The EDN1 Missense Variant rs5370G > T Regulates Adaptation and Maladaptation under Hypobaric Hypoxia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Clinical Characteristics
2.3. Selection and Genotyping of Polymorphism
2.4. Quantitative RT-PCR
2.5. Site-Directed Mutagenesis of EDN1
2.6. Cell Culture, Transient Transfections and Hypoxia Treatment
2.7. Quantification of Circulating Plasma and Cellular ET-1 Levels
2.8. Statistical Analyses
2.9. In Silico Analyses
2.9.1. Data Collection
2.9.2. Sequence-Based Analysis
2.9.3. Aggregation Propensity, Conservation and Frustration Analysis
2.9.4. Molecular Dynamics Simulations
3. Results
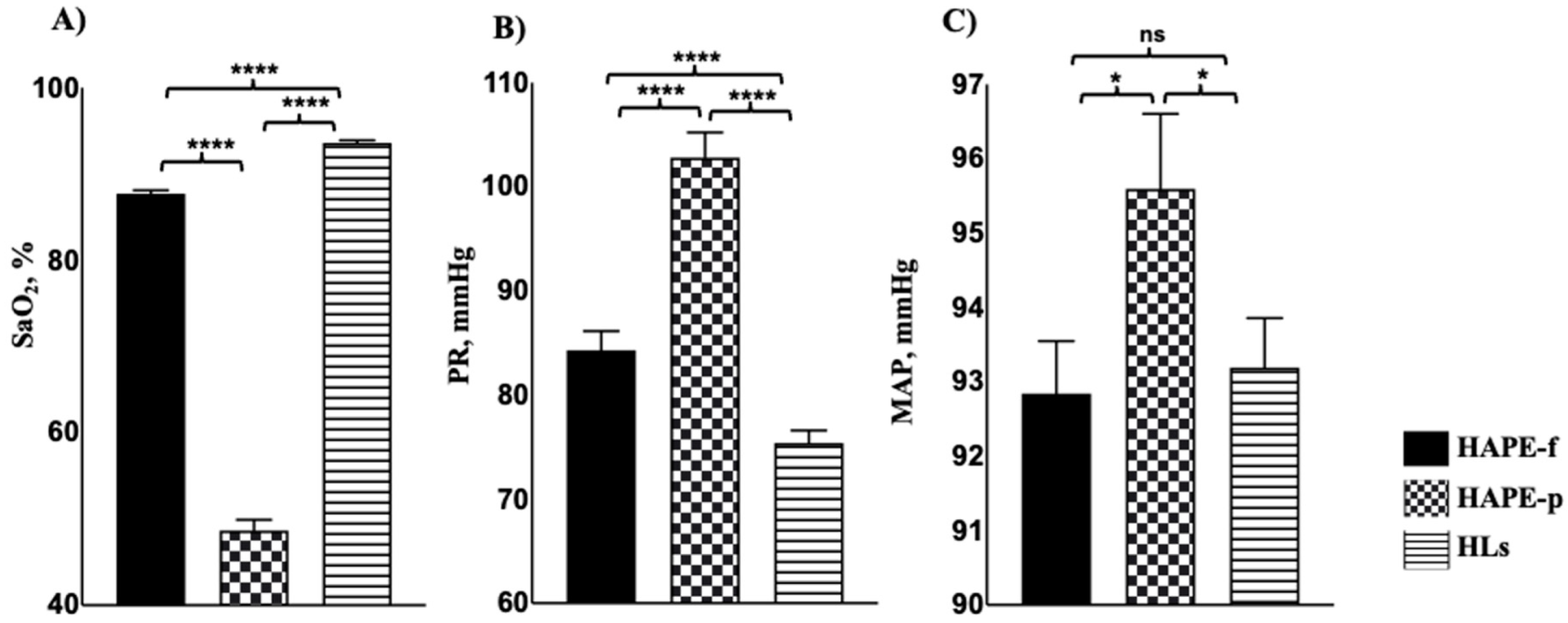
3.1. Clinical Assessment Showed Significant Variations in Patients
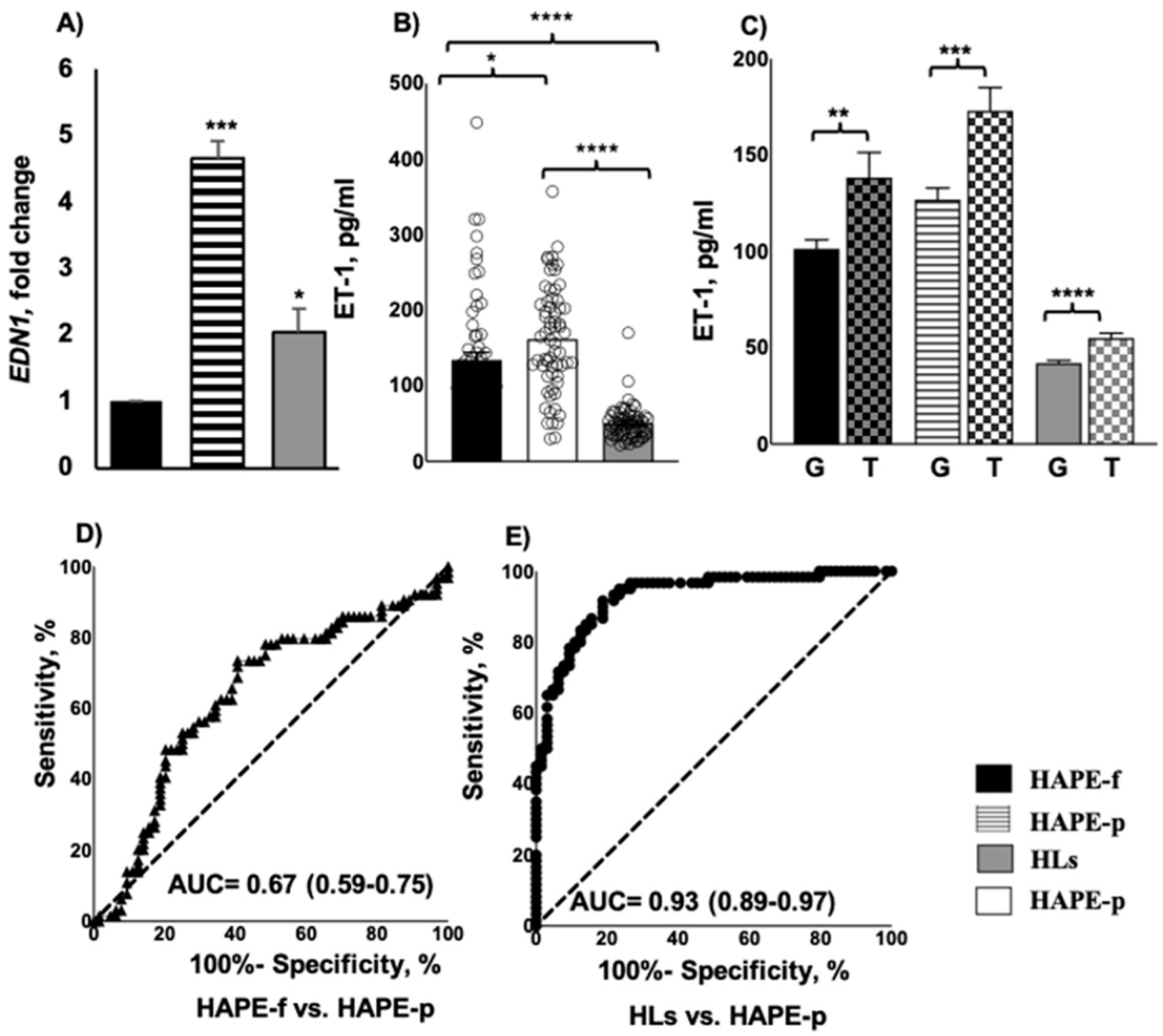
3.2. Genotype and Allele Distribution Differed in Patients and Healthy Subjects
3.3. Elevated ET-1 Expression and Protein Levels Associated with HAPE
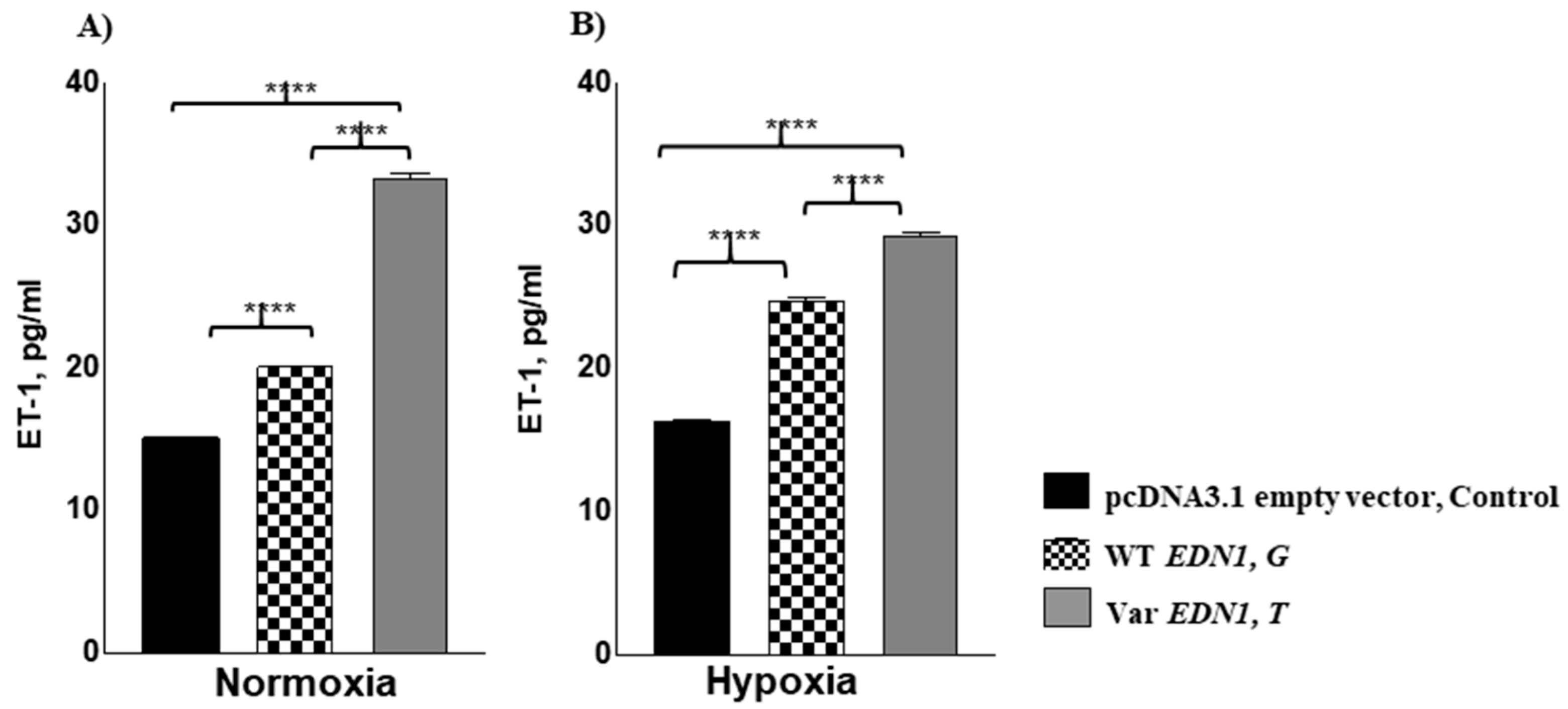
3.4. Allele rs5370T Associated with Enhanced Cellular ET1 Levels under Normoxia and Hypoxia
3.5. Sequence-Based Analysis Favours a Deleterious Effect of the Variant Allele
3.6. Aggregation Propensity, Conservation and Frustration Analyses
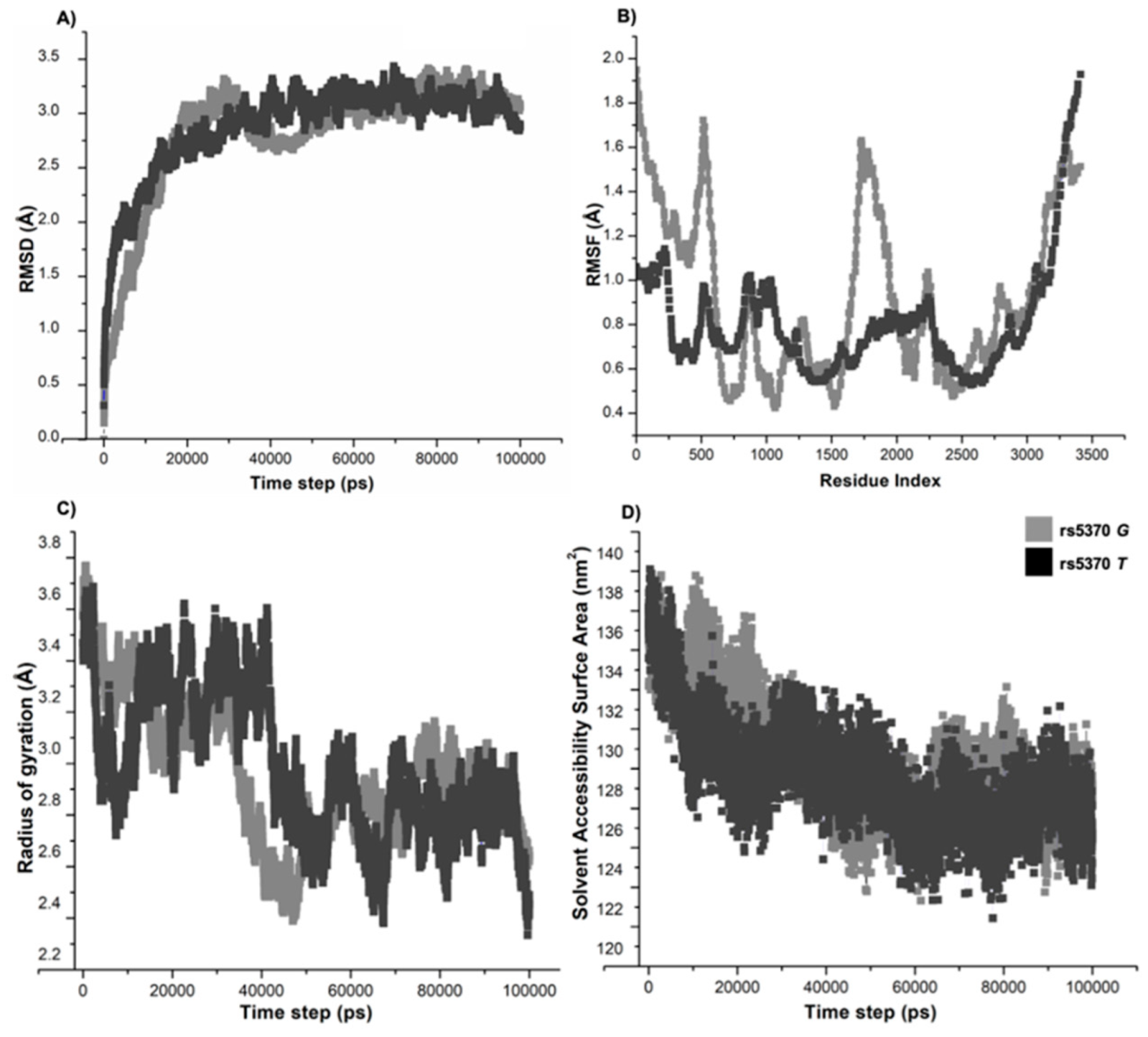
3.7. Molecular Dynamics Simulations Confirmed the Structural Flexibility of 198N Mutation
4. Discussion
5. Conclusions
6. Limitation of the Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Scheinfeldt, L.B.; Soi, S.; Thompson, S.; Ranciaro, A.; Woldemeskel, D.; Beggs, W.; Lambert, C.; Jarvis, J.P.; Abate, D.; Belay, G.; et al. Genetic Adaptation to High Altitude in the Ethiopian Highlands. Genome Biol. 2012, 13, R1. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.G. Measuring High-Altitude Adaptation. J. Appl. Physiol. 2017, 123, 1371–1385. [Google Scholar] [CrossRef]
- Beall, C.M.; Decker, M.J.; Brittenham, G.M.; Kushner, I.; Gebremedhin, A.; Strohl, K.P. An Ethiopian Pattern of Human Adaptation to High-Altitude Hypoxia. Proc. Natl. Acad. Sci. USA 2002, 99, 17215–17218. [Google Scholar] [CrossRef] [PubMed]
- Beall, C.M. Two Routes to Functional Adaptation: Tibetan and Andean High-Altitude Natives. Proc. Natl. Acad. Sci. USA 2007, 104, 8655–8660. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, A.; Mohd, G.; Norboo, T.; Baig, M.A.; Qadar Pasha, M.A. Heterozygotes of NOS3 Polymorphisms Contribute to Reduced Nitrogen Oxides in High-Altitude Pulmonary Edema. Chest 2006, 130, 1511–1519. [Google Scholar] [CrossRef]
- Hultgren, H.N. High-Altitude Pulmonary Edema: Current Concepts. Annu. Rev. Med. 1996, 47, 267–284. [Google Scholar] [CrossRef]
- West, J.B. High-Altitude Medicine. Am. J. Respir. Crit. Care Med. 2012, 186, 1229–1237. [Google Scholar] [CrossRef]
- Bärtsch, P.; Mairbäurl, H.; Maggiorini, M.; Swenson, E.R. Physiological Aspects of High-Altitude Pulmonary Edema. J. Appl. Physiol. 2005, 98, 1101–1110. [Google Scholar] [CrossRef]
- Qadar Pasha, M.A.; Newman, J.H. High-Altitude Disorders: Pulmonary Hypertension: Pulmonary Vascular Disease: The Global Perspective. Chest 2010, 137, 13S–19S. [Google Scholar] [CrossRef]
- O’Brien, R.F.; Robbins, R.J.; McMurtry, I.F. Endothelial Cells in Culture Produce a Vasoconstrictor Substance. J. Cell. Physiol. 1987, 132, 263–270. [Google Scholar] [CrossRef]
- Schneider, M.P.; Boesen, E.I.; Pollock, D.M. Contrasting Actions of Endothelin ETA and ETB Receptors in Cardiovascular Disease. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 731–759. [Google Scholar] [CrossRef]
- Hon, W.-C.; Wilson, M.I.; Harlos, K.; Claridge, T.D.W.; Schofield, C.J.; Pugh, C.W.; Maxwell, P.H.; Ratcliffe, P.J.; Stuart, D.I.; Jones, E.Y. Structural Basis for the Recognition of Hydroxyproline in HIF-1 Alpha by PVHL. Nature 2002, 417, 975–978. [Google Scholar] [CrossRef] [PubMed]
- Pullamsetti, S.S.; Mamazhakypov, A.; Weissmann, N.; Seeger, W.; Savai, R. Hypoxia-Inducible Factor Signaling in Pulmonary Hypertension. J. Clin. Investig. 2020, 130, 5638–5651. [Google Scholar] [CrossRef]
- Maeda, S.; Miyauchi, T.; Iemitsu, M.; Tanabe, T.; Irukayama-Tomobe, Y.; Goto, K.; Yamaguchi, I.; Matsuda, M. Involvement of Endogenous Endothelin-1 in Exercise-Induced Redistribution of Tissue Blood Flow: An Endothelin Receptor Antagonist Reduces the Redistribution. Circulation 2002, 106, 2188–2193. [Google Scholar] [CrossRef]
- Legakis, I.; Mantzouridis, T.; Mountokalakis, T. Increased Endothelin-1 Levels in Athletes. Br. J. Sports Med. 2003, 37, 92. [Google Scholar] [CrossRef][Green Version]
- Barrett-O’Keefe, Z.; Ives, S.J.; Trinity, J.D.; Morgan, G.; Rossman, M.J.; Donato, A.J.; Runnels, S.; Morgan, D.E.; Gmelch, B.S.; Bledsoe, A.D.; et al. Taming the “Sleeping Giant”: The Role of Endothelin-1 in the Regulation of Skeletal Muscle Blood Flow and Arterial Blood Pressure during Exercise. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H162–H169. [Google Scholar] [CrossRef]
- Semenza, G.L. Hypoxia-Inducible Factor 1 and Cardiovascular Disease. Annu. Rev. Physiol. 2014, 76, 39–56. [Google Scholar] [CrossRef]
- Minchenko, A.; Caro, J. Regulation of Endothelin-1 Gene Expression in Human Microvascular Endothelial Cells by Hypoxia and Cobalt: Role of Hypoxia Responsive Element. Mol. Cell. Biochem. 2000, 208, 53–62. [Google Scholar] [CrossRef]
- Gampenrieder, S.P.; Hufnagl, C.; Brechelmacher, S.; Huemer, F.; Hackl, H.; Rinnerthaler, G.; Romeder, F.; Monzo Fuentes, C.; Morre, P.; Hauser-Kronberger, C.; et al. Endothelin-1 Genetic Polymorphism as Predictive Marker for Bevacizumab in Metastatic Breast Cancer. Pharm. J. 2017, 17, 344–350. [Google Scholar] [CrossRef]
- Li, J.; Yin, W.; Liu, M.-S.; Mao, L.-J.; Wang, X.-H. Potential Correlation between EDN1 Gene Polymorphisms with Preeclampsia. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1602–1608. [Google Scholar] [CrossRef] [PubMed]
- Panoulas, V.F.; Douglas, K.M.J.; Smith, J.P.; Taffé, P.; Stavropoulos-Kalinoglou, A.; Toms, T.E.; Elisaf, M.S.; Nightingale, P.; Kitas, G.D. Polymorphisms of the Endothelin-1 Gene Associate with Hypertension in Patients with Rheumatoid Arthritis. Endothelium 2008, 15, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sui, R. Effect of SNP Polymorphisms of EDN1, EDNRA, and EDNRB Gene on Ischemic Stroke. Cell Biochem. Biophys. 2014, 70, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Charu, R.; Stobdan, T.; Ram, R.B.; Khan, A.P.; Norboo, T.; Afrin, F.; Qadar Pasha, M.A. Susceptibility to High Altitude Pulmonary Oedema: Role of ACE and ET-1 Polymorphisms. Thorax 2006, 61, 1011–1012. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Sim, N.-L.; Kumar, P.; Hu, J.; Henikoff, S.; Schneider, G.; Ng, P.C. SIFT Web Server: Predicting Effects of Amino Acid Substitutions on Proteins. Nucleic Acids Res. 2012, 40, W452–W457. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Chan, A.P. PROVEAN Web Server: A Tool to Predict the Functional Effect of Amino Acid Substitutions and Indels. Bioinformatics 2015, 31, 2745–2747. [Google Scholar] [CrossRef]
- Adzhubei, I.; Jordan, D.M.; Sunyaev, S.R. Predicting Functional Effect of Human Missense Mutations Using PolyPhen-2. Curr. Protoc. Hum. Genet. 2013, 76, 7.20.1–7.20.41. [Google Scholar] [CrossRef]
- Paladin, L.; Piovesan, D.; Tosatto, S.C.E. SODA: Prediction of Protein Solubility from Disorder and Aggregation Propensity. Nucleic Acids Res. 2017, 45, W236–W240. [Google Scholar] [CrossRef]
- Ashkenazy, H.; Abadi, S.; Martz, E.; Chay, O.; Mayrose, I.; Pupko, T.; Ben-Tal, N. ConSurf 2016: An Improved Methodology to Estimate and Visualize Evolutionary Conservation in Macromolecules. Nucleic Acids Res. 2016, 44, W344–W350. [Google Scholar] [CrossRef]
- Rausch, A.O.; Freiberger, M.I.; Leonetti, C.O.; Luna, D.M.; Radusky, L.G.; Wolynes, P.G.; Ferreiro, D.U.; Parra, R.G. FrustratometeR: An R-Package to Compute Local Frustration in Protein Structures, Point Mutants and MD Simulations. Bioinformatics 2021, 37, 3038–3040. [Google Scholar] [CrossRef]
- Sastry, G.M.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and Ligand Preparation: Parameters, Protocols, and Influence on Virtual Screening Enrichments. J. Comput. Aided Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and Testing of the OPLS All-Atom Force Field on Conformational Energetics and Properties of Organic Liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Harrach, M.F.; Drossel, B. Structure and Dynamics of TIP3P, TIP4P, and TIP5P Water near Smooth and Atomistic Walls of Different Hydroaffinity. J. Chem. Phys. 2014, 140, 174501. [Google Scholar] [CrossRef]
- Parrinello, M.; Rahman, A. Polymorphic Transitions in Single Crystals: A New Molecular Dynamics Method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle Mesh Ewald: An N⋅log(N) Method for Ewald Sums in Large Systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Rajput, C.; Najib, S.; Norboo, T.; Afrin, F.; Qadar Pasha, M.A. Endothelin-1 Gene Variants and Levels Associate with Adaptation to Hypobaric Hypoxia in High-Altitude Natives. Biochem. Biophys. Res. Commun. 2006, 341, 1218–1224. [Google Scholar] [CrossRef]
- Sartori, C.; Vollenweider, L.; Löffler, B.M.; Delabays, A.; Nicod, P.; Bärtsch, P.; Scherrer, U. Exaggerated Endothelin Release in High-Altitude Pulmonary Edema. Circulation 1999, 99, 2665–2668. [Google Scholar] [CrossRef]
- Barker, K.R.; Conroy, A.L.; Hawkes, M.; Murphy, H.; Pandey, P.; Kain, K.C. Biomarkers of Hypoxia, Endothelial and Circulatory Dysfunction among Climbers in Nepal with AMS and HAPE: A Prospective Case-Control Study. J. Travel. Med. 2016, 23, taw005. [Google Scholar] [CrossRef][Green Version]
- Ali, Z.; Mishra, A.; Kumar, R.; Alam, P.; Pandey, P.; Ram, R.; Thinlas, T.; Mohammad, G.; Qadar Pasha, M.A. Interactions among Vascular-Tone Modulators Contribute to High Altitude Pulmonary Edema and Augmented Vasoreactivity in Highlanders. PLoS ONE 2012, 7, e44049. [Google Scholar] [CrossRef]
- Mishra, A.; Mohammad, G.; Norboo, T.; Newman, J.H.; Qadar Pasha, M.A. Lungs at High-Altitude: Genomic Insights into Hypoxic Responses. J. Appl. Physiol. 2015, 119, 1–15. [Google Scholar] [CrossRef]
- Li, L.; Fink, G.D.; Watts, S.W.; Northcott, C.A.; Galligan, J.J.; Pagano, P.J.; Chen, A.F. Endothelin-1 Increases Vascular Superoxide via Endothelin(A)-NADPH Oxidase Pathway in Low-Renin Hypertension. Circulation 2003, 107, 1053–1058. [Google Scholar] [CrossRef]
- Sud, N.; Black, S.M. Endothelin-1 Impairs Nitric Oxide Signaling in Endothelial Cells Through a Protein Kinase Cδ-Dependent Activation of STAT3 and Decreased Endothelial Nitric Oxide Synthase Expression. DNA Cell Biol. 2009, 28, 543–553. [Google Scholar] [CrossRef]
- Burtscher, J.; Millet, G.P.; Burtscher, M. Sex-Dependent Blood Pressure Regulation in Acute Hypoxia. Hypertens. Res. 2021, 44, 1689. [Google Scholar] [CrossRef] [PubMed]
- Droma, Y.; Hayano, T.; Takabayashi, Y.; Koizumi, T.; Kubo, K.; Kobayashi, T.; Sekiguchi, M. Endothelin-1 and Interleukin-8 in High Altitude Pulmonary Oedema. Eur. Respir. J. 1996, 9, 1947–1949. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.M.; Dehnert, C.; Bailey, D.M.; Luks, A.M.; Menold, E.; Castell, C.; Schendler, G.; Faoro, V.; Mairbäurl, H.; Bärtsch, P.; et al. Transpulmonary Plasma ET-1 and Nitrite Differences in High Altitude Pulmonary Hypertension. High Alt. Med. Biol. 2009, 10, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Carlsen, K.; Carlsen, K.-H.; Lenney, W.; Silverman, M.; Whyte, M.K.; Hosking, L.; Helms, P.; Roses, A.D.; Hay, D.W.; et al. Polymorphisms in the Endothelin-1 (EDN1) Are Associated with Asthma in Two Populations. Genes Immun. 2008, 9, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Lilevska, A.; Sierkova, V.; Savytska, O. Relationship of the Polymorphism Lys198Asn Endothelin-1 Gene with COPD and Coronary Heart Disease. Eur. Respir. J. 2020, 56, 2982. [Google Scholar] [CrossRef]
- Tomar, A.; Malhotra, S.; Sarkar, S. Polymorphism Profiling of Nine High Altitude Relevant Candidate Gene Loci in Acclimatized Sojourners and Adapted Natives. BMC Genet. 2015, 16, 112. [Google Scholar] [CrossRef]
- Minchenko, D.O.; Tsymbal, D.O.; Riabovol, O.O.; Viletska, Y.M.; Lahanovska, Y.O.; Sliusar, M.Y.; Bezrodnyi, B.H.; Minchenko, O.H. Hypoxic Regulation of EDN1, EDNRA, EDNRB, and ECE1 Gene Expressions in ERN1 Knockdown U87 Glioma Cells. Endocr. Regul. 2019, 53, 250–262. [Google Scholar] [CrossRef]
- Mishra, A.; Mohammad, G.; Thinlas, T.; Qadar Pasha, M.A. EGLN1 variants influence expression and S ao2 levels to associate with high-altitude pulmonary oedema and adaptation. Clin. Sci. 2013, 124, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Kohli, S.; Dua, S.; Thinlas, T.; Mohammad, G.; Qadar Pasha, M.A. Genetic differences and aberrant methylation in the apelin system predict the risk of high-altitude pulmonary edema. Proc. Natl. Acad. Sci. USA 2015, 112, 6134–6139. [Google Scholar] [CrossRef] [PubMed]
- Qadar Pasha, M.A.; Kocherlakota, K.S.; Khan, A.P.; Norboo, T.; Grover, S.K.; Baig, M.A.; Selvamurthy, W.; Brahmachari, S.K. Arterial Oxygen Saturation under Hypoxic Environment of High-Altitude Associates with Routine Physical Activities of Natives. Curr. Sci. 2003, 85, 502–506. [Google Scholar]
- Burtscher, M.; Niedermeier, M.; Burtscher, J.; Pesta, D.; Suchy, J.; Strasser, B. Preparation for Endurance Competitions at Altitude: Physiological, Psychological, Dietary and Coaching Aspects. A Narrative Review. Front. Physiol. 2018, 9, 1504. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, M.; Millet, G.P.; Burtscher, J. Hypoxia Conditioning for High-Altitude Pre-Acclimatization. J. Sci. Sport Exerc. 2022, 1–15. [Google Scholar] [CrossRef]
- Neri Serneri, G.G.; Cecioni, I.; Migliorini, A.; Vanni, S.; Galanti, G.; Modesti, P.A. Both Plasma and Renal Endothelin-1 Participate in the Acute Cardiovascular Response to Exercise. Eur. J. Clin. Investig. 1997, 27, 761–766. [Google Scholar] [CrossRef] [PubMed]
| Genetic Model | rs ID | HAPE-f | HAPE-p | HLs | HAPE-f vs. HAPE-p | HAPE-f vs. HLs | HLs vs. HAPE-p | |||
|---|---|---|---|---|---|---|---|---|---|---|
| rs5370 | Total Count (%) | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |||
| Co-dominant | GG | 128 (41.2) | 87 (28.0) | 168 (54.1) | Ref | - | Ref | - | Ref | - |
| GT | 151 (48.7) | 204 (65.8) | 130 (41.9) | 2.17 (1.52–3.10) | 1.75 × 10−5 | 0.60 (0.41–0.90) | 0.01 | 3.37 (2.27–5.01) | 1.78 × 10−9 | |
| TT | 31 (10) | 19 (6.12) | 12 (3.8) | 0.90 (0.47–1.72) | 0.76 | 2.66 (1.08–6.54) | 0.03 | 0.13 (0.14–0.71) | 0.006 | |
| Dominant | GG | 128 (41.2) | 87 (28.0) | 168 (54.1) | Ref | - | Ref | - | Ref | - |
| GT+TT | 182 (58.7) | 223 (71.9) | 142 (45.8) | 1.95 (1.38–2.76) | 1.39 × 10−4 | 0.56 (0.38–0.82) | 0.003 | 3.31 (2.24–4.89) | 1.57 × 10−9 | |
| Recessive | TT | 31 (10) | 19 (6.12) | 12 (3.8) | Ref | - | Ref | - | Ref | - |
| GG+GT | 279 (90.0) | 291 (93.8) | 298 (96.1) | 0.55 (0.30–1.01) | 0.05 | 0.45 (0.20–0.98) | 0.04 | 1.20 (0.52–2.79) | 0.65 | |
| Allelic | G | 407 (65.6) | 378 (60.9) | 466 (75.1) | Ref | - | Ref | - | Ref | - |
| T | 213 (34.4) | 242 (39.1) | 154 (24.9) | 1.25 (0.99–1.59) | 0.05 | 0.63 (0.47–0.84) | 0.002 | 1.96 (1.48–2.61) | 3.09 × 10−6 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmo, T.; Abbasi, B.A.; Chanana, N.; Sharma, K.; Faruq, M.; Thinlas, T.; Abdin, M.Z.; Pasha, Q. The EDN1 Missense Variant rs5370G > T Regulates Adaptation and Maladaptation under Hypobaric Hypoxia. Int. J. Environ. Res. Public Health 2022, 19, 11174. https://doi.org/10.3390/ijerph191811174
Palmo T, Abbasi BA, Chanana N, Sharma K, Faruq M, Thinlas T, Abdin MZ, Pasha Q. The EDN1 Missense Variant rs5370G > T Regulates Adaptation and Maladaptation under Hypobaric Hypoxia. International Journal of Environmental Research and Public Health. 2022; 19(18):11174. https://doi.org/10.3390/ijerph191811174
Chicago/Turabian StylePalmo, Tsering, Bilal Ahmed Abbasi, Neha Chanana, Kavita Sharma, Mohammed Faruq, Tashi Thinlas, Malik Z. Abdin, and Qadar Pasha. 2022. "The EDN1 Missense Variant rs5370G > T Regulates Adaptation and Maladaptation under Hypobaric Hypoxia" International Journal of Environmental Research and Public Health 19, no. 18: 11174. https://doi.org/10.3390/ijerph191811174
APA StylePalmo, T., Abbasi, B. A., Chanana, N., Sharma, K., Faruq, M., Thinlas, T., Abdin, M. Z., & Pasha, Q. (2022). The EDN1 Missense Variant rs5370G > T Regulates Adaptation and Maladaptation under Hypobaric Hypoxia. International Journal of Environmental Research and Public Health, 19(18), 11174. https://doi.org/10.3390/ijerph191811174






