Early-Stage High-Concentration Thiacloprid Exposure Induced Persistent Behavioral Alterations in Zebrafish
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Exposure-Recovery Experiments of Zebrafish Embryos
2.3. Quantification of THCP in Exposure Solution
2.4. Growth and Developmental Toxicity
2.5. Locomotor Activity Measurement
2.6. Five-Fish Behavioral Assay
2.7. Image Analysis in ImageJ
2.8. Biochemical Analysis
2.9. Gene Expression Measurement
2.10. Statistical Analysis
3. Results
3.1. Growth and Developmental Toxicity
3.2. Effects of THCP on Larval Locomotor Activity
3.3. Larval Avoidance Behavior and Edge Preference
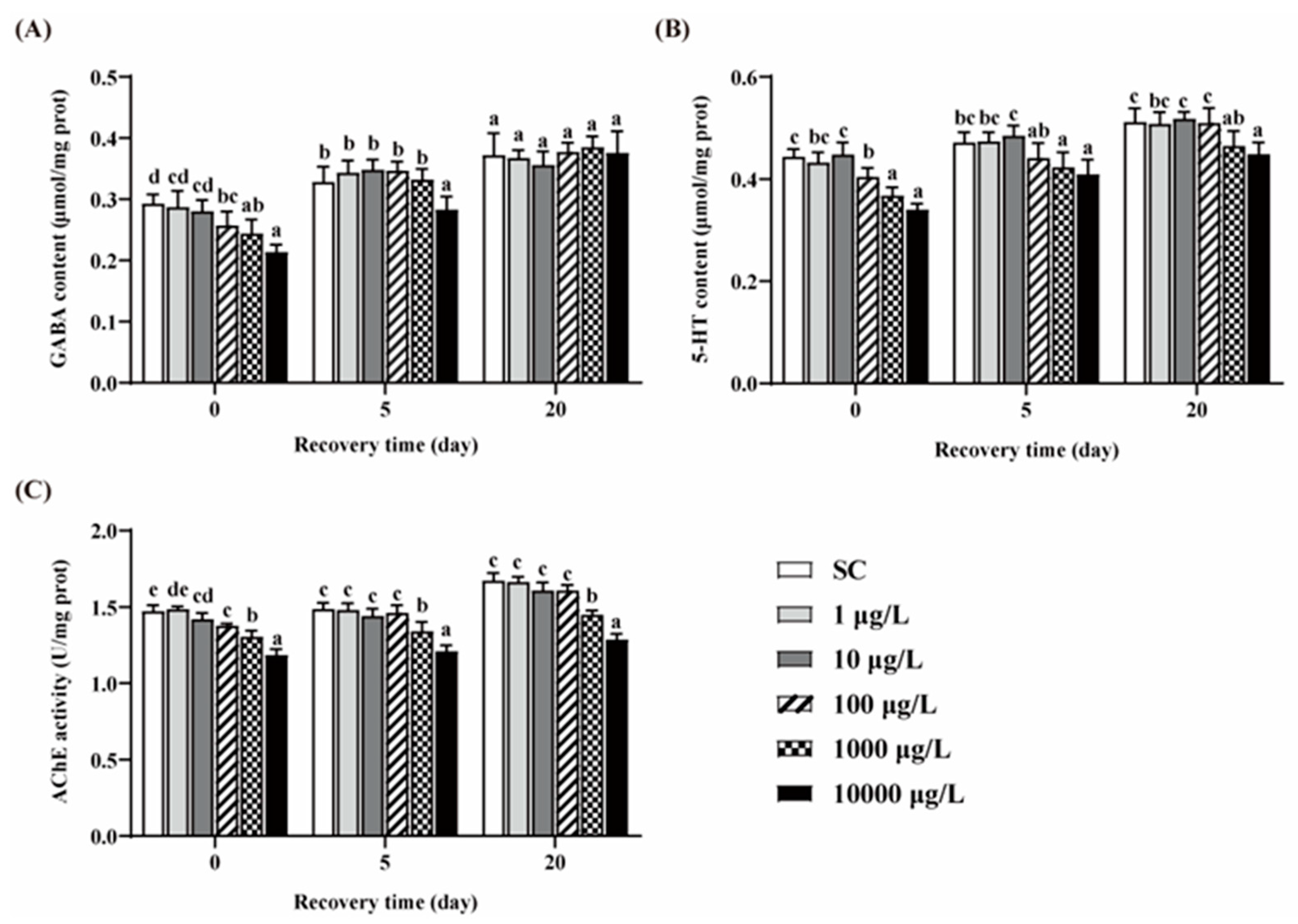
3.4. Changes of GABA, 5-HT Contents and AChE Activity
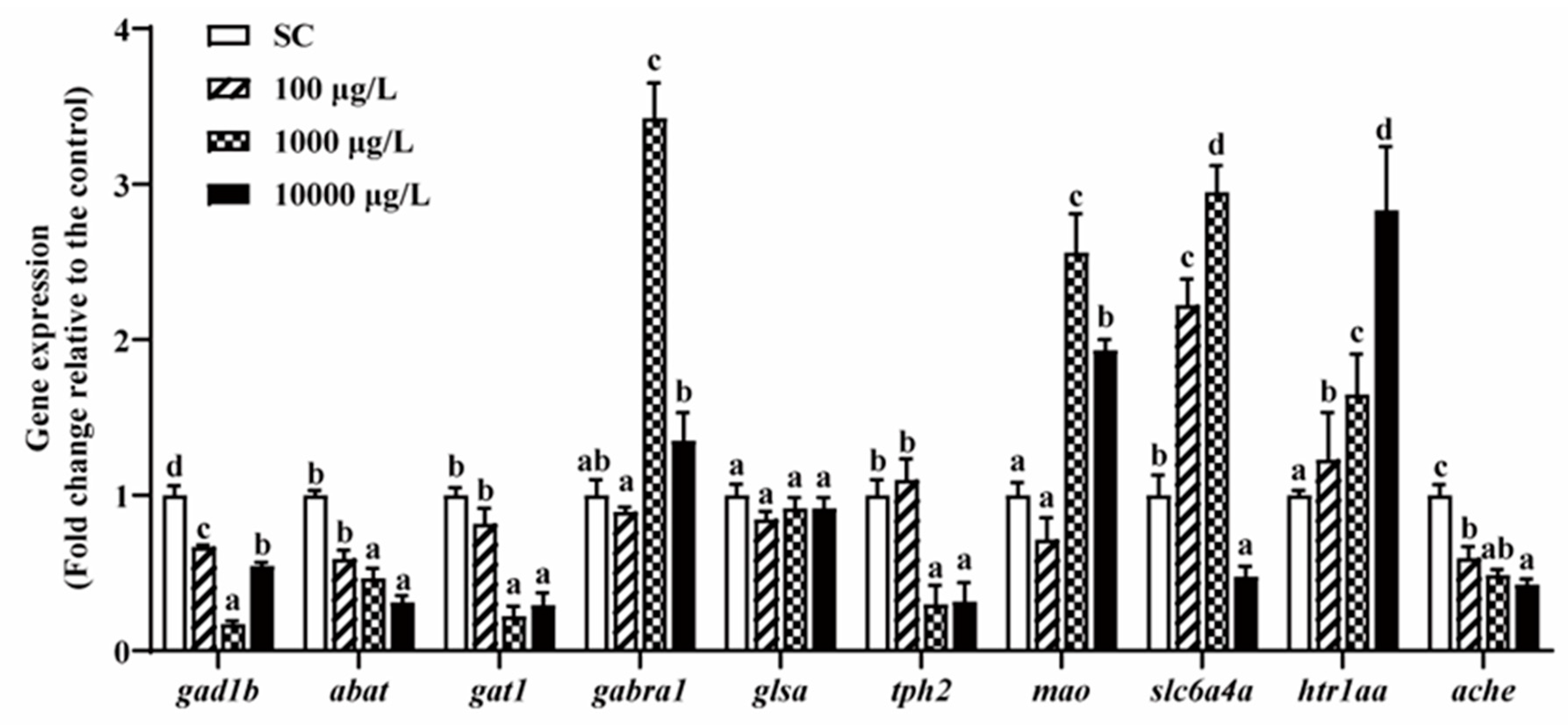
3.5. Neurotransmitters-Related Genes Expressions
3.6. THCP Induced Oxidative Stress in Zebrafish Larvae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeschke, P.; Nauen, R.; Schindler, M.; Elbert, A. Overview of the status and global strategy for neonicotinoids. J. Agric. Food Chem. 2011, 59, 2897–2908. [Google Scholar] [CrossRef] [PubMed]
- Bass, C.; Denholm, I.; Williamson, M.S.; Nauen, R. The global status of insect resistance to neonicotinoid insecticides. Pestic. Biochem. Physiol. 2015, 121, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, M.; Casida, J.E. Neonicotinoid insecticide toxicology: Mechanisms of selective action. Annu. Rev. Pharmacol. 2005, 45, 247–268. [Google Scholar] [CrossRef]
- Goulson, D.; Kleijn, D. An overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 2013, 50, 977–987. [Google Scholar] [CrossRef]
- Marins, A.T.; Cerezer, C.; Leitemperger, J.W.; Severo, E.S.; Costa, M.D.; Fontoura, D.O.; Nunes, M.E.M.; Ribeiro, L.C.; Zanella, R.; Loro, V.L. A mixture of pesticides at environmental concentrations induces oxidative stress and cholinergic effects in the neotropical fish Rhamdia quelen. Ecotoxicology 2021, 30, 164–174. [Google Scholar] [CrossRef]
- Raby, M.; Zhao, X.; Hao, C.; Poirier, D.G.; Sibley, P.K. Chronic effects of an environmentally-relevant, short-term neonicotinoid insecticide pulse on four aquatic invertebrates. Sci. Total Environ. 2018, 639, 1543–1552. [Google Scholar] [CrossRef]
- Toghan, R.; Amin, Y.A.; Ali, R.A.; Fouad, S.S.; Ahmed, M.A.-E.B.; Saleh, S.M.M. Protective effects of folic acid against reproductive, hematological, hepatic, and renal toxicity induced by acetamiprid in male Albino rats. Toxicology 2022, 469, 153115. [Google Scholar] [CrossRef]
- Main, A.R.; Michel, N.L.; Cavallaro, M.C.; Headley, J.V.; Peru, K.M.; Morrissey, C.A. Snowmelt transport of neonicotinoid insecticides to Canadian Prairie wetlands. Agric. Ecosyst. Environ. 2016, 215, 76–84. [Google Scholar] [CrossRef]
- Zhou, Y.T.; Wu, J.X.; Wang, B.; Duan, L.; Zhang, Y.Z.; Zhao, W.X.; Wang, F.; Sui, Q.; Chen, Z.Y.; Xu, D.J.; et al. Occurrence, source and ecotoxicological risk assessment of pesticides in surface water of Wujin District (northwest of Taihu Lake), China. Environ. Pollut. 2020, 265 Pt A, 114953. [Google Scholar] [CrossRef]
- Chen, Y.C.; Zhang, L.; Hu, H.M.; Wu, R.X.; Ling, J.; Yue, S.Q.; Yang, D.; Yu, W.F.; Du, W.; Shen, G.F.; et al. Neonicotinoid pollution in marine sediments of the East China Sea. Sci. Total Environ. 2022, 842, 156658. [Google Scholar] [CrossRef]
- Naumann, T.; Bento, C.P.M.; Wittmann, A.; Gandrass, J.; Tang, J.; Zhen, X.; Liu, L.; Ebinghaus, R. Occurrence and ecological risk assessment of neonicotinoids and related insecticides in the Bohai Sea and its surrounding rivers, China. Water Res. 2021, 209, 117912. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, T.; Motoyama, N.; Ambrose, J.T.; Roe, R.M. Mechanism for the differential toxicity of neonicotinoid insecticides in the honey bee, Apis mellifera. Crop. Prot. 2004, 23, 371–378. [Google Scholar] [CrossRef]
- Sanchez-Bayo, F.; Hyne, R.V. Detection and analysis of neonicotinoids in river waters-development of a passive sampler for three commonly used insecticides. Chemosphere 2014, 99, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Struger, J.; Grabuski, J.; Cagampan, S.; Sverko, E.; McGoldrick, D.; Marvin, C.H. Factors influencing the occurrence and distribution of neonicotinoid insecticides in surface waters of southern Ontario, Canada. Chemosphere 2017, 169, 516–523. [Google Scholar] [CrossRef]
- Süß, A.; Bischoff, G.; Mueller, A.C.W.; Buhr, L. Chemical and biological monitoring of the load of plant protection products and of zoocoenoses in ditches of the orchard region “Altes Land”. Nachrichtenbl. Deut. Pflanzenschutzd. 2006, 58, 28–42. [Google Scholar]
- Barmentlo, S.H.; Schrama, M.; de Snoo, G.R.; van Bodegom, P.M.; van Nieuwenhuijzen, A.; Vijver, M.G. Experimental evidence for neonicotinoid driven decline in aquatic emerging insects. Proc. Natl. Acad. Sci. USA 2021, 118, e2105692118. [Google Scholar] [CrossRef]
- Wang, Y.H.; Li, X.F.; Yang, G.L.; Weng, H.B.; Wang, X.Q.; Wang, Q. Changes of enzyme activity and gene expression in embryonic zebrafish co-exposed to beta-cypermethrin and thiacloprid. Environ. Pollut. 2020, 256, 113437. [Google Scholar] [CrossRef]
- Velisek, J.; Stara, A. Effect of thiacloprid on early life stages of common carp (Cyprinus carpio). Chemosphere 2018, 194, 481–487. [Google Scholar] [CrossRef]
- Xie, Z.T.; Lu, G.H.; Zhou, R.R.; Ma, Y.C. Thiacloprid-induced hepatotoxicity in zebrafish: Activation of the extrinsic and intrinsic apoptosis pathways regulated by p53 signaling pathway. Aquat. Toxicol. 2022, 246, 106147. [Google Scholar] [CrossRef]
- Krnjevic, K. When and why amino acids? J. Physiol. 2010, 588 Pt 1, 33–44. [Google Scholar] [CrossRef]
- Tang, Y.Q.; Li, Z.R.; Zhang, S.Z.; Mi, P.; Chen, D.Y.; Feng, X.Z. Venlafaxine plus melatonin ameliorate reserpine-induced depression-like behavior in zebrafish. Neurotoxicol. Teratol. 2019, 76, 106835. [Google Scholar] [CrossRef] [PubMed]
- Herculano, A.M.; Maximino, C. Serotonergic modulation of zebrafish behavior: Towards a paradox. Prog. Neuro-Psychoph. 2014, 55, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Nordquist, N.; Oreland, L. Serotonin, genetic variability, behaviour, and psychiatric disorders-a review. Upsala J. Med. Sci. 2010, 115, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.; Dong, T.; Keerthisinghe, T.P.; Chen, H.; Li, M.; Chu, W.Q.; Yang, J.F.; Hu, Z.F.; Snyder, S.A.; Dong, W.; et al. Long-term oxytetracycline exposure potentially alters brain thyroid hormone and serotonin homeostasis in zebrafish. J. Hazard. Mater. 2020, 399, 123061. [Google Scholar] [CrossRef]
- Cao, Z.G.; Su, M.L.; Wang, H.L.; Zhou, L.Q.; Meng, Z.; Xiong, G.H.; Liao, X.J.; Lu, H.Q. Carboxyl graphene oxide nanoparticles induce neurodevelopmental defects and locomotor disorders in zebrafish larvae. Chemosphere 2021, 270, 128611. [Google Scholar] [CrossRef]
- Zhang, J.G.; Ma, D.D.; Xiong, Q.; Qiu, S.Q.; Huang, G.Y.; Shi, W.J.; Ying, G.G. Imidacloprid and thiamethoxam affect synaptic transmission in zebrafish. Ecotoxicol. Environ. Saf. 2021, 227, 112917. [Google Scholar] [CrossRef]
- Yang, L.H.; Ho, N.Y.; Alshut, R.; Legradi, J.; Weiss, C.; Reischl, M.; Mikut, R.; Liebel, U.; Müller, F.; Strähle, U. Zebrafish embryos as models for embryotoxic and teratological effects of chemicals. Reprod. Toxicol. 2009, 28, 245–253. [Google Scholar] [CrossRef]
- Konemann, S.; von Wyl, M.; vom Berg, C. Zebrafish larvae rapidly recover from locomotor effects and neuromuscular alterations induced by cholinergic insecticides. Environ. Sci. Technol. 2022, 56, 8449–8462. [Google Scholar] [CrossRef]
- Pelkowski, S.D.; Kapoor, M.; Richendrfer, H.A.; Wang, X.; Colwill, R.M.; Creton, R. A novel high-throughput imaging system for automated analyses of avoidance behavior in zebrafish larvae. Behav. Brain Res. 2011, 223, 135–144. [Google Scholar] [CrossRef]
- Richendrfer, H.; Pelkowski, S.D.; Colwill, R.M.; Creton, R. On the edge: Pharmacological evidence for anxiety-related behavior in zebrafish larvae. Behav. Brain Res. 2012, 228, 99–106. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Góth, L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta 1991, 196, 143–152. [Google Scholar] [CrossRef]
- Habig, W.H.; Jakoby, W.B. Assays for differentiation of glutathione S-transferases. Method. Enzymol. 1981, 77, 398–405. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, H.; Lim, W.; Song, G. Benfuresate induces developmental toxicity in zebrafish larvae by generating apoptosis and pathological modifications. Pestic. Biochem. Physiol. 2021, 172, 104751. [Google Scholar] [CrossRef]
- Zhou, R.R.; Lu, G.H.; Yan, Z.H.; Jiang, R.R.; Sun, Y.; Zhang, P. Interactive transgenerational effects of polystyrene nanoplastics and ethylhexyl salicylate on zebrafish. Environ. Sci. Nano 2021, 8, 146–159. [Google Scholar] [CrossRef]
- Ran, L.L.; Yang, Y.; Zhou, X.; Jiang, X.X.; Hu, D.Y.; Lu, P. The enantioselective toxicity and oxidative stress of dinotefuran on zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2021, 226, 112809. [Google Scholar] [CrossRef]
- Ma, X.; Li, H.Z.; Xiong, J.J.; Mehler, W.T.; You, J. Developmental toxicity of a neonicotinoid insecticide, acetamiprid to zebrafish embryos. J. Agric. Food Chem. 2019, 67, 2429–2436. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.F.; Zhao, Y.Q.; Liu, W.; Li, Z.T.; Souders, C.L., 2nd; Martyniuk, C.J. Butylated hydroxytoluene induces hyperactivity and alters dopamine-related gene expression in larval zebrafish (Danio rerio). Environ. Pollut. 2020, 257, 113624. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, K.; Ekvall, M.T.; Hansson, L.A.; Linse, S.; Malmendal, A.; Cedervall, T. Altered behavior, physiology, and metabolism in fish exposed to polystyrene nanoparticles. Environ. Sci. Technol. 2015, 49, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Creton, R. Automated analysis of behavior in zebrafish larvae. Behav. Brain Res. 2009, 203, 127–136. [Google Scholar] [CrossRef]
- Colwill, R.M.; Creton, R. Imaging escape and avoidance behavior in zebrafish larvae. Rev. Neurosci. 2011, 22, 63–73. [Google Scholar] [CrossRef]
- Mineur, Y.S.; Obayemi, A.; Wigestrand, M.B.; Fote, G.M.; Calarco, C.A.; Li, A.M.; Picciotto, M.R. Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety- and depression-like behavior. Proc. Natl. Acad. Sci. USA 2013, 110, 3573–3578. [Google Scholar] [CrossRef]
- Horzmann, K.A.; Freeman, J.L. Zebrafish get connected: Investigating neurotransmission targets and alterations in chemical toxicity. Toxics 2016, 4, 19. [Google Scholar] [CrossRef]
- Oliveri, A.N.; Levin, E.D. Dopamine D1 and D2 receptor antagonism during development alters later behavior in zebrafish. Behav. Brain Res. 2019, 356, 250–256. [Google Scholar] [CrossRef]
- Tufi, S.; Leonards, P.; Lamoree, M.; de Boer, J.; Legler, J.; Legradi, J. Changes in neurotransmitter profiles during early zebrafish (Danio rerio) development and after pesticide exposure. Environ. Sci. Technol. 2016, 50, 3222–3230. [Google Scholar] [CrossRef]
- Saint-Amant, L.; Drapeau, P. Motoneuron activity patterns related to the earliest behavior of the zebrafish embryo. J. Neurosci. 2000, 20, 3964–3972. [Google Scholar] [CrossRef]
- Gong, G.Y.; Chen, H.B.; Kam, H.; Chan, G.; Tang, Y.X.; Wu, M.; Tan, H.S.; Tse, Y.C.; Xu, H.X.; Lee, S.M. In vivo screening of xanthones from Garcinia oligantha identified oliganthin H as a novel natural inhibitor of convulsions. J. Nat. Prod. 2020, 83, 3706–3716. [Google Scholar] [CrossRef] [PubMed]
- Jager, A.; Amiri, H.; Bielczyk, N.; van Heukelum, S.; Heerschap, A.; Aschrafi, A.; Poelmans, G.; Buitelaar, J.K.; Kozicz, T.; Glennon, J.C. Cortical control of aggression: GABA signalling in the anterior cingulate cortex. Eur. Neuropsychopharm. 2020, 30, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Li, L.; Li, G.Y.; Zhao, S.J. Microcystin-LR induces changes in the GABA neurotransmitter system of zebrafish. Aquat. Toxicol. 2017, 188, 170–176. [Google Scholar] [CrossRef]
- Rowley, N.M.; Madsen, K.K.; Schousboe, A.; Steve White, H. Glutamate and GABA synthesis, release, transport and metabolism as targets for seizure control. Neurochem. Int. 2012, 61, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Goodman, A.C.; Wong, R.Y. Differential effects of ethanol on behavior and GABAA receptor expression in adult zebrafish (Danio rerio) with alternative stress coping styles. Sci. Rep. 2020, 10, 13076. [Google Scholar] [CrossRef]
- Thompson, W.A.; Vijayan, M.M. Zygotic venlafaxine exposure impacts behavioral programming by disrupting brain serotonin in zebrafish. Environ. Sci. Technol. 2020, 54, 14578–14588. [Google Scholar] [CrossRef]
- Daubert, E.A.; Condron, B.G. Serotonin: A regulator of neuronal morphology and circuitry. Trends Neurosci. 2010, 33, 424–434. [Google Scholar] [CrossRef]
- Norton, W.H.; Folchert, A.; Bally-Cuif, L. Comparative analysis of serotonin receptor (HTR1A/HTR1B families) and transporter (slc6a4a/b) gene expression in the zebrafish brain. J. Comp. Neurol. 2008, 511, 521–542. [Google Scholar] [CrossRef]
- Bellipanni, G.; Rink, E.; Bally-Cuif, L. Cloning of two tryptophan hydroxylase genes expressed in the diencephalon of the developing zebrafish brain. Mech. Develop. 2002, 119, S215–S220. [Google Scholar] [CrossRef]
- Migliarini, S.; Pacini, G.; Pelosi, B.; Lunardi, G.; Pasqualetti, M. Lack of brain serotonin affects postnatal development and serotonergic neuronal circuitry formation. Mol. Psychiatr. 2013, 18, 1106–1118. [Google Scholar] [CrossRef]
- Airhart, M.J.; Lee, D.H.; Wilson, T.D.; Miller, B.E.; Miller, M.N.; Skalko, R.G.; Monaco, P.J. Adverse effects of serotonin depletion in developing zebrafish. Neurotoxicol. Teratol. 2012, 34, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Yang, L.H.; Wang, Q.W.; Guo, Y.Y.; Li, N.; Ma, M.; Zhou, B.S. The neurotoxicity of DE-71: Effects on neural development and impairment of serotonergic signaling in zebrafish larvae. J. Appl. Toxicol. 2016, 36, 1605–1613. [Google Scholar] [CrossRef]
- Lucki, I. The spectrum of behaviors influenced by serotonin. Biol. Psychiat. 1998, 44, 151–162. [Google Scholar] [CrossRef]
- Crosby, E.B.; Bailey, J.M.; Oliveri, A.N.; Levin, E.D. Neurobehavioral impairments caused by developmental imidacloprid exposure in zebrafish. Neurotoxicol. Teratol. 2015, 49, 81–90. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: From basic research to clinical application. Am. J. Med. 1991, 91, S31–S38. [Google Scholar] [CrossRef]
- Yan, S.H.; Wang, J.H.; Zhu, L.S.; Chen, A.M.; Wang, J. Thiamethoxam induces oxidative stress and antioxidant response in zebrafish (Danio rerio) livers. Environ. Toxicol. 2016, 31, 2006–2015. [Google Scholar] [CrossRef] [PubMed]
- Gobi, N.; Vaseeharan, B.; Rekha, R.; Vijayakumar, S.; Faggio, C. Bioaccumulation, cytotoxicity and oxidative stress of the acute exposure selenium in Oreochromis mossambicus. Ecotoxicol. Environ. Saf. 2018, 162, 147–159. [Google Scholar] [CrossRef]
- da Silva Barreto, J.; de Melo Tarouco, F.; da Rosa, C.E. Chlorothalonil causes redox state change leading to oxidative stress generation in Danio rerio. Aquat. Toxicol. 2020, 225, 105527. [Google Scholar] [CrossRef]
- Zhang, C.N.; Zhang, J.L.; Ren, H.T.; Zhou, B.H.; Wu, Q.J.; Sun, P. Effect of tributyltin on antioxidant ability and immune responses of zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2017, 138, 1–8. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, Q.; Shi, X.; Guo, Y.; Xu, T.; Zhou, B. Effect of combined exposure to lead and decabromodiphenyl ether on neurodevelopment of zebrafish larvae. Chemosphere 2016, 144, 1646–1654. [Google Scholar] [CrossRef]
- Behra, M.; Cousin, X.; Bertrand, C.; Vonesch, J.L.; Biellmann, D.; Chatonnet, A.; Strahle, U. Acetylcholinesterase is required for neuronal and muscular development in the zebrafish embryo. Nat. Neurosci. 2002, 5, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Salminen, L.E.; Paul, R.H. Oxidative stress and genetic markers of suboptimal antioxidant defense in the aging brain: A theoretical review. Rev. Neurosci. 2014, 25, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Q.; Gundlach, M.; Yang, S.Y.; Jiang, J.; Velki, M.; Yin, D.Q.; Hollert, H. Quantitative investigation of the mechanisms of microplastics and nanoplastics toward zebrafish larvae locomotor activity. Sci. Total Environ. 2017, 584–585, 1022–1031. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Z.; Lu, G.; Yu, Y. Early-Stage High-Concentration Thiacloprid Exposure Induced Persistent Behavioral Alterations in Zebrafish. Int. J. Environ. Res. Public Health 2022, 19, 10920. https://doi.org/10.3390/ijerph191710920
Xie Z, Lu G, Yu Y. Early-Stage High-Concentration Thiacloprid Exposure Induced Persistent Behavioral Alterations in Zebrafish. International Journal of Environmental Research and Public Health. 2022; 19(17):10920. https://doi.org/10.3390/ijerph191710920
Chicago/Turabian StyleXie, Zhongtang, Guanghua Lu, and Yeting Yu. 2022. "Early-Stage High-Concentration Thiacloprid Exposure Induced Persistent Behavioral Alterations in Zebrafish" International Journal of Environmental Research and Public Health 19, no. 17: 10920. https://doi.org/10.3390/ijerph191710920
APA StyleXie, Z., Lu, G., & Yu, Y. (2022). Early-Stage High-Concentration Thiacloprid Exposure Induced Persistent Behavioral Alterations in Zebrafish. International Journal of Environmental Research and Public Health, 19(17), 10920. https://doi.org/10.3390/ijerph191710920








