Acute Effect of Exposure to Extreme Heat (100 ± 3 °C) on Lower Limb Maximal Resistance Strength
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Guidelines
2.2. Health Security Protocol
2.3. Participants
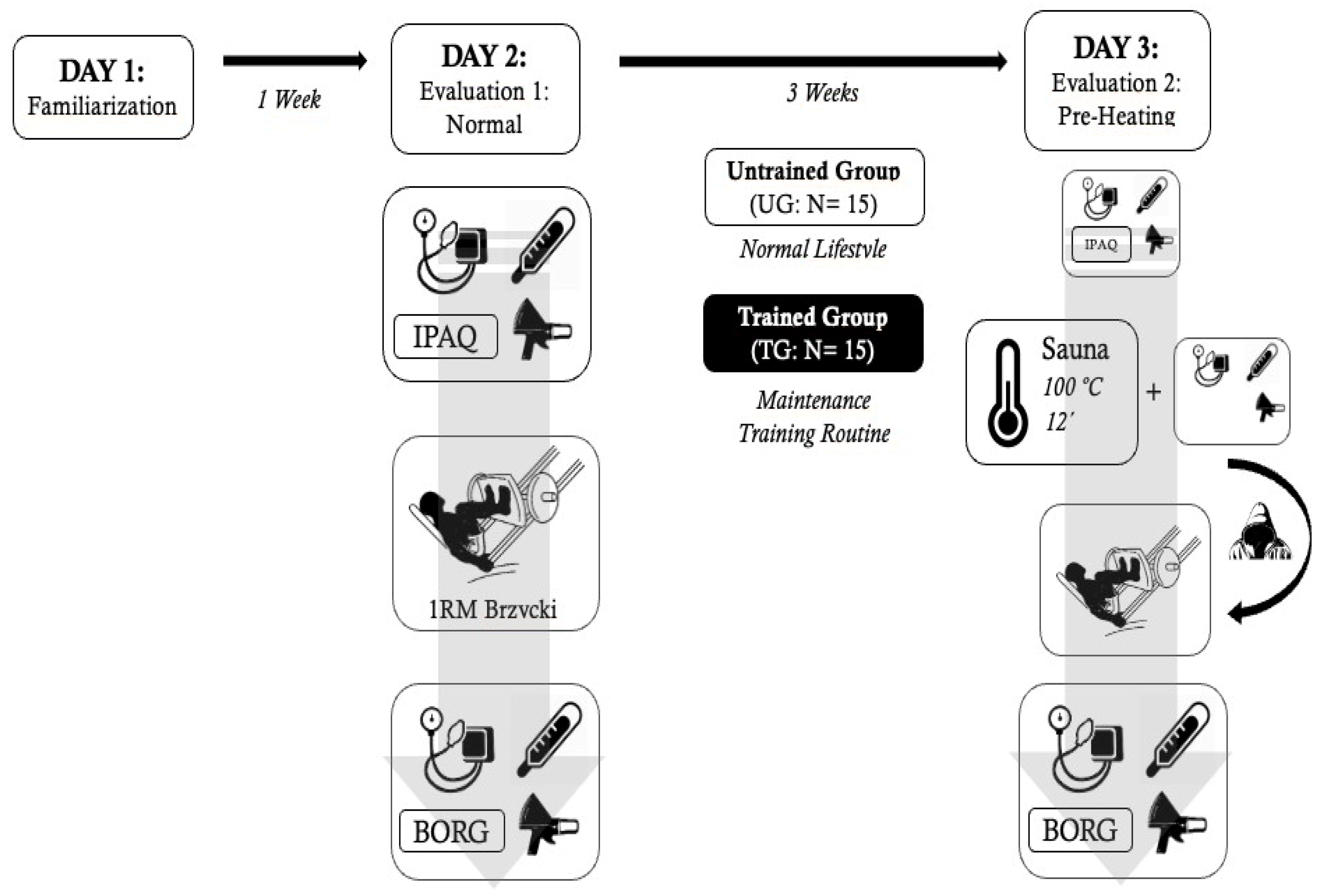
2.4. Experimental Protocol
2.5. Anthropometric and Body Composition Evaluations
2.6. Physical Activity Evaluation
2.7. Cardiovascular Evaluation
2.8. Body Temperatures Evaluation
2.9. Strength Evaluation and Maximum Repetition Calculation
2.10. Rating of Perceived Exertion
2.11. Sauna Bathing
2.12. Moving from the Sauna to the Gym
2.13. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McGorm, H.; Roberts, L.A.; Coombes, J.S.; Peake, J.M. Turning up the heat: An evaluation of the evidence for heating to promote exercise recovery, muscle rehabilitation and adaptation. Sports Med. 2018, 48, 1311–1328. [Google Scholar] [CrossRef]
- Yoon, S.J.; Lee, M.J.; Lee, H.M.; Lee, J.S. Effect of low-intensity resistance training with heat stress on the HSP72, anabolic hormones, muscle size, and strength in elderly women. Aging Clin. Exp. Res. 2016, 29, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Pilch, W.; Szygula, Z.; Palka, T.; Pilch, P.; Cison, T.; Wiecha, S. Comparison of physiological reactions and physiological strain in healthy men under heat stress in dry and steam heat saunas. Biol. Sport 2014, 31, 145. [Google Scholar] [CrossRef] [PubMed]
- Corbett, J.; Neal, R.A.; Lunt, H.C.; Tipton, M.J. Adaptation to heat and exercise performance under cooler conditions: A new hot topic. Sports Med. 2014, 44, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Parsons, K. Human Heat Stress; Parsons, K., Ed.; CRC Press: Boca Ratón, FL, USA, 2019; ISBN 042902083X. [Google Scholar]
- Périard, J.; Racinais, S. Heat Stress in Sport and Exercise; Springer: Cham, Switzerland, 2019; ISBN 3319935143. [Google Scholar]
- Bishop, D. Warm up I: Potential mechanisms and the effects of passive warm up on exercise performance. Sports Med. 2003, 33, 439–454. [Google Scholar] [CrossRef]
- Racinais, S.; Wilson, M.G.; Périard, J.D. Passive heat acclimation improves skeletal muscle contractility in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R101–R107. [Google Scholar] [CrossRef]
- Kukkonen-Harjula, K.; Kauppinen, K. How the sauna affects the endocrine system. Ann. Clin. Res. 1988, 20, 262–266. [Google Scholar] [PubMed]
- Jezová, D.; Kvetnanský, R.; Vigas, M. Sex differences in endocrine response to hyperthermia in sauna. Acta Physiol. Scand. 1994, 150, 293–298. [Google Scholar] [CrossRef]
- Kakigi, R.; Naito, H.; Ogura, Y.; Kobayashi, H.; Saga, N.; Ichinoseki-Sekine, N.; Yoshihara, T.; Katamoto, S. Heat stress enhances mTOR signaling after resistance exercise in human skeletal muscle. J. Physiol. Sci. 2011, 61, 131–140. [Google Scholar] [CrossRef]
- Heathcote, S.L.; Hassmén, P.; Zhou, S.; Stevens, C.J. Passive Heating: Reviewing Practical Heat Acclimation Strategies for Endurance Athletes. Front. Physiol. 2018, 9, 1851. [Google Scholar] [CrossRef] [Green Version]
- Bartolomé, I.; Siquier-Coll, J.; Pérez-Quintero, M.; Robles-Gil, M.C.; Muñoz, D.; Maynar-Mariño, M. Effect of handgrip training in extreme heat on the development of handgrip maximal isometric strength among young males. Int. J. Environ. Res. Public Health 2021, 18, 5240. [Google Scholar] [CrossRef] [PubMed]
- McLellan, T.M. The importance of aerobic fitness in determining tolerance to uncompensable heat stress. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2001, 128, 691–700. [Google Scholar] [CrossRef]
- McArdle, W.D.; Katch, F.I.; Katch, V.L. Exercise Physiology: Nutrition, Energy, and Human Performance; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2010; ISBN 0781797810. [Google Scholar]
- Avellini, B.A.; Shapiro, Y.; Fortney, S.M.; Wenger, C.B.; Pandolf, K.B. Effects on heat tolerance of physical training in water and on land. J. Appl. Physiol. 1982, 53, 1291–1298. [Google Scholar] [CrossRef]
- Marfell-Jones, M.; Stewart, A.; Olds, T. Kinanthropometry IX: Proceedings of the 9th International Conference of the International Society for the Advancement of Kinanthropometry; Routledge: London, UK, 2006; p. 156. [Google Scholar]
- Aibar, A.; García González, L.; Abarca Sos, A.; Murillo, B.; Zaragoza, J. Testing the validity of the International Physical Activity Questionnaire in early spanish adolescent: A modified protocol for data collection. Sport TK Rev. Euroam. Cienc. Deport. 2016, 5, 1–10. [Google Scholar]
- Brzycki, M. Strength testing—Predicting a one-rep max from reps-to-fatigue. J. Phys. Educ. Recreat. Dance 1993, 64, 88–90. [Google Scholar] [CrossRef]
- Borg, G. Borg’s Perceived Exertion and Pain Scales; Human Kinetics: Vancouver, BC, Canada, 1998. [Google Scholar]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect size estimates: Current use, calculations, and interpretation. J. Exp. Psychol. Gen. 2012, 141, 2. [Google Scholar] [CrossRef]
- Binkhorst, R.A.; Hoofd, L.; Vissers, A.C. Temperature and force-velocity relationship of human muscles. J. Appl. Physiol. 1977, 42, 471–475. [Google Scholar] [CrossRef]
- Davies, C.T.M.; Young, K. Effect of temperature on the contractile properties and muscle power of triceps surae in humans. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1983, 55, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Falk, B.; Radom-lsaac, S.; Hoffmann, J.R.; Wang, Y.; Yarom, Y.; Magazanik, A.; Weinstein, Y. The effect of heat exposure on performance of and recovery from high-intensity, intermittent exercise. Int. J. Sports Med. 1998, 19, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hedley, A.M.; Climstein, M.; Hansen, R. The effects of acute heat exposure on muscular strength, muscular endurance, and muscular power in the euhydrated athlete. J. Strength Cond. Res. 2002, 16, 353–358. [Google Scholar]
- Kukkonen-Harjula, K.; Oja, P.; Laustiola, K.; Vuori, I.; Jolkkonen, J.; Siitonen, S.; Vapaatalo, H. Haemodynamic and hormonal responses to heat exposure in a Finnish sauna bath. Eur. J. Appl. Physiol. Occup. Physiol. 1989, 58, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S. Catecholamines and stress. Endocr. Regul. 2003, 37, 69–80. [Google Scholar] [PubMed]
- Costill, D.L.; Kenney, W.L.; Wilmore, J. Physiology of Sport and Exercise; Human Kinetics: Champaign, IL, USA, 2008; Volume 448. [Google Scholar]
- Leppäluoto, J.; Huttunen, P.; Hirvonen, J.; Väänänen, A.; Tuominen, M.; Vuori, J. Endocrine effects of repeated sauna bathing. Acta Physiol. Scand. 1986, 128, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Racinais, S.; Oksa, J. Temperature and neuromuscular function. Scand. J. Med. Sci. Sport. 2010, 20, 1–18. [Google Scholar] [CrossRef]
- De Jesus, P.V.; Hausmanowa-Petrusewicz, I.; Barchi, R.L. The effect of cold on nerve conduction of human slow and fast nerve fibers. Neurology 1973, 23, 1182–1189. [Google Scholar] [CrossRef]
- Rutkove, S.B.; Kothari, M.J.; Shefner, J.M. Nerve, muscle, and neuromuscular junction electrophysiology at high temperature. Muscle Nerve 1997, 20, 431–436. [Google Scholar] [CrossRef]
- Kelty, J.D.; Noseworthy, P.A.; Feder, M.E.; Robertson, R.M.; Ramirez, J.M. Thermal preconditioning and heat-shock protein 72 preserve synaptic transmission during thermal stress. J. Neurosci. 2002, 22, RC193. [Google Scholar] [CrossRef]
- Rutkove, S.B. Effects of temperature on neuromuscular electrophysiology. Muscle Nerve 2001, 24, 867–882. [Google Scholar] [CrossRef]
- Faulkner, J.A.; Zerba, E.; Brooks, S.V. Muscle temperature of mammals: Cooling impairs most functional properties. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1990, 259, R259–R265. [Google Scholar] [CrossRef]
- North, R.A. Molecular physiology of P2X receptors. Physiol. Rev. 2002, 82, 1013–1067. [Google Scholar] [CrossRef]
- McCord, J.L.; Kaufman, M.P. Reflex autonomic responses evoked by group III and IV muscle afferents. In Translational Pain Research: From Mouse to Man; CRC Press: Boca Raton, FL, USA, 2009; ISBN 9781439812105. [Google Scholar]
- Kluess, H.A.; Buckwalter, J.B.; Hamann, J.J.; Clifford, P.S. Elevated temperature decreases sensitivity of P2X purinergic receptors in skeletal muscle arteries. J. Appl. Physiol. 2005, 99, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Tominaga, T. Structure and function of TRPV1. Pflugers Arch. Eur. J. Physiol. 2005, 451, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wen, H. Heat activation mechanism of TRPV1: New insights from molecular dynamics simulation. Temperature 2019, 6, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Amann, M.; Blain, G.M.; Proctor, L.T.; Sebranek, J.J.; Pegelow, D.F.; Dempsey, J.A. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J. Appl. Physiol. 2010, 109, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Toth, A.; Tran, R.; Szabo, T.; Welter, J.D.; Blumberg, P.M.; Lee, J.; Kang, S.U.; Lim, J.O.; Lee, J. High-affinity partial agonists of the vanilloid receptor. Mol. Pharmacol. 2003, 64, 325–333. [Google Scholar] [CrossRef]
- Amann, M.; Runnels, S.; Morgan, D.E.; Trinity, J.D.; Fjeldstad, A.S.; Wray, D.W.; Reese, V.R.; Richardson, R.S. On the contribution of group III and IV muscle afferents to the circulatory response to rhythmic exercise in humans. J. Physiol. 2011, 589, 3855–3866. [Google Scholar] [CrossRef]
- Bennett, A.F. Thermal dependence of muscle function. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1984, 247, R217–R229. [Google Scholar] [CrossRef]
- Komi, P. Strength and Power in Sport; John Wiley & Sons: Hoboken, NJ, USA, 2008; Volume 3, ISBN 1405140593. [Google Scholar]
- Schoenfeld, B. Potential mechanisms for a role of metabolic stress in hypertrophic adaptations to resistance training. Sports Med. 2013, 43, 179–194. [Google Scholar] [CrossRef]
- Morton, J.P.; Kayani, A.C.; McArdle, A.; Drust, B. The Exercise-Induced stress response of skeletal muscle, with specific emphasis on humans. Sports Med. 2009, 39, 643–662. [Google Scholar] [CrossRef]
- Stetler, R.A.; Gan, Y.; Zhang, W.; Liou, A.K.; Gao, Y.; Cao, G.; Chen, J. Heat shock proteins: Cellular and molecular mechanisms in the central nervous system. Prog. Neurobiol. 2010, 92, 184–211. [Google Scholar] [CrossRef] [PubMed]
- Karunanithi, S.; Barclay, J.W.; Robertson, R.M.; Brown, I.R.; Atwood, H.L. Neuroprotection at Drosophila synapses conferred by prior heat shock. J. Neurosci. 1999, 19, 4360–4369. [Google Scholar] [CrossRef] [PubMed]
- Roatta, S.; Farina, D. Sympathetic actions on the skeletal muscle. Exerc. Sport Sci. Rev. 2010, 38, 31–35. [Google Scholar] [CrossRef] [PubMed]
- MacLaren, D.; Morton, J. Biochemistry for Sport and Exercise Metabolism; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 1119967821. [Google Scholar]
- Hedrick, A. Exercise physiology: Physiological responses to warm-up. Strength Cond. J. 1992, 14, 25–27. [Google Scholar] [CrossRef]
- Febbraio, M.A.; Snow, R.J.; Stathis, C.G.; Hargreaves, M.; Carey, M.F. Effect of heat stress on muscle energy metabolism during exercise. J. Appl. Physiol. 1994, 77, 2827–2831. [Google Scholar] [CrossRef]
- Tesch, P.A.; Colliander, E.B.; Kaiser, P. Muscle metabolism during intense, heavy-resistance exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1986, 55, 362–366. [Google Scholar] [CrossRef]
- Guyton, A.C.; Hall, J.E. Textbook of Medical Physiology; Saunders: Philadelphia, PA, USA, 1986; Volume 548. [Google Scholar]
- Girard, O.; Bishop, D.J.; Racinais, S. Hot conditions improve power output during repeated cycling sprints without modifying neuromuscular fatigue characteristics. Eur. J. Appl. Physiol. 2013, 113, 359–369. [Google Scholar] [CrossRef]
- Savoie, F.-A.; Kenefick, R.W.; Ely, B.R.; Cheuvront, S.N.; Goulet, E.D.B. Effect of hypohydration on muscle endurance, strength, anaerobic power and capacity and vertical jumping ability: A meta-analysis. Sports Med. 2015, 45, 1207–1227. [Google Scholar] [CrossRef]
- Cheuvront, S.N.; Kenefick, R.W.; Montain, S.J.; Sawka, M.N. Mechanisms of aerobic performance impairment with heat stress and dehydration. J. Appl. Physiol. 2010, 109, 1989–1995. [Google Scholar] [CrossRef]
- Buchthal, F.; Kaiser, E.; Knappeis, G. Elasticity, Viscosity and Plasticity in the Cross Striated Muscle Fibre. Acta Physiol. Scand. 1944, 8, 16–37. [Google Scholar] [CrossRef]
- Stephenson, D.G.; Williams, D.A. Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. J. Physiol. 1981, 317, 281–302. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.F.; Masock, A.J.; Warren, C.G.; Koblanski, J.N. Effect of therapeutic temperatures on tendon extensibility. Arch. Phys. Med. Rehabil. 1970, 6, 185. [Google Scholar]
- Brunt, V.E.; Weidenfeld-Needham, K.M.; Comrada, L.N.; Francisco, M.A.; Eymann, T.M.; Minson, C.T. Serum from young, sedentary adults who underwent passive heat therapy improves endothelial cell angiogenesis via improved nitric oxide bioavailability. Temperature 2019, 6, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Brunt, V.E.; Howard, M.J.; Francisco, M.A.; Ely, B.R.; Minson, C.T. Passive heat therapy improves endothelial function, arterial stiffness and blood pressure in sedentary humans. J. Physiol. 2016, 594, 5329–5342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Evaluation 1: Normothermia | Evaluation 2: Pre-Heating | |||
|---|---|---|---|---|
| Untrained Group (n = 15) | Trained Group (n = 15) | Untrained Group (n = 15) | Trained Group (n = 15) | |
| Weight (kg) | 66.45 ± 8.03 | 80.26 ± 12.44 + | 66.56 ± 8.22 | 80.58 ± 12.34 + |
| Height (m) | 1.74 ± 0.05 | 1.76 ± 0.09 | 1.74 ± 0.05 | 1.76 ± 0.09 |
| BMI | 21.82 ± 2.46 | 24.97 ± 2.21 + | 21.83 ± 2.55 | 25.05 ± 2.17 + |
| BM (Kcal/day) | 1706 ± 111 | 1925 ± 210 + | 1707 ± 121 | 1930 ± 208 + |
| Training (hours/week) | 4.56 ± 0.92 | 9.17 ± 1.02 ++ | 4.72 ± 0.71 | 10.22 ± 0.43 ++ |
| Perimeter-Arm (cm) | 28.97 ± 1.73 | 33.70 ± 2.79 +++ | 28.72 ± 1.75 | 33.73 ± 3.03 +++ |
| Perimeter-Tight (cm) | 51.65 ± 3.16 | 57.57 ± 4.70 ++ | 51.12 ± 4.17 | 57.58 ± 3.99 ++ |
| Perimeter-Calf (cm) | 36.12 ± 2.62 | 38.36 ± 2.27 + | 35.82 ± 2.53 | 38.79 ± 1.93 + |
| Perimeter-Waist (cm) | 75.93 ± 3.96 | 79.04 ± 6.23 | 75.81 ± 4.42 | 79.66 ± 5.43 |
| Perimeter-Hip (cm) | 83.42 ± 7.51 | 85.20 ± 7.31 | 81.66 ± 6.78 | 84.73 ± 6.96 |
| Waist-to-hip index | 0.91 ± 0.06 | 0.92 ± 0.02 | 0.93 ± 0.03 | 0.92 ± 0.02 |
| Fold-Abdominal (cm) | 19.45 ± 8.62 | 18.13 ± 8.44 | 19.71 ± 7.56 | 18.41 ± 9.37 |
| Fold-Suprailiac (cm) | 16.43 ± 7.35 | 17.20 ± 7.76 | 16.01 ± 8.10 | 18.95 ± 7.93 |
| Fold-Subscapular (cm) | 13.31 ± 5.38 | 13.37 ± 4.96 | 13.42 ± 6.34 | 13.51 ± 3.77 |
| Fold-Tricipital (cm) | 11.80 ± 4.78 | 12.16 ± 4.34 | 11.56 ± 4.30 | 13.75 ± 5.75 |
| Fold-Tight(cm) | 17.62 ± 7.64 | 2086 ± 7.83 | 17.35 ± 6.62 | 19.55 ± 6.29 |
| Fold-Twin (cm) | 9.67 ± 4.33 | 11.16 ± 4.39 | 9.15 ± 4.46 | 11.94 ± 5.64 |
| Fat weight (kg) | 8.16 ± 2.61 | 10.45 ± 4.25 | 8.08 ± 2.51 | 10.71 ± 4.34 |
| Fat weight (%) | 12.20 ± 3.48 | 12.27 ± 3.62 | 12.10 ± 3.28 | 12.96 ± 3.51 |
| Muscle weight (kg) | 31.54 ± 4.69 | 38.21 ± 4.25 + | 31.52 ± 5.03 | 38.00 ± 4.57 + |
| Muscle weight (%) | 51.28 ± 2.65 | 51.14 ± 3.06 | 50.29 ± 2.80 | 50.66 ± 3.42 |
| Lean weight (kg) | 58.36 ± 7.21 | 69.81 ± 8.74 + | 58.46 ± 7.22 | 69.86 ± 8.92 + |
| Lean body Mass (%) | 87.02 ± 4.08 | 83.31 ± 3.64 | 86.21 ± 4.50 | 83.55 ± 4.84 |
| Bone weight (kg) | 11.04 ± 0.92 | 12.25 ± 1.89 | 11.03 ± 0.74 | 12.44 ± 1.84 |
| Bone weight (%) | 16.73 ± 1.45 | 15.30 ± 1.19 | 16.70 ± 1.41 | 15.47 ± 0.96 |
| Body water (kg) | 42.16 ± 3.74 | 48.77 ± 6.44 + | 41.96 ± 3.66 | 48.98 ± 5.54 + |
| Body Water (%) | 63.68 ± 2.97 | 60.97 ± 2.68 | 63.32 ± 3.27 | 61.17 ± 3.55 |
| Evaluation 1: Normothermia | Evaluation 2: Pre-Heating | ||||
|---|---|---|---|---|---|
| Pre-RM | Post-RM | Pre-Sauna | Post-Sauna- Pre-RM | Post-RM | |
| Untrained Group (n = 15) | |||||
| Weight (kg) | 66.4 ± 8.0 | 66.4 ± 7.5 | 66.4 ± 8.1 | 66.2 ± 8.1 * | 66.6 ± 8.8 |
| SBP (mmHg) | 125 ± 17 | 136 ± 21 | 120 ± 5 | 123 ± 12 | 122 ± 12 |
| DBP (mmHg) | 73 ± 7 | 78 ± 10 | 73 ± 12 | 73 ± 6 | 68 ± 8 ° |
| Heart Rate (bpm) | 82 ± 17 | 93 ± 19 | 73 ± 12 | 125 ± 20 * | 123 ± 25 ^° |
| Temp-Mouth (°C) | 36.6 ± 0.5 | 37.1 ± 0.7 | 36.9 ± 0.4 | 39.0 ± 0.8 * | 38.2 ± 0.9 *° |
| Temp-Forehead (°C) | 36.7 ± 0.5 | 36.5 ± 0.4 | 36.8 ± 0.4 | 39.6 ± 2.1 * | 36.8 ± 1.4 * |
| Temp-Tight (°C) | 35.7 ± 0.5 | 35.6 ± 0.5 | 34.9 ± 1.8 | 39.1 ± 2.1 * | 36.5 ± 1.5 |
| Trained group (n = 15) | |||||
| Weight (kg) | 80.3 ± 12.4 + | 80.4 ± 10.1 + | 80.5 ± 12.2 + | 80.2 ± 12.2 +** | 80.1 ± 12.4 + |
| SBP (mmHg) | 128 ± 12 | 128 ± 9 | 122 ± 9 | 119 ± 11 | 123 ± 9 |
| DBP (mmHg) | 75 ± 6 | 74 ± 9 | 72 ± 14 | 76 ± 18 | 71 ± 9 ° |
| Heart Rate (bpm) | 73 ± 10 | 84 ± 16 * | 66 ± 7 | 113 ± 19 ** | 106 ± 17 ^^° |
| Temp-Mouth (°C) | 36.4 ± 0.6 | 36.7 ± 0.4 | 36.6 ± 0.5 | 38.5 ± 0.9 ** | 37.3 ± 0.44 +**^^° |
| Temp-Forehead (°C) | 36.7 ± 0.4 | 36.5 ± 0.5 | 36.6 ± 0.5 | 39.8 ± 1.4 ** | 36.3 ± 0.3 ** |
| Temp-Tight (°C) | 34.4 ± 1.9 + | 35.4 ± 0.6 | 34.3 ± 2.3 | 39.4 ± 1.7 ** | 35.7 ± 0.3 **^ |
| Evaluation 1: Normothermia | Evaluation 2: Pre-Heating | Differences. | % Change | R | |
|---|---|---|---|---|---|
| Untrained Group (n = 15) | |||||
| Load (kg) | 161.9 ± 49.7 | 198.8 ± 50.7 * | +36.8 ± 27.6 | 26.9 ± 28.9 | 0.797 |
| Repetitions | 4.3 ± 0.9 | 4.0 ± 1.2 | −0.3 ± 1.4 | −2.3 ± 35.1 | 0.169 |
| RM (kg) | 178.5 ± 56.7 | 217.6 ± 59.2 * | +39.1 ± 34.5 | 26.7 ± 33.5 | 0.798 |
| Relative RM | 2.7 ± 0.6 | 3.2 ± 0.6 * | +0.6 ± 0.6 | 26.4 ± 33.0 | 0.798 |
| Muscular RM | 5.6 ± 1.2 | 6.8 ± 1.1 * | +1.1 ± 1.1 | 23.8 ± 32.3 | 0.797 |
| RPE | 8.0 ± 0.9 | 8.4 ± 0.9 | +0.4 ± 0.7 | 5.1 ± 9.8 | 0.421 |
| Trained group (n = 15) | |||||
| Load (kg) | 257.054.9 ++ | 282.0 ± 59.8 ++** | +25.0 ± 6.5 | 9.9 ± 6.2 + | 0.888 |
| Repetitions | 4.5 ± 0.7 | 4.8 ± 0.4 | +0.3 ± 0.7 | 8.8 ± 17.0 | 0.424 |
| RM (kg) | 285.0 ± 62.4 +++ | 314.9 ± 1.0 +** | +30.0 ± 21.0 | 11.0 ± 7.2 | 0.886 |
| Relative RM | 3.6 ± 0.9 ++ | 4.00 ± 1.9 +** | +0.4 ± 0.3 | 10.5 ± 7.2 | 0.886 |
| Muscular RM | 7.8 ± 1.7 ++ | 8.7 ± 1.9 +** | +0.9 ± 0.5 | 11.4 ± 6.6 | 0.854 |
| RPE | 7.5 ± 0.7 | 8.3 ± 0.8 * | +0.80 ± 1.0 | 11.5 ± 14.7 | 0.630 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartolomé, I.; Toro-Román, V.; Siquier-Coll, J.; Muñoz, D.; Robles-Gil, M.C.; Maynar-Mariño, M. Acute Effect of Exposure to Extreme Heat (100 ± 3 °C) on Lower Limb Maximal Resistance Strength. Int. J. Environ. Res. Public Health 2022, 19, 10934. https://doi.org/10.3390/ijerph191710934
Bartolomé I, Toro-Román V, Siquier-Coll J, Muñoz D, Robles-Gil MC, Maynar-Mariño M. Acute Effect of Exposure to Extreme Heat (100 ± 3 °C) on Lower Limb Maximal Resistance Strength. International Journal of Environmental Research and Public Health. 2022; 19(17):10934. https://doi.org/10.3390/ijerph191710934
Chicago/Turabian StyleBartolomé, Ignacio, Víctor Toro-Román, Jesús Siquier-Coll, Diego Muñoz, María C. Robles-Gil, and Marcos Maynar-Mariño. 2022. "Acute Effect of Exposure to Extreme Heat (100 ± 3 °C) on Lower Limb Maximal Resistance Strength" International Journal of Environmental Research and Public Health 19, no. 17: 10934. https://doi.org/10.3390/ijerph191710934






