1. Introduction
Fetal growth restriction (FGR) is a pregnancy complication that occurs in approximately 10% of pregnancies worldwide. In this complication, fetuses cannot reach their predestinated genetic weight. Its multiple causes could be divided into two groups: due to placental insufficiency supply and non-placental origin (genetics or chromosomal disorders, congenital infections, or metabolic disorders). The classical definition of FGR was a fetus whose estimated weight was below the 10th percentile. Doppler study incorporation allows for differentiation between genetic small for gestational age fetuses and real FGR due to insufficiency of placental supply [
1,
2].
FGR could be divided into two groups depending on its onset, placental ischemic degree, and chronicity: early-onset and late-onset. Early-onset FGR is associated with early and severe ischemic placental involvement. It is associated with a high uterine artery pulsatility index measurement [
3] and an elevated rate of early-onset preeclampsia, close to 70% [
4]. Usually, umbilical artery (UA) Doppler is pathological at diagnosis, and associated with elevated severe neonatal outcomes [
5]. Induced prematurity is a frequent outcome due to fetal deterioration, with high morbidity and mortality rates. Late-onset FGR has the same etiopathogenic basis, although it is a minor, late, and acute condition [
6]. Its association with preeclampsia is less frequent, close to 8–15% [
4,
7]. UA usually is normal at diagnosis, even if they have circulation redistribution as a middle cerebral artery (MCA) or the ratio measurement is abnormal [
7,
8]. Commonly, neonatal morbidity rates are lower, but stillbirth and mortality are higher [
7].
Adverse neonatal outcome in FGR has been exhaustively researched. TRUFFLE study described short-term outcomes in a cohort of fetuses with FGR. Prematurity was strongly associated with adverse neonatal outcomes, being more frequent neonatal sepsis and bronchopulmonary dysplasia. Adverse outcomes were more frequent at a lower gestational age at delivery or associated with hypertensive states [
2].
Childhood and adulthood development could be affected by FGR [
9]. Recently, interest in long-term outcomes is increasing. Specifically, cognitive and motor development, as well as brain structure development, is a primary field of study. The fetal period and early childhood are sensitive stages where genomic interactions with the environment occur as organs and systems acquire their long-term functions. Induced prematurity is a risk factor for adverse neurological outcomes. Those children could be affected by visual and auditory disabilities, coordination disorders, cognitive, and behavioral conditions and, in extreme cases, cerebral palsy [
10]. Adverse neurological outcomes could be due to prematurity or as a consequence of FGR, although this connection is complex to link. In both cases, motor, cognitive and behavioral development could be affected by a hostile environment.
Multiple studies in animal models have demonstrated changes in brain structure. More precisely, induced hypoxia in animals has shown a reduction in neuron quantity [
11] and a significant modification of dendritic arbors [
12]. Other studies have demonstrated a delay in myelin production that could affect normal nervous conduction in the early stages [
13,
14]. This process is crucial to cognitive development in childhood. Furthermore, those changes have been appreciated in different concentrations of metabolites and neurotransmitters [
15,
16,
17]. Studies in human models have also demonstrated a decrease in brain volume [
18,
19,
20], a reduction in grey matter volume [
18,
21,
22], as well as differences in white matter [
23,
24] and gyrification patterns [
25] in FGR children. These modifications could affect cognitive, motor, and behavior development.
Poor cognitive development, behavioral disabilities, and academic difficulties have been related to FGR. Different studies have shown a higher incidence of neurodevelopmental disabilities in premature infants with FGR compared with preterm infants with adequate growth [
26,
27,
28,
29]. Late-onset FGR has also been related to cognitive disabilities and academic difficulties, but published data are contradictory and differences between groups tend to be minimal [
30,
31,
32,
33,
34].
Even though the relationship between Doppler alterations and neonatal outcomes has been well established, the relationship with neurodevelopment has not been yet elucidated. Different studies have tried to link umbilical and middle cerebral artery alterations to cognitive and motor disabilities in childhood [
35,
36,
37]. Classically, brain sparing has been associated with adaptative phenomena, but more recent studies have associated this process with poor cognitive and behavioral development [
38,
39,
40,
41,
42,
43,
44]. In a recent systematic review, we were able to connect poor intelligence quotient results in children with brain sparing, but an association with motor or behavioral disabilities was difficult to link. These could be due to heterogeneity in the studies analyzed, great variety in both specific tests and ages of assessment, different definitions of brain sparing and lack of control of confounders [
45]. In severe cases, the deleterious consequences of brain sparing on neurodevelopment could overpass the benefits of the sparing, leading to a wide spectrum of clinical manifestations [
45].
We believe that FGR causes development disabilities in adaptative, motor, communication, and cognitive spheres during childhood. This study aimed to describe neurodevelopment in FGR children at six years of age. Secondly, we tried to demonstrate influencing factors that can improve or exacerbate this development, as well as predictive factors that might help us select a population at risk to assist with early childhood support.
2. Materials and Methods
2.1. Design and Population
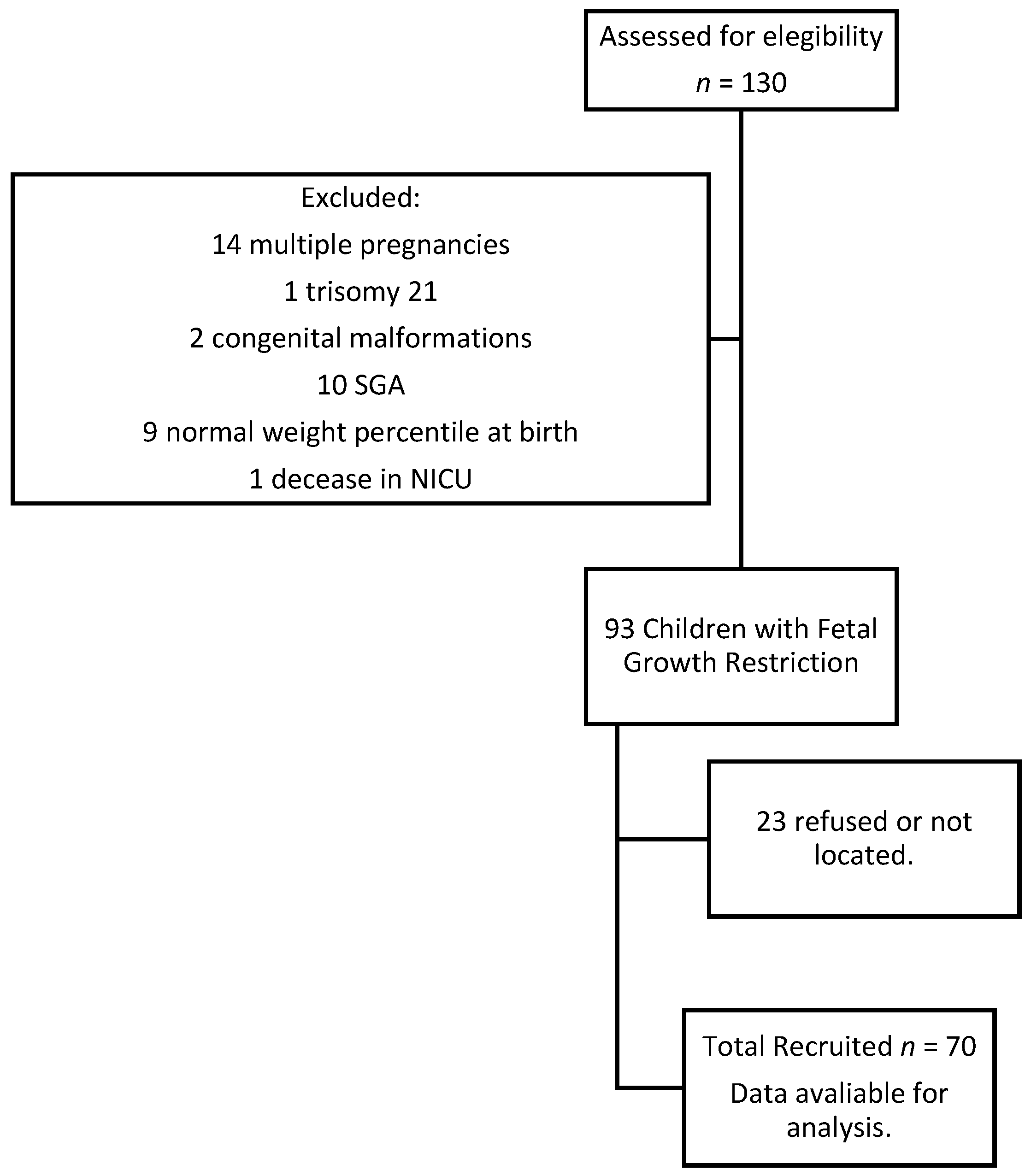
This study was designed as a retrospective cohort study, in which we selected a group of children with fetal growth restriction born in 2015 at University Hospital Carlos Haya, Málaga, Spain. Inclusion criteria were based on FGR definition: less than the 3rd percentile birth weight or less than the 10th percentile birth weight with abnormal hemodynamic Doppler study. The abnormal hemodynamic study was defined as a pulsatility index (PI) of umbilical artery (UA) above the 95th percethe ntile, PI of middle cerebral artery (MCA) below the 5th percentile, cerebroplacental ratio (CPR) below the 5th percentile, or PI of uterine arteries above the 95th percentile. The CPR was calculated by dividing the PI of the MCA by the PI of the UA. A CPR below the 5th percentile was defined as brain sparing effect. Exclusion criteria were structural and chromosomal abnormalities, multiple pregnancies and small for gestational age. Once approval was obtained from the regional ethics committee, recruitment started in 2021. Parental consent was obtained before starting the procedure. Data were collected from clinical records, parents’ reports, and individually assessed children with Battelle Developmental Inventory (screening test).
2.2. Neurodevelopment Follow-Up at 6 Years Old
At the age of six, a Batelle Developmental Inventory (BDI) screening test (Spanish Edition) was performed prospectively [
46]. We selected this screening test because it has a good correlation with the total inventory. The correlation level was 0.96 on all scales, except for the cognitive scale which was 0.92 [
47]. This battery includes 96 items divided into five scales: personal-social, adaptive, motor, communicative and cognitive scale. Finally, a global score was obtained and converted into an equivalent developmental age on each scale. The items are presented in a standardized format, specifying the behavior or characteristic to be evaluated. This evaluation was performed individually and the average time to complete the test was approximately one hour. Information was obtained by means of direct observation, parental interviews, and direct children assessment. The global development quotient was obtained using Moraleda’s formula as follows: dividing the equivalent developmental age by the real age multiplied by 100 [
47]. We considered the upper limit of the range at the final equivalent age of the BDI to calculate this ratio. Children were considered to have a developmental delay if their score was lower than 100 [
48].
2.3. Parent Reports
At the same time that the children were assessed, a questionary was provided to the parents. In this report, they were requested to provide information about sociodemographic items and children’s issues during the first years of life (necessity of early child support, academic difficulties, and illnesses during childhood). Completion of the questionary required circa 15 min.
2.4. Data Collection
Medical records were searched for data about pregnancy and neonatal care during the first days of life. We registered variables about pregnancy care, delivery episode, postpartum, as well as weight, length, and head circumference of the neonate. We recorded the days of neonatal intensive care unit (NICU) admission and adverse neonatal outcomes if this was the case. NICU admission was considered when newborns required invasive care or close monitoring by neonatal pediatricians. Those children who remained with their mothers in the maternity ward were not considered in this group.
2.5. Statistical Analysis
Firstly, we carried out a descriptive analysis to detail the frequency distribution of the different variables in the cohort, as well as the frequency distribution of the developmental delay on each scale and global scale. Secondly, we explored the association between global development quotient and sociodemographic, pregnancy, Doppler measurement, delivery, and neonatal variables using the Student’s t-test or ANOVA test analysis.
Thirdly, multivariate analysis was accomplished to analyze moderating factors. Linear regression analyses were performed to examine the effect of (1) sociodemographic factors, (2) Doppler measurements, birth weight and age at delivery, and (3) postnatal factors. We incorporated all sociodemographic factors (maternal and paternal studies level, socioeconomic status, maternal and paternal occupational status, and separated parent status) in the first model. In the second model we included doppler measurements of UA, MCA, and CPR, birth weight and gestational age at delivery. In the third model, we included gestational age at delivery, gender, adverse neonatal outcomes, early childcare, academic difficulties, and nursery assistance. For all analyses, a p-value below 0.05 was considered significant. Data were processed and analyzed using the Statistical Package for the Social Sciences (SPSS), Version 22.0 (SPSS Inc., Chicago, IL, USA).
4. Discussion
We performed a study to assess the neurodevelopment of children with FGR at 6 years of age. We have demonstrated higher neurodevelopment delay rates in these children. At the same time, we have proved a positive relationship between gestational age at delivery and MCA percentile with the global development quotient.
The relationship between hemodynamics disturbances, prematurity in FGR, and adverse neonatal outcomes has been well established. Assessing neurodevelopment in children with a history of FGR is more intricate. Neurodevelopment is a continuous process with different stages that can be influenced by multiple postnatal factors, both protective and risk factors. In the same way, the severity of hemodynamic alterations in FGR, as well as in prematurity, could involve a deleterious effect in the process. Our global developmental delay was 57.1%, higher than that found in other studies (close to 10–20%) [
35,
49,
50]. This divergence could be due to different ages and methods of assessment. We evaluated the children at a late age (6 years old) by means of BDI, which assesses completely all neurodevelopment areas.
Baschat et al. (2009) found an increased risk of global retardation, cerebral palsy, and neurosensory abnormalities among FGR children with the reverse flow of the UA. They also determined that birthweight, gestational age at delivery, and neonatal adverse outcomes were strong predictors of adverse neurodevelopment [
35]. In our bivariate analysis, similar results were found. A significantly lower gestational age and birth weight were found in children with developmental delays. Length of stay in the NICU was longer in children with developmental delays. We only could find a relationship between NRDS, neonatal sepsis, and BPD with worse scores in the global development coefficient. However, we could not associate perinatal outcomes with the global neurodevelopment coefficient in the multivariate analysis. This finding could be due to the low perinatal morbidity rates recorded in our study cohort.
We found that prematurity and brain sparing could be risk factors for impaired neurodevelopment. We saw a statistical association between global development quotient and gestational age at delivery and MCA pulsatility index percentile. Previous studies have related prematurity with poor neurodevelopment. In the GRIT study, the investigators evaluated the possibility of immediate or late delivery, always under safety conditions for the fetus. They found similar mortality rates in both groups. When they assessed neurodevelopment at 2 years of age, they observed comparable rates of neurodevelopment disabilities. However, when they evaluated extreme prematurity (24–30 weeks) they encountered higher rates of neurodevelopment disabilities in the immediate delivery group than in the late delivery one (13% versus 5%), as well as a poorer development quotient. A tendency to reduce morbidity and mortality in late delivery was noted [
51]. When they assessed children at 6–13 years of age, they could not find differences in motor, cognitive development, or behavioral disturbances [
52].
Therefore, in early-onset FGR, prematurity plays a role in neurodevelopment, mainly in psychomotor development, independently of the severity of the FGR and hemodynamic disturbances [
49]. The impact on psychomotor development is more important in extreme prematurity (before 28–29 weeks of gestation) [
50,
53]. Besides cerebral palsy rate is between 4–18% before 32 weeks of gestational age, being higher in early prematurity [
35,
53]. In the same manner, Guellec et al. (2011) found a higher impact on cognitive development in FGR fetuses born before 28 weeks (37.5%) than in older fetuses. However, the result was similar to fetuses with adequate weight born before 28 weeks of gestation (38.2%). This result was non-significant [
53]. Our percentage of cognitive delay was 17.1% across the entire cohort. For us, psychomotor and cognitive consequences are difficult to demonstrate because our prematurity rate before the 28th week of gestation was 2.9%.
Contrary, in late-onset FGR, gestational age is not a determinant for neurodevelopment. The DIGITAT study found that birthweight below the 2.3rd percentile is the strongest predictor for abnormal neurodevelopment in fetuses born between 36–41 weeks. They conclude that expectant management could deteriorate birthweight and neurodevelopment at 2 years of age [
54].
The brain sparing effect is more controversial. Classically, brain sparing has been defined as a protective phenomenon by means of which the brain obtains nutrients and oxygen for the maintenance of its proper function. Recent studies have demonstrated the contrary. Brain sparing is a risk factor for brain development, specifically for adaptative, motor, communicative and cognitive development. Brain sparing has been related to smaller head circumferences [
43] and smaller brain volume at delivery [
55,
56]. We could not find this relationship in our cohort, in which head circumference was similar to non-brain-sparing FGR fetuses at delivery (data not shown).
Scherjon’s group study could not relate the umbilical-cerebral ratio (UCR) with cognitive disabilities at 12 months and three years of age. However, these children had higher hyperactivity disorder rates and fewer words in their vocabulary. When children were assessed at five years of age, they could find visual potentials suggesting worse maturation profile and slower responses, as well as poor cognitive development with lower scores in intelligence quotient [
41,
42,
43]. They only could find differences in behavior at the age of 11 [
44].
Other studies have shown cognitive disturbances in brain sparing group at early ages: lower scores in habituation, motor, social interaction, and attention areas at birth [
57]; poorest cognitive development at 2 years old [
55] and at 3 years of age [
40]. Monteith et al. (2019) could demonstrate that FGR with brain sparing resulted in lower scores in motor development tests than FGR without this condition [
40].
In our systematic review, published the last year, we could connect poor intelligence quotient results to brain sparing in children with FGR. The relationship between brain sparing and motor or behavioral disabilities was difficult to assess. A good reason for that lies in methodological differences as children were assessed at different ages, when disabilities might not have yet appeared or may have already improved. On the other hand, the lack of control of the confounder could affect the results [
45]. Our findings in this cohort are consistent with this trend. We have found that the MCA pulsatility index is positively related to the global development quotient.
Beukers et al. (2017) and Richter et al. (2020) could not find any association between brain sparing and cognitive development. They could not find differences in intelligence quotient either [
58,
59]. Specifically, the umbilico-cerebral ratio was not associated with adverse outcomes, and birth weight and sociodemographic variables seemed to take a more important role [
58]. Although we reached a relationship between MCA and the global neurodevelopment quotient, our cognitive delay rate was low (17.1%).
We noted a worse quotient in those children with an AU pulsatility index above the 95th percentile. However, we could not link the AU percentile to this quotient in the multivariate analysis. This could be due to fetuses with AU pathological measurements usually being preterm, with significant deterioration. Prematurity could be more decisive for neurodevelopment than the measurement of the UA pulsatility index itself. Studies have shown controversy about this connection. Some studies determine that it is a good predictor of early neurological complications or cerebral palsy but not for adverse cognitive outcomes [
37,
60,
61]. Other studies relate it to worse cognitive development and motor outcomes, as well as an increased rate of cerebral palsy, especially in cases where diastolic flow is absent or reversed [
36,
62]. We could not prove it. We only had 12 cases of absent end diastolic flow velocity in the umbilical artery. At the same time, non-cerebral palsy was found in our cohort. A larger sample size would be necessary to demonstrate an association between the measurement of UA PI and its characteristics and neurodevelopmental outcomes.
Despite the importance of fetal maturation, neurodevelopment is a complex and continuous process in which multiple factors could influence the progress. In our study, we have associated higher socioeconomic status with better global development quotients. Other studies identified a higher parental educational level [
63,
64] and socioeconomic status [
58,
65] as linked to better cognitive results. These findings could be due to higher implications and knowledge of the neurological stimulation process by parents.
On the other hand, we could not identify early stimulation in early child support as a protective factor in this group of children. Initially, we have recognized a negative association between early child support, academic disabilities, and global development quotient. These findings could be due to children attending early stimulation being a group with severe FGR and prematurity with worse results. But finally, early stimulation is a protective factor for this subgroup of children in a certain way. Other studies could identify early stimulation as a protective factor [
64].
We could not relate breastfeeding as a protective factor or postpartum depression as a risk factor against other studies that succeeded to do so. Breastfeeding has been associated with better cognitive development in term and preterm infants, specifically when its use is prolonged. However, this effect is moderate when confounders are adjusted [
66]. Nevertheless, breastfeeding improves neurological development, being the effect more powerful in low-birth-weight children [
67] and children with lower cognitive test scores [
68]. A systematic review has shown worse cognitive, language, and behavioral development in children with mothers affected by postpartum depression [
69]. Postpartum depression is usually associated with worse caregiving, affecting thus neurodevelopment. Its implications for motor development are more controversial. We could not find any link between these variables and global development. Although children with breastfeeding or non-depression postpartum had better scores, this difference was minimal and non-significant. Further studies and larger sample sizes are necessary to demonstrate these associations.