 nm. By comparison, it can be found that the specific surface area, total pore volume and micropore volume of Fe3O4@ZIF-8 after adsorption of arsenic are significantly reduced, indicating that arsenic is adsorbed to the surface of the material.
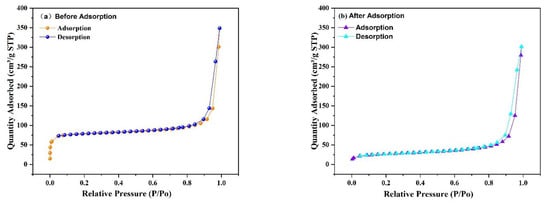
Figure 12.
N2 adsorption-desorption isotherms of Fe3O4@ZIF-8.
Magnetic Fe3O4 nanoparticles have been used for the treatment of arsenic from wastewater. Ref. [42] reported the maximum adsorption capacity of magnetite Fe3O4 nanoparticles occurred at pH 2, with a value of 3.70 mg/g for As (V). Due to aggregation effect, magnetic Fe3O4 nanoparticles are difficult to use in continuous flow systems [43]. Consequently, some researchers had encapsulated nanoparticles into metal organic frameworks to resolve above problem. Iron and 1,3,5-benzenetricarboxylic (Fe-BTC) used for As(V) removal from waters, and its adsorption capacity can reach 12.3 mg/g, more than 6 times that of iron oxide nanoparticles with a size of 50 nm [19]. Studies have shown that ZIF-8 demonstrated an adsorption amount of 60.03 mg/g [44] in comparison to that of MIL-53(Fe) (21.27 mg/g) [45]. Furthermore, the activated indium MOF (AUBM-1) was applied for As(V) removal from water and showed a high arsenic uptake capacity of 103.1 mg/g at a neutral pH. In this study, the maximum adsorption capacity of Fe3O4@ZIF-8 for As(V) can reach 116.114 mg/g; the result implies that the Fe3O4@ZIF-8 could be as a potential candidate for removing As(V) pollution in wastewater.
4. Conclusions
Characterization analysis shows that the Fe3O4@ZIF-8 materials prepared in this study have different shapes and small particle sizes, and the specific surface area can reach 316 m2/g. Through various characterization analyses, it can be proved that the metal-organic framework composite material was successfully prepared in the experiment; comparing the characterization results of Fe3O4@ZIF-8 before and after adsorption, it can be found that the material has a good adsorption effect on arsenic. At 25 °C, the initial concentration of arsenic is 46.916 mg/L, and the initial pH is 3. When the dosage of adsorbent is 0.4 g/L, the adsorption rate at equilibrium is 76.29%. When the dosage increases to 1.0 g/L, the adsorption rate at equilibrium increases to 99.29%. However, when the dosage of adsorbent increases to 0.6 g/L, the equilibrium adsorption rate can reach 97.20%, which is not much different from the equilibrium adsorption rate when the dosage is 1.0 g/L. Therefore, from the economic benefit to the perspective of resource saving, the optimal dosage is 0.6 g/L. When the pH value is 3, the adsorption effect of Fe3O4@ZIF-8 on arsenic can reach the best, the adsorption amount reach 59.80 mg/g, and the removal rate is 99.93%. When pH > 3, the equilibrium adsorption capacity of Fe3O4@ZIF-8 to arsenic decreases with the increase of pH, and when pH > 9.46, the decreasing rate is faster. The adsorption process of Fe3O4@ZIF-8 for arsenic has the best fit with the Langmuir adsorption isotherm equation. When the temperature is 298 K, the maximum adsorption capacity of Fe3O4@ZIF-8 for arsenic can reach 116.114 mg/g. For the adsorption kinetics, the second-order kinetic equation has the best fitting result for the adsorption kinetics of arsenic on Fe3O4@ZIF-8, with R2 > 0.999. By analyzing the fitting curve of the second-order kinetics, it can be found that the adsorption process of Fe3O4@ZIF-8 to arsenic-containing wastewater mainly occurs through chemical adsorption. From the analysis of thermodynamic parameters, it can be concluded that the adsorption process of Fe3O4@ZIF-8 to arsenic is a spontaneous endothermic reaction.
Author Contributions
All authors make significant contributions to this article. Conceptualization, X.H., L.H., T.X., D.L. and H.Z.; Methodology, X.H., L.H., T.X., D.L., Y.L. and H.Z.; Investigation, X.H., X.W., L.Z., J.J., Y.H., M.Y. and D.L.; Resources, X.H., L.H., T.X., D.L., Y.L. and H.Z.; Data Curation, X.W., L.Z., J.J., Y.H., M.Y. and D.L.; Writing—Original Draft Preparation, X.H., Y.L., X.W. and L.H.; Writing—Review & Editing, X.H., L.H., T.X., D.L., Y.L. and H.Z.; Supervision, X.H., L.H., T.X. and H.Z.; Project Administration, L.H., M.Y., Y.H., J.J. and T.X.; Funding Acquisition, D.L., L.H., H.Z. and T.X. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by the National Natural Science Foundation of China (NSFC) (41830753, 41301348) and the Natural Science Foundation of Guangdong Province (2022A1515010764).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sikdar, S.; Kundu, M. A Review on Detection and Abatement of Heavy Metals. ChemBioEng Rev. 2018, 5, 18–29. [Google Scholar] [CrossRef]
- Senila, M.; Levei, E.; Cadar, O.; Senila, L.R.; Roman, M.; Puskas, F.; Sima, M. Assessment of Availability and Human Health Risk Posed by Arsenic Contaminated Well Waters from Timis-Bega Area, Romania. J. Anal. Methods Chem. 2017, 2017, 3037651. [Google Scholar] [CrossRef] [PubMed]
- Alkurdi, S.S.A.; Herath, I.; Bundschuh, J.; Al-Juboori, R.A.; Vithanage, M.; Mohan, D. Biochar versus bone char for a sustainable inorganic arsenic mitigation in water: What needs to be done in future research? Environ. Int. 2019, 127, 52–69. [Google Scholar] [CrossRef]
- Ravi, R.; Mishra, A. Preparation of Iron Nanoparticles and Composites for Arsenic Removal: An Updated Review. Biosci. Biotechnol. Res. Commun. 2021, 14, 83–89. [Google Scholar] [CrossRef]
- Palma-Lara, I.; Martinez-Castillo, M.; Quintana-Perez, J.C.; Arellano-Mendoza, M.G.; Tamay-Cach, F.; Valenzuela-Limon, O.L.; Garcia-Montalvo, E.A.; Hernandez-Zavala, A. Arsenic exposure: A public health problem leading to several cancers. Regul. Toxicol. Pharmacol. 2020, 110, 104539. [Google Scholar] [CrossRef] [PubMed]
- Ricci Nicomel, N.; Leus, K.; Folens, K.; Van der Voort, P.; Du Laing, G. Technologies for Arsenic Removal from Water: Current Status and Future Perspectives. Int. J. Environ. Res. Public Health 2016, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Asere, T.G.; Stevens, C.V.; Du Laing, G. Use of (modified) natural adsorbents for arsenic remediation: A review. Sci. Total Environ. 2019, 676, 706–720. [Google Scholar] [CrossRef]
- Martinez-Castillo, M.; Garcia-Montalvo, E.A.; Arellano-Mendoza, M.G.; Sanchez-Pena, L.d.C.; Soria Jasso, L.E.; Izquierdo-Vega, J.A.; Valenzuela, O.L.; Hernandez-Zavala, A. Arsenic exposure and non-carcinogenic health effects. Hum. Exp. Toxicol. 2021, 40, S826–S850. [Google Scholar] [CrossRef]
- Singh, R.; Singh, S.; Parihar, P.; Singh, V.P.; Prasad, S.M. Arsenic contamination, consequences and remediation techniques: A review. Ecotoxicol. Environ. Saf. 2015, 112, 247–270. [Google Scholar] [CrossRef]
- Sanjrani, M.A.; Zhou, B.; Zhao, H.; Bhutto, S.A.; Muneer, A.S.; Xia, S.B. Arsenic Contaminated Groundwater in China and its Treatment Options, a Review. Appl. Ecol. Environ. Res. 2019, 17, 1655–1683. [Google Scholar] [CrossRef]
- Tokoro, C.; Yatsugi, Y.; Koga, H.; Owada, S. Sorption Mechanisms of Arsenate during Coprecipitation with Ferrihydrite in Aqueous Solution. Environ. Sci. Technol. 2010, 44, 638–643. [Google Scholar] [CrossRef]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A state-of-the-art review on wastewater treatment techniques: The effectiveness of adsorption method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef]
- Anil, I.; Gunday, S.T.; Bozkurt, A.; Alagha, O. Design of Crosslinked Hydrogels Comprising Poly(Vinylphosphonic Acid) and Bis 2-(Methacryloyloxy)Ethyl Phosphate as an Efficient Adsorbent for Wastewater Dye Removal. Nanomaterials 2020, 10, 131. [Google Scholar] [CrossRef]
- Mueller-Buschbaum, K.; Beuerle, F.; Feldmann, C. MOF based luminescence tuning and chemical/physical sensing. Microporous Mesoporous Mater. 2015, 216, 171–199. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Y.; Lu, J.; Zhou, Y. Novel cyclodextrin-based adsorbents for removing pollutants from wastewater: A critical review. Chemosphere 2020, 241, 125043. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Yuan, X.; Zhang, J.; Chew, J.W. Metal-organic framework membranes for wastewater treatment and water regeneration. Coord. Chem. Rev. 2020, 404, 213116. [Google Scholar] [CrossRef]
- Rezk, A.; Al-Dadah, R.; Mahmoud, S.; Elsayed, A. Characterisation of metal organic frameworks for adsorption cooling. Int. J. Heat Mass Transf. 2012, 55, 7366–7374. [Google Scholar] [CrossRef]
- Ma, X.; Chai, Y.; Li, P.; Wang, B. Metal-Organic Framework Films and Their Potential Applications in Environmental Pollution Control. Acc. Chem. Res. 2019, 52, 1461–1470. [Google Scholar] [CrossRef]
- Zhu, B.-J.; Yu, X.-Y.; Jia, Y.; Peng, F.-M.; Sun, B.; Zhang, M.-Y.; Luo, T.; Liu, J.-H.; Huang, X.-J. Iron and 1,3,5-Benzenetricarboxylic Metal-Organic Coordination Polymers Prepared by Solvothermal Method and Their Application in Efficient As(V) Removal from Aqueous Solutions. J. Phys. Chem. C 2012, 116, 8601–8607. [Google Scholar] [CrossRef]
- Wang, C.; Luan, J.; Wu, C. Metal-organic frameworks for aquatic arsenic removal. Water Res. 2019, 158, 370–382. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, M.; Hao, Y. Study on the adsorption of Cu(II) by EDTA functionalized Fe3O4 magnetic nano-particles. Chem. Eng. J. 2013, 218, 46–54. [Google Scholar] [CrossRef]
- Yang, H.; Hu, S.; Zhao, H.; Luo, X.; Liu, Y.; Deng, C.; Yu, Y.; Hu, T.; Shan, S.; Zhi, Y.; et al. High-performance Fe-doped ZIF-8 adsorbent for capturing tetracycline from aqueous solution. J. Hazard. Mater. 2021, 416, 126046. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.S.; Itoh, H. Iron oxide-loaded slag for arsenic removal from aqueous system. Chemosphere 2005, 60, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Burton, E.D.; Bush, R.T.; Johnston, S.G.; Watling, K.M.; Hocking, R.K.; Sullivan, L.A.; Parker, G.K. Sorption of Arsenic(V) and Arsenic(III) to Schwertmannite. Environ. Sci. Technol. 2009, 43, 9202–9207. [Google Scholar] [CrossRef]
- Wang, Y.; Li, K.; Fang, D.; Ye, X.; Liu, H.; Tan, X.; Li, Q.; Li, J.; Wu, Z. Ammonium molybdophosphate/metal-organic framework composite as an effective adsorbent for capture of Rb+ and Cs+ from aqueous solution. J. Solid State Chem. 2022, 306, 122767. [Google Scholar] [CrossRef]
- Morris, W.; Stevens, C.J.; Taylor, R.E.; Dybowski, C.; Yaghi, O.M.; Garcia-Garibay, M.A. NMR and X-ray Study Revealing the Rigidity of Zeolitic Imidazolate Frameworks. J. Phys. Chem. C 2012, 116, 13307–13312. [Google Scholar] [CrossRef]
- Max, J.J.; Chapados, C. Infrared spectroscopy of aqueous carboxylic acids: Comparison between different acids and their salts. J. Phys. Chem. A 2004, 108, 3324–3337. [Google Scholar] [CrossRef]
- Kim, C.; Ahn, J.-Y.; Kim, T.Y.; Shin, W.S.; Hwang, I. Activation of Persulfate by Nanosized Zero-Valent Iron (NZVI): Mechanisms and Transformation Products of NZVI. Environ. Sci. Technol. 2018, 52, 3625–3633. [Google Scholar] [CrossRef]
- Miao, D.; Zhao, S.; Zhu, K.; Zhang, P.; Wang, T.; Jia, H.; Sun, H. Activation of persulfate and removal of ethyl-parathion from soil: Effect of microwave irradiation. Chemosphere 2020, 253, 126679. [Google Scholar] [CrossRef]
- Kim, E.J.; Batchelor, B. X-Ray Photoelectron Spectroscopic Investigation of Interactions of Arsenic with Microwave Synthesized Pyrite as a Function of pH. Environ. Eng. Sci. 2009, 26, 1785–1793. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Wang, J. Iron and sulfur co-doped graphite carbon nitride (FeOy/S-g-C3N4) for activating peroxymonosulfate to enhance sulfamethoxazole degradation. Chem. Eng. J. 2020, 382, 122836. [Google Scholar] [CrossRef]
- Du, J.; Bao, J.; Liu, Y.; Kim, S.H.; Dionysiou, D.D. Facile preparation of porous Mn/Fe3O4 cubes as peroxymonosulfate activating catalyst for effective bisphenol A degradation. Chem. Eng. J. 2019, 376, 119193. [Google Scholar] [CrossRef]
- Sahar, S.; Zeb, A.; Liu, Y.; Ullah, N.; Xu, A. Enhanced Fenton, photo-Fenton and peroxidase-like activity and stability over Fe3O4/g-C3N4 nanocomposites. Chin. J. Catal. 2017, 38, 2110–2119. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Lau, L.W.M.; Gerson, A.; Smart, R.S.C. X-ray photoelectron spectroscopic chemical state quantification of mixed nickel metal, oxide and hydroxide systems. Surf. Interface Anal. 2009, 41, 324–332. [Google Scholar] [CrossRef]
- Wang, M.; Cui, S.; Yang, X.; Bi, W. Synthesis of g-C3N4/Fe3O4 nanocomposites and application as a new sorbent for solid phase extraction of polycyclic aromatic hydrocarbons in water samples. Talanta 2015, 132, 922–928. [Google Scholar] [CrossRef]
- Ding, Y.; Pan, C.; Peng, X.; Mao, Q.; Xiao, Y.; Fu, L.; Huang, J. Deep mineralization of bisphenol A by catalytic peroxymonosulfate activation with nano CuO/Fe3O4 with strong Cu-Fe interaction. Chem. Eng. J. 2020, 384, 123378. [Google Scholar] [CrossRef]
- Dong, L.; Li, Y.; Chen, D.; Chen, X.; Zhang, D. Facilitated Activation of Peroxymonosulfate by Loading ZIF-8 on Fe3O4-MnO2 for Deep Mineralization of Bisphenol A. ACS EST Water 2021, 1, 417–429. [Google Scholar] [CrossRef]
- Huo, J.-B.; Xu, L.; Yang, J.-C.E.; Cui, H.-J.; Yuan, B.; Fu, M.-L. Magnetic responsive Fe3O4-ZIF-8 core-shell composites for efficient removal of As(III) from water. Colloids Surf. A-Physicochem. Eng. Asp. 2018, 539, 59–68. [Google Scholar] [CrossRef]
- Yang, D.-H.; Zhao, B.-J.; Chen, B.-J.; Zhu, S.-F.; He, Z.-Y.; Zhao, Z.-R.; Liu, M.-D. Study on impurities of ZnGeP2 single crystal and its effect on infrared optical property. Mater. Res. Express 2017, 4, 075906. [Google Scholar] [CrossRef]
- Yang, D.; Zhao, B.; Chen, B.; Zhu, S.; He, Z.; Huang, W.; Zhao, Z.; Liu, M. Impurity phases analysis of ZnGeP2 single method crystal grown by Bridgman. J. Alloys Compd. 2017, 709, 125–128. [Google Scholar] [CrossRef]
- Yang, D.-H.; Cao, X.-L. Effect of weak Mn doping on optical properties of ZnGeP2 single crystal grown by vertical gradient freezing method. Mater. Res. Express 2020, 7, 105905. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Yanful, E.K. Arsenic removal from aqueous solutions by adsorption on magnetite nanoparticles. Water Environ. J. 2011, 25, 429–437. [Google Scholar] [CrossRef]
- Luo, X.; Wang, C.; Luo, S.; Dong, R.; Tu, X.; Zeng, G. Adsorption of As (III) and As (V) from water using magnetite Fe3O4-reduced graphite oxide-MnO2 nanocomposites. Chem. Eng. J. 2012, 187, 45–52. [Google Scholar] [CrossRef]
- Jian, M.; Liu, B.; Zhang, G.; Liu, R.; Zhang, X. Adsorptive removal of arsenic from aqueous solution by zeolitic imidazolate framework-8 (ZIF-8) nanoparticles. Colloids Surf. A-Physicochem. Eng. Asp. 2015, 465, 67–76. [Google Scholar] [CrossRef]
- Vu, T.A.; Le, G.H.; Dao, C.D.; Dang, L.Q.; Nguyen, K.T.; Nguyen, Q.K.; Dang, P.T.; Tran, H.T.K.; Duong, Q.T.; Nguyen, T.V.; et al. Arsenic removal from aqueous solutions by adsorption using novel MIL-53(Fe) as a highly efficient adsorbent. RSC Adv. 2015, 5, 5261–5268. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).