Removal of Polytungstate from Mine Wastewater Using a Flat Renewal Membrane Reactor with N1633 as a Carrier
Abstract
:1. Introduction
2. Materials and Methods
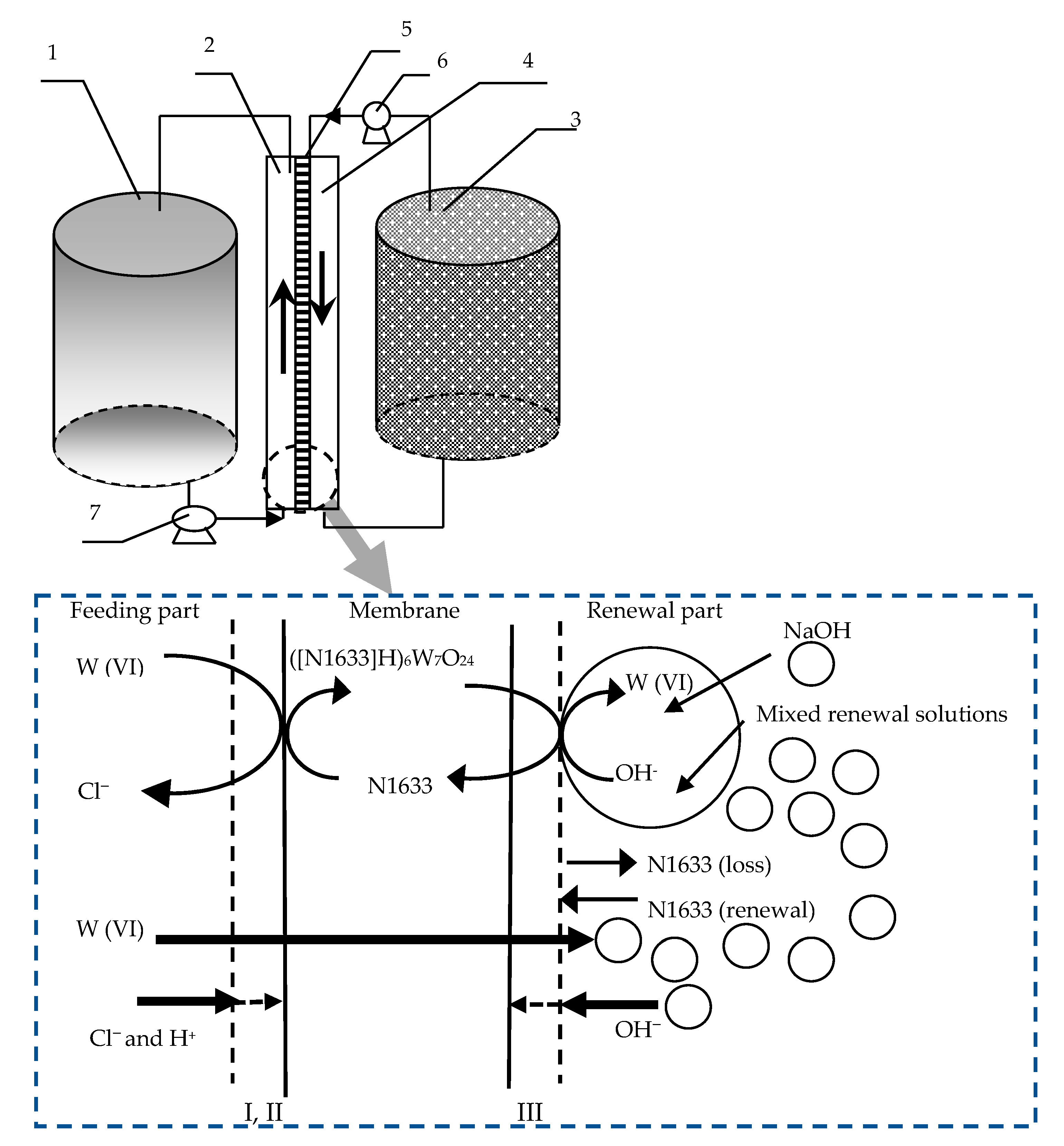
2.1. The Design of the FRMR and Reaction Mechanisms
2.2. Materials and Reagent
2.3. Test Method
2.4. Experimental Procedure
3. Results and Discussion
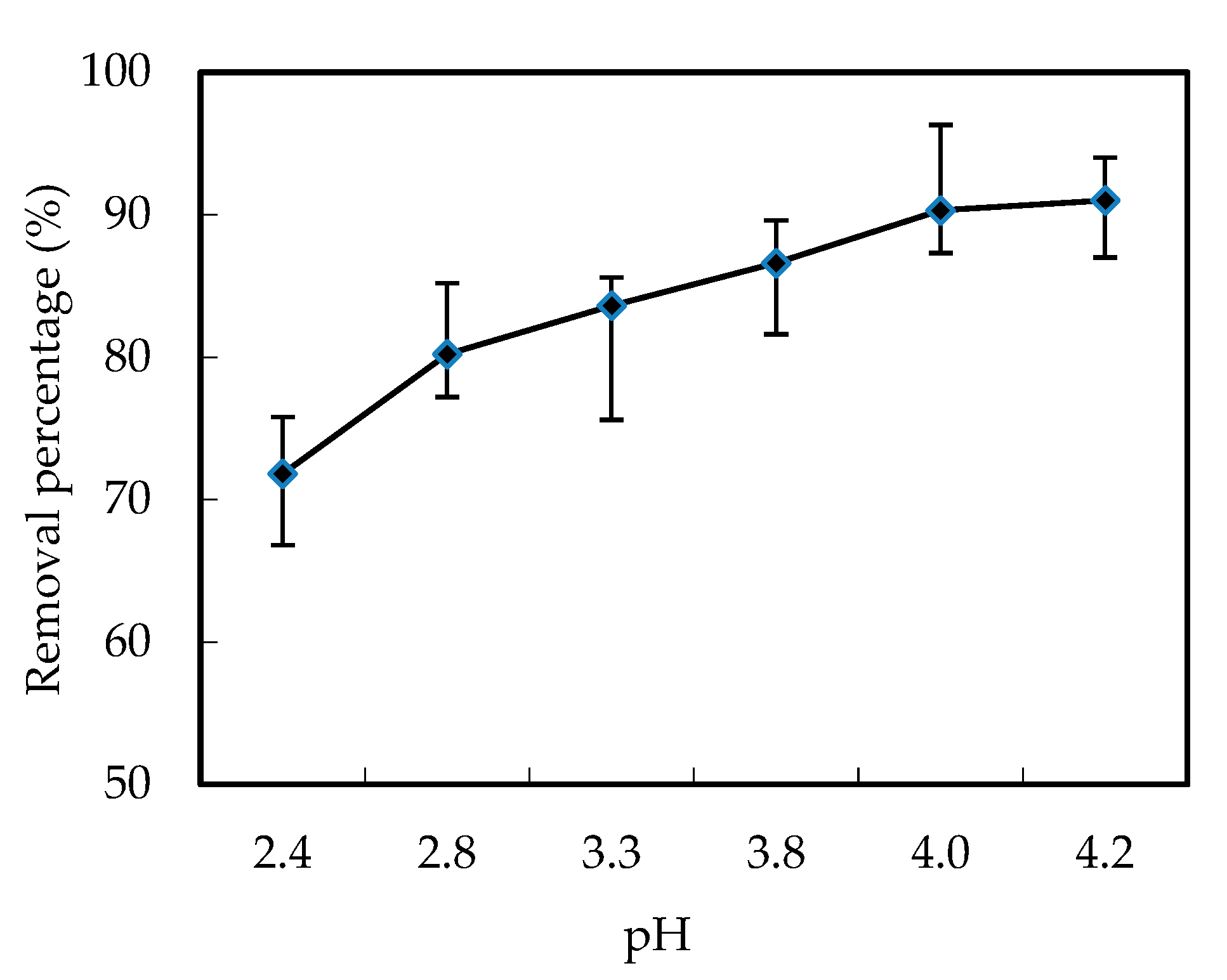
3.1. Effects of pH and Ion Strength in the Feeding Cell
3.2. Effects of the Volume Ratio and Carrier Concentration in the Renewal Cell
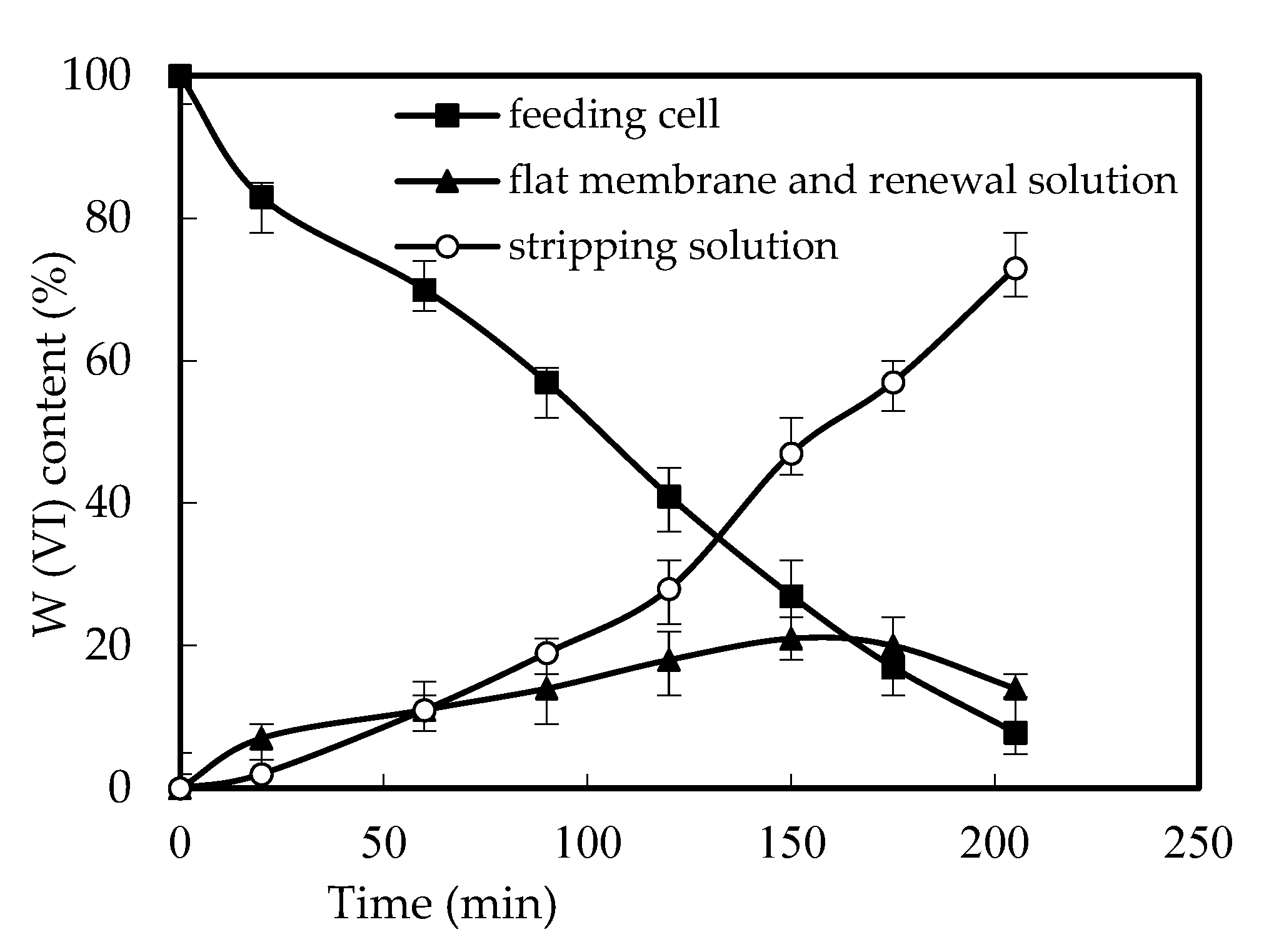
3.3. Effects of W (VI) Retention and the Reuse of the Membrane
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tejado-Ramos, J.-J.; Chocarro-Leon, M.; Barrero-Bejar, I.; Valverde-Calvo, A.; Ferreras-Moreno, M.; Giraldo-Pavon, F.; Tarragona-Perez, C. Enhancement of the sustainability of wolfram mining using drone remote sensing technology. Remote Sens. Appl. -Soc. Environ. 2021, 23, 100542. [Google Scholar] [CrossRef]
- Zoroddu, M.A.; Medici, S.; Peana, M.; Nurchi, V.M.; Lachowicz, J.I.; Laulicht-Glick, F.; Costa, M. Tungsten or Wolfram: Friend or Foe? Curr. Med. Chem. 2018, 25, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Oliver, W.C.; Pharr, G.M. An Improved Technique For Determining Hardness And Elastic-Modulus Using Load And Displacement Sensing Indentation Experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Park, J.H.; Han, H.-J. Effect of tungsten-resistant bacteria on uptake of tungsten by lettuce and tungsten speciation in plants. J. Hazard. Mater. 2019, 379, 120825. [Google Scholar] [CrossRef]
- Bailey, S.E.; Olin, T.J.; Bricka, R.M.; Adrian, D.D. A review of potentially low-cost sorbents for heavy metals. Water Res. 1999, 33, 2469–2479. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, Z.; Zhi, C. Analysis of Structural Superimposed Halos and Ore Prospecting Prediction of the Xiaoliugou Wolfram-Molybdenum Polymetallic Ore Field in Gansu Province. Geol. Explor. 2016, 52, 874–884. [Google Scholar]
- Plattes, M.; Bertrand, A.; Schmitt, B.; Sinner, J.; Verstraeten, F.; Welfring, J. Removal of tungsten oxyanions from industrial wastewater by precipitation, coagulation and flocculation processes. J. Hazard. Mater. 2007, 148, 613–615. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, K.; Mo, L.; Li, J.; Xu, J. Removal of tungsten from electroplating wastewater by acid- and heat-treated sepiolite. Desalination Water Treat. 2015, 56, 232–238. [Google Scholar] [CrossRef]
- Shen, L.; Li, X.; Lindberg, D.; Taskinen, P. Tungsten extractive metallurgy: A review of processes and their challenges for sustainability. Miner. Eng. 2019, 142, 105934. [Google Scholar] [CrossRef]
- Han, Z.; Golev, A.; Edraki, M. A Review of Tungsten Resources and Potential Extraction from Mine Waste. Minerals 2021, 11, 701. [Google Scholar] [CrossRef]
- Tkaczyk, A.H.; Bartl, A.; Amato, A.; Lapkovskis, V.; Petranikova, M. Sustainability evaluation of essential critical raw materials: Cobalt, niobium, tungsten and rare earth elements. J. Phys. D-Appl. Phys. 2018, 51, 203001. [Google Scholar] [CrossRef] [Green Version]
- Yang, X. Beneficiation studies of tungsten ores—A review. Miner. Eng. 2018, 125, 111–119. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Z.; Huo, G. Deep separation of resemble elements. Chin. J. Nonferrous Met. 2003, 13, 234–240. [Google Scholar]
- Lende, A.B.; Kulkarni, P.S. Selective recovery of tungsten from printed circuit board recycling unit wastewater by using emulsion liquid membrane process. J. Water Process. Eng. 2015, 8, 75–81. [Google Scholar] [CrossRef]
- Orefice, M.; Nguyen, V.T.; Raiguel, S.; Jones, P.T.; Binnemans, K. Solvometallurgical Process for the Recovery of Tungsten from Scheelite. Ind. Eng. Chem. Res. 2022, 61, 754–764. [Google Scholar] [CrossRef]
- Adams, E.; Chaban, V.; Khandelia, H.; Shin, R. Selective chemical binding enhances cesium tolerance in plants through inhibition of cesium uptake. Sci. Rep. 2015, 5, 1–10. [Google Scholar]
- Riedl, W. Membrane-Supported Liquid-Liquid Extraction—Where Do We Stand Today? Chembioeng Rev. 2021, 8, 6–14. [Google Scholar] [CrossRef]
- Youzhi, L.I.U.; Deyu, Z.; Weizhou, J. Further development of phenol removal by emulsion liquid membrane. Membr. Sci. Technol. 2006, 26, 66–71. [Google Scholar]
- Volkov, A.V.; Korneeva, G.A.; Tereshchenko, G.F. Organic Solvent Nanofiltration: Prospects and Applications. Russ. Chem. Rev. 2008, 77, 983. [Google Scholar] [CrossRef]
- Chen, D.; Sirkar, K.K.; Jin, C.; Singh, D.; Pfeffer, R. Membrane-Based Technologies in the Pharmaceutical Industry and Continuous Production of Polymer-Coated Crystals/Particles. Curr. Pharm. Des. 2017, 23, 242–249. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Li, D.-S.; Bu, X.; Feng, P. Metal-Organic Frameworks for Separation. Adv. Mater. 2018, 30, 1705189. [Google Scholar] [CrossRef] [PubMed]
- Albaraka, Z. Carrier-mediated liquid membrane systems for lead (II) ion separations. Chem. Pap. 2020, 74, 77–88. [Google Scholar] [CrossRef]
- Pei, L.; Wang, L.M.; Ma, Z.Y. Modeling of Ce(IV) transport through a dispersion flat combined liquid membrane with carrier P507. Front. Environ. Sci. Eng. 2014, 8, 503–509. [Google Scholar] [CrossRef]
- Pei, L.; Yao, B.H.; Zhang, C.J. Transport of Tm(III) through dispersion supported liquid membrane containing PC-88A in kerosene as the carrier. Sep. Purif. Technol. 2009, 65, 220–227. [Google Scholar] [CrossRef]
- Valenzuela, F.; Covarrubias, C.; Martinez, C.; Smith, P.; Diaz-Dosque, M.; Yazdani-Pedram, M. Preparation and bioactive properties of novel bone-repair bionanocomposites based on hydroxyapatite and bioactive glass nanoparticles. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1672–1682. [Google Scholar] [CrossRef]
- Yang, X.J.; Fane, A.G.; MacNaughton, S. Removal and recovery of heavy metals from wastewaters by supported liquid membranes. Water Sci. Technol. 2001, 43, 341–348. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, L.; Duo, J.; Chu, L. Removal of Polytungstate from Mine Wastewater Using a Flat Renewal Membrane Reactor with N1633 as a Carrier. Int. J. Environ. Res. Public Health 2022, 19, 11092. https://doi.org/10.3390/ijerph191711092
Pei L, Duo J, Chu L. Removal of Polytungstate from Mine Wastewater Using a Flat Renewal Membrane Reactor with N1633 as a Carrier. International Journal of Environmental Research and Public Health. 2022; 19(17):11092. https://doi.org/10.3390/ijerph191711092
Chicago/Turabian StylePei, Liang, Jia Duo, and Linlin Chu. 2022. "Removal of Polytungstate from Mine Wastewater Using a Flat Renewal Membrane Reactor with N1633 as a Carrier" International Journal of Environmental Research and Public Health 19, no. 17: 11092. https://doi.org/10.3390/ijerph191711092




