Abstract
Background: There is little information on the feasibility and benefit of therapeutic exercise (TE) in women with metastatic breast cancer (MBC). The aim of this article is to describe the implementation of a TE intervention in MBC patients, and to determine the recruitment, compliance and improvement in outcomes after its completion. Methods: The “Therapeutic Exercise program in MBC” (TEP-MBC) consists of 1 h of individualized TE supervised by a physiotherapist in a group format, consisting of four groups of seven to eight participants. TEP-MBC was delivered twice a week, lasting 12 weeks (22 sessions), with patients considered to have completed the program when attending at least 17 sessions (>75% attendance). After referral, patients underwent a clinical interview and a physical and functional assessment. This information was complemented with patient-reported outcomes. Data about referral, compliance and assessment were collected. Results: Only 11 of the 30 patients completed the program. Drop-out was mainly related to personal issues and symptoms arising from the disease or treatment. All patients who completed the program improved cancer-related fatigue and increased their functional parameters. Conclusions: The TEP-MBC was safe and feasible in patients with MBC, although with low compliance. The high variability in baseline measures reflects the heterogeneous level of function.
1. Introduction
Physical activity (PA) displays great benefits in breast cancer (BC) patients and survivors. PA levels are associated with a lower risk of relapse [1], greater BC-specific survival and greater overall survival independent of body mass index or menopausal status [2,3]. PA displays multiple mediated pathways such as metabolism, inflammatory and immunomodulatory effects [4], and essentially lowers all obesity-related mediators of cancer [5]. Besides these benefits, BC patients decrease their PA levels after surgical treatment [6].
Exercise is a planned, structured and repetitive form of physical activity aimed at improving a specific physical benefit [7]. There has been growing interest in therapeutic exercise (TE). In BC patients, TE interventions improve quality of life (QoL) [8], mobility [9] and cancer-related fatigue (CRF). The safety, feasibility and benefit of TE is robust in BC and advanced cancer patients [10]. However, there is little information on the feasibility and benefit of exercise in women with metastatic breast cancer (MBC).
A prospective randomized study with 100 patients with MBC showed no impact on functionality after an unsupervised distance-based PA program vs. the wait-list control group [11]. Another distance-based study evaluated the impact of a seated exercise DVD program in 38 MBC patients, reporting an improvement in QOL compared to control participants [12]. The literature is limited about the benefit of survival. Analysis of secondary data with more than 100 MBC patients showed that greater PA level at baseline was significantly associated with longer survival, with the major limitation of relying on self-reported questionnaires to draw these conclusions [13].
New treatments allow MBC patients to live for several years after diagnosis of metastatic disease. However, many women experience significant side effects from systemic treatments and cancer symptoms in this period, hindering and preventing PA programs and lowering their functional capacity [14]. In this regard, data from a systematic review and meta-analysis have shown that distance-based interventions have a very small, limited impact on PA behavior in BC survivors [15]. Therefore, new approaches are needed to facilitate and support PA levels in MBC patients to achieve more reliable, accurate information on its impact on functionality, quality of life and prognosis.
Current exercise therapy prescription in the oncology setting is limited to generic guidelines, and there is interest in tailoring TE interventions thanks to its effectiveness [7]. Another limitation of exercise therapy prescription is implementation, as there is a lack of accessible exercise therapy prescription in cancer patients in the real-world setting [16]. Some institutions, such as the Canadian Cancer Society [17] and the Australian public hospitals [18] have developed real-world settings. Still, current research is limited to cancer survivors or people diagnosed with cancer [19,20]. The aim of this article was (a) to describe the process behind a free, not-for-profit community-based therapeutic exercise program (TEP) for MBC patients in the clinical setting and (b) to determine the recruitment, compliance and improvement in outcomes after its completion.
2. Materials and Methods
2.1. Program Design and Description
The present program started in May 2017 as The School of Healthy Habits for Women Operated for Breast Cancer, known colloquially as The Onco-Health Club (OHC), providing TE and educational interventions in BCS that have been surgically treated for their primary tumor with no evidence presence of tumor or metastatic disease [21]. Later, TE intervention was offered to patients diagnosed with MBC: The Therapeutic Exercise program in MBC (TEP-MBC).
TEP-MBC was a result of the research network between the Translation Research in Cancer B-01 and Clinimetric F-14 research groups at Málaga Biomedical Research Institute (IBIMA), accredited for healthcare research in Spain by Carlos III Institute of Health (www.ibima.eu/en). The main goal of TEP-MBC was to provide MBC patients the opportunity to benefit from an exercise tailored to their needs that was supervised by a physiotherapist (CRJ).
2.2. Participant Referral and Eligibility
Women were recruited by Medical Oncologists from the Medical Oncology Unit at the hospital (BCP, EA), who were in close contact with physiotherapists (CRJ, ACV). Participants included in this study were diagnosed with metastatic breast cancer, not amenable to curative treatment. Patients were excluded if they had suffered any cardiovascular event defined as stable or unstable angor, acute pulmonary edema, cardiac rhythm disorders or syncope of cause not affiliated in the year prior to inclusion.
At the beginning of the program, a baseline assessment was carried out.
2.3. Clinical Data Collection
Once eligibility was confirmed, all participants in this study signed an informed consent form prior to inclusion. The University Clinical Hospital gave ethical clearance for the study, following the Declaration of Helsinki. The oncologists collected clinical data on tumor subtype and type of surgery (breast-conserving or mastectomy), line of treatment, type of ongoing systemic therapy (endocrine therapy (ET), ET-cyclin-dependent kinases (CDKs) inhibitors, chemotherapy (CT), monoclonal antibody (MA) and CT-MA, type of metastatic disease (oligometastatic or multiple metastasis) and location of metastatic disease (visceral, not visceral, or both). The presence of bone metastases, axial bone metastasis, spine stabilization surgery and type and metastatic bone pattern (osteolytic, osteoblast or mixed) were also collected. This allowed screening precautions, assessing possible risk and tailoring intervention. For example, modifications in cases of bone metastases based on location [22,23].
2.4. Clinical Interview
Patients underwent an interview with the physiotherapist (CRJ), reporting their clinical history, ensuring personalized intervention based on the clinical information, the current interview, and further physical testing to establish baseline levels [22,24]. Patients were given personalized information about ET benefits [25], and any questions were answered, e.g., the effects of lifting weights [26] and the safety of upper limb strength exercises on lymphedema [27]. This allowed patients’ interests and preferences to be considered [28]. Furthermore, patients were asked about prior or current exercise behavior to establish intensity levels [24].
2.5. Physical and Functional Assessment
Assessment consisted of assessing the musculoskeletal system, the cardiorespiratory capacity and muscular strength. The physical assessment allowed musculoskeletal signs and symptoms [24], range of motion limitations and motor control to be taken into account to establish adaptations, loads and targeted muscle groups. For example, the range of motion in upper limb exercises was modified in cases of pectoral shortening and/or skin retraction on the affected side. To assess cardiorespiratory capacity, a submaximal oncology ergometry was carried out following a protocol tested in BC survivors [29]. It consisted of a multistage treadmill test, increasing speed gradually until the patients reached 85% of their maximum predicted heart rate (HR). According to the literature, muscular strength was assessed in the major muscle groups [30]. The program’s weight was based on the estimated percentage of one-repetition maximum (1-RM). A weight load that produces fatigue in the 12 repetitions (12-RM) was calculated for better strength gains [31,32].
Besides the widely-known 30-Second sit-to-stand test (30-STS) [33] and handgrip strength test [34], the functional assessment was provided by the following test:
- Lie-to-sit (LTS) transfer: Patients were asked to transfer from lying to sitting. Patients started from a supine position with the head resting and arms parallel to the body. The patient should turn right, supporting the right arm to arise from the sitting position. They were allowed to use a hand and a pillow if necessary. The number of repetitions performed during 30 s was counted [35]
- Adapted burpees (AB): Furthermore, patients who were able to complete the 30-STS test with repetitions ≥15 and BPE ≤ 7 (strong) were asked to perform adapted burpees (AB) for two minutes, following a protocol tested in BCS [29].
This information was complemented with the following questionnaires: Piper Fatigue Scale-Revised (PFS-R) [36] to measure cancer-related fatigue (CRF), the Upper Limb Functional Index (ULFI) [37], the Lower Limb Functional Index (LLFI) [38], the International Physical Activity Questionnaire–Short Form (IPAQ–SF) [39], the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ–C30) [40] and the European Organization for Research and Treatment of Cancer Breast Cancer-Specific Quality of Life Questionnaire (EORTC QLQ–BR23) [41].
2.6. Therapeutic Exercise Intervention
The intervention consisted of 1 h of individualized TE supervised by a physiotherapist. It was delivered twice a week, lasting 12 weeks. The intervention was in a group format, consisting of 4 groups of 7–8 participants. Thus, the complete program consisted of 22 TE sessions, with patients being taken to have completed the program when attending at least 17 sessions (>75% attendance). It was considered that patients who had missed more than five sessions did not complete the program.
Exercises to generate neuromuscular and cardiovascular adaptations were carried out, accounting for training principles [7] and current recommendations in the oncology field [9]. Intensity, time and time prescription were individualized based on evaluations of muscular strength, endurance and patient needs [24], and followed the FIIT formula, as detailed in Appendix A. The TE intervention consisted mainly of strength exercises and endurance with aerobic training.
2.7. Funding and Sustainability
Contract Nº PS16060 in IBIMA between Novartis-IBIMA funded the TEP-MBC, consisting of payment for CRJ as the physiotherapist. University Clinical Hospital Virgen de la Victoria provided the rehabilitation room, equipped with bicycles, dumbbells, weights, mats and treatment tables for those patients unable to lay on the floor. The Chair of Physiotherapy at the University of Málaga provided material for assessment.
2.8. Statistical Analysis
Descriptive analyses were used to present the mean and standard deviation of quantitative variables and number (percentage) for qualitative variables. Patients’ functional and self-reported outcome data were calculated at baseline and post-intervention. The following groups were considered: baseline group (all patients who attended the assessment, left or not started group, compliance < 75% group and intervention group (Compliance > 75%). Mean changes were calculated in the intervention group. All analyses were performed using SPSS 22.0 for Windows.
3. Results
3.1. Recruitment
A total of 30 women who were MBC patients were recruited as volunteers between February 2018 and April 2019 by Medical Oncologists from the Medical Oncology Unit at University Clinical Hospital Virgen de la Victoria (Málaga, Spain).
3.2. Compliance
Of the 30 patients initially recruited, only 11 completed the program with attendance at 17 sessions or more (75% of attendance). In total, 19 patients abandoned the program due to different reasons: 11 patients dropped out because of personal matters (transport, family problems, distance to hospital, lack of motivation); 5 patients presented tumor symptoms (3 bone pain, 1 liver pain, and 1 thrombosis) that prevented them from attending the sessions; 2 patients had serious treatment side effects; and 1 patient presented major depressive symptoms that made it impossible to complete the program.
Overall, 11 patients completed the program, representing 36% compliance. Lack of attendance was due to personal matters and health status, namely hematological compromise (absolute neutrophil counts), pharyngitis and wound infection. In total, 4 out of 11 patients lacked some functional parameters because they were unable to attend on the day of the pre- or post-evaluation and seven out of 11 patients completed the program having attended all the evaluation appointments (pre- and post-intervention), meaning complete data are available.
3.3. Clinical and Oncology Variables
The average age of participants was 52.46 years. Affected breast side and comorbidities from the baseline and intervention group are shown in Table 1.

Table 1.
Patients’ clinical variables at baseline.
Regarding treatment (n = 30), the majority presented Hormone Receptor (HR) positive—HER2 negative tumors (21 patients, 70%) and were treated in the 1st line of treatment (20 patients, 67%). Three patients presented HR negative—HER2 positive tumors, three presented HR positive—HER 2 positive tumors and three presented triple negative (TN) tumors. Six patients were treated in the 2nd line and four in the 3rd line of treatment. More details from the baseline and intervention group are given in Table 2.

Table 2.
Patients’ oncological characteristics at baseline.
3.4. Patient-Reported and Functional Outcomes at Baseline
Baseline levels from the whole sample are provided in Table 3. As can be observed, the group who left or did not start the program had lower levels of physical activity measured by IPAQ (2.351 METS), and slightly lower levels of function measured by 30-STS and LLFI.

Table 3.
Patients’ baseline functional and self-reported outcomes in each attendance group.
3.5. Improvements in Outcomes
Differences between baseline and final assessment were calculated in those patients with Compliance > 75% (intervention group). Regarding CRF, the intervention group showed lower levels after intervention (4.33). With regards to QoL, results varied depending on the questionnaire used. All patients who completed the program objectively increased their functional parameters concerning 30-STS and LTS. In the intervention group, 30-STS increased from 14.50 repetitions to 19.61 repetitions. Regarding lie-to-sit (LTS) transfer, data could only be attained pre- and post-intervention from 6 of the 11 patients, and all of them showed an increase in their scores. Regarding handgrip strength, values remained stable. More details are shown in Table 4.

Table 4.
Patients’ functional and self-reported outcomes from intervention group (n = 11).
4. Discussion
As far as the authors are aware, this is the first study that provides a supervised TE program in MBC patients, which has shown to be safe and feasible in our study population, with no adverse events related to exercise reported in the group. Moreover, this study provides the descriptive functional status of MBC patients based on patient-reported outcomes and functional assessment, as well as data in terms of recruitment, compliance and improvement in outcomes after its completion. It should be noted that, while exercise in BCS under adjuvant therapy and beyond is widely studied, this study is the second with a supervised design in metastatic cancer patients [42], and the first in a homogeneous cohort of MBC patients. Therefore, the results of this study may be of interest to clinicians and researchers when implementing and designing TEP in MBC patients in a real-world setting.
4.1. Recruitment
One strength of the present study was that oncologists recruited patients interested in the intervention. Current guidelines highlight that oncologists’ advice and referral to exercise programs are essential for patient engagement [43]. Furthermore, oncologists provided oncology and clinical data to the physiotherapist, guaranteeing screening precautions, such as bone metastases (Table 2).
4.2. Compliance
Drop-out was the main obstacle in developing our TE program, with only 11 out of 30 patients recruited completing the program, representing 36% compliance. Most patients who dropped out did so for reasons unrelated to disease progression or treatment side effects. This is essential, as most women who dropped out did so mainly due to reasons unrelated to exercise (Figure 1).
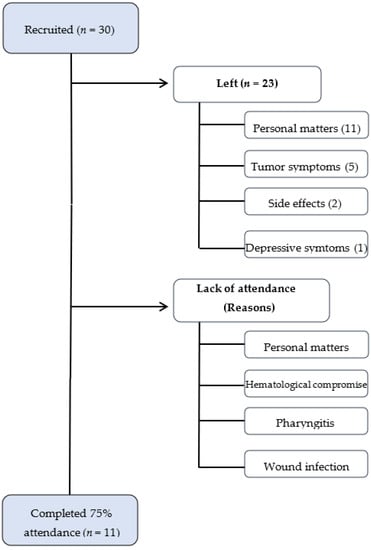
Figure 1.
Flowchart from compliance.
Similar interventions in BCS have shown compliance of 67%, and drop-outs and lack of attendance were mainly related to personal matters (incompatibility with work and family life), health problems and transport barriers [21]. Data from other randomized studies in metastatic patients report higher rates of completion (64–70%) [11,42], but recent reports from other hospital distance-based PA programs prescribed at home are around 35–52% [44], with abandonment due mainly to tumor progression [42]. The reason for our low compliance rate may be the cohort heterogeneity, with significant differences in extension of metastatic disease and line of treatment. Although compliance in MBC population is still known, it should be highlighted that this population suffers from greater side effects and complications. Future inventions should analyze compliance in home-based in-person interventions.
4.3. Assessment and Intervention
One of the strengths of this study is that intervention was tailored based on pre-assessment, taking into account the oncology process, physical status and capacity, as well as patients’ interests and preferences, following current literature in the oncology field [22,23,24,25,29,32]. This allowed patients to be stratified in groups based on physical activity level and functional status to prioritize intervention type. The FIIT (Frequency, Intensity, Time and Type) formula was also followed [7]. Furthermore, training principles were further considered [7] and explained in the present manuscript (Appendix A). Concerning the strengths of this study, it should be noted that our intervention was safe, and no adverse events were reported in the study participants.
Pre-assessment allowed a supervised, tailored prospective design: while the physical and functional assessment paid attention to the patient’s physical status, the clinical interview allowed personal interests, needs and preferences to be taken into account. A patient-centred intervention is considered the best clinical practice in exercise intervention in women with advanced BC [28].
There is limited information evaluating the prognostic value of a TE intervention in women with MBC, in contrast to the growing work in early BC that reports a decreased risk of cancer-related and overall mortality. One study analyzed secondary data of a psychotherapy clinical trial with more than 100 MBC patients and showed a benefit in survival, with the major limitation of relying on self-reported questionnaires to draw these conclusions [13].
The intensity of the present TE program was moderate, consisting of aerobic exercise at 60–80% age-predicted maximum HR and resistance exercise at 70% of estimated 1-RM. This matches the combined exercise type intervention, with aerobic training ranging from 55–85% maximum HR and resistance exercise ranging from 40–90% of RM [28]. Apart from the similarities, this intervention was modified as needed in order to guarantee safety (Appendix A).
4.4. Patient-Reported and Functional Outcomes
Regarding functional outcomes, handgrip strength at baseline ranged from 3.33 to 29.33 kg. A group of 71 MBC had a mean value of 26.6 (6.0) kg, significantly lower than their matched controls [45]. Lower values in handgrip strength are associated with physical frailty and are predictive of disability in older people [46]. For example, a cut-off point of 17.4 kg identifies patients with mobility limitations in older women [47].
The number of repetitions performed during 30-STS ranged from one single repetition to 26 repetitions at baseline levels, while 2 out of 30 patients were not able to perform the test. Regarding 30-STS, older metastasis patients (mean age = 62.6 years) have shown lower levels of 30-STS repetitions (11.6 [0.38]) [42] when compared to matched controls (22 [7]) [48]. In the group that completed the TE program (n = 11), 30-STS repetitions increased from 14.50 to 19.61. In patients with spinal metastases, a TE program involving isometric spinal muscle strengthening increased 30-sts values from 5.1 (1.4) to 9.0 (2.6) [49]. Increasing lower limb strength is vital, as 30-STS repetitions below 15 predicts risks of falls and fractures in older healthy patients [50].
It should be highlighted that the level of physical activity (IPAQ) from the left or not started group (2.351 METS) and the compliance < 75% group (3.583 METS) was lower than the group with higher compliance (6.675) measured by IPAQ–SF (Table 3). A study with a sample of 85 patients with bone metastases (45 of them with breast cancer) found an inverse relationship between the level of physical activity and outcomes such as pain score or perceived physical function [51].
4.5. Improvements in Outcomes
Patients who completed the program (n = 11) decreased their CRF and improved their physical function measured by handgrip strength and functional tests such as 30-STS, lie-to-sit transition and adapted burpees. However, the lack of a control group did not allow comparison. The heterogeneity was also a limitation, as women undergoing various forms of systemic therapy and who were at different points during their metastatic disease were included. This contributed to the variation in functional parameters. However, findings in terms of low compliance and sample heterogeneity represent the implementation of TE in the real-world setting.
5. Conclusions
This study showed that an individualized, supervised TE program is safe and feasible in MBC, although with low compliance due to personal matters and tumor/treatment-related issues. Patients who completed the program decreased their CRF and improved their physical function measured by handgrip strength and functional tests such as 30-STS, lie-to-sit transition and adapted burpees. Given the heterogeneity in the clinical status of patients and the degree of compliance, future research should include a wider sample to further analyze the effects of TE programs in this population.
Author Contributions
Conceptualization, A.I.C.-V. and E.A.; methodology, A.I.C.-V.; validation, A.I.C.-V., B.P. and E.A.; formal analysis, C.R.-J. and A.I.C.-V.; investigation, A.I.C.-V. and C.R.-J.; resources, E.A., B.P. and A.I.C.-V.; data curation; A.I.C.-V. and C.R.-J.; writing—original draft preparation, B.P., C.R.-J. and A.I.C.-V.; writing—review and editing, A.I.C.-V. and C.R.-J.; visualization, A.I.C.-V. and C.R.-J.; supervision, A.I.C.-V.; project administration, E.A., B.P. and A.I.C.-V.; funding acquisition, E.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research project was partially funded by Contract Nº PS16060 in IBIMA between Novartis-IBIMA (Translation Research in Cancer B-01 and Clinimetric F-14) for the physiotherapist in the assessment.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by Portal de Ética de la Investigación Biomédica de Andalucía Ethics Committee, Spain (protocol code 28042016). Trial registration: NCT03879096, Registered 18th March 2019.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors are grateful to the volunteers for their participation. The assistance provided by the Chair of Physiotherapy at University of Málaga was greatly appreciated.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Appendix A
Appendix A.1. Therapeutic Exercise Intervention
The following FITT formula was followed:
- Frequency: 22 sessions (2 sessions per week);
- Intensity: Moderate; more details are given along with this file;
- Time: A total of 1 h per session (2 h per week).;
- Type: Exercise modalities consisted of muscular strength training to induce neuromuscular adaptations with endurance and aerobic training to induce cardiovascular adaptations. Strength training consisted of free-weight exercises of major muscle groups. Aerobic training consisted of a treadmill or cycling ergometer. Warm-up and cool-down exercises were included.
Individually tailored TE prescription was based on physical activity level, functional status and patients’ preferences, as follows:
- Physical activity level: Meeting the American College of Sports Medicine (ACSM) guidelines: ≥150 min per week of moderate or ≥75 min per week of vigorous exercise [52]. This information was obtained from pre-intervention assessment (clinical interview). Aerobic training was mainly used for patients who did not meet ACSM levels.
- Functional status: Lower and upper limb function obtained from LLFI and ULFI questionnaires, respectively. Strength training was prioritized in patients with functionality below 50%. Lower limb strength exercises were targeted mainly in those patients unable to perform more than 15 repetitions in 30-STS test [50]. Upper limb strength training was targeted mainly in those patients with handgrip strength lower than 16 kg [53,54].
- Patients’ preferences: Physical activity level and functional status were complemented by patients’ needs [24]. For example, if the patient’s main goal is to improve standing transition, knee extensor strength exercises such as sit-to-stand transitions will be implemented; if the patient’s main goal is getting up when lying on the floor, triceps strength exercises will be implemented.
The following guide was developed:
| Patient Subgroup | Level of Functionality and Independence | Main Adaptation Targeted | Physical Activity Level | Functional Status | ||
| Meeting ACSM Guidelines | No Meeting ACSM Guidelines | >50% | <50% | |||
| A | High | Neuromuscular and cardiovascular | + | + | ||
| B | Low | Neuromuscular | + | + | ||
| C | Medium | Cardiovascular | + | + | ||
- Group A: Patients had function > 50% and meet the ACSM physical activity guidelines. TE intervention will consist of both muscular strength and aerobic training (neuromuscular and cardiovascular adaptations).
- Group B: Patients had lower function (<50%) and do not meet the ACSM physical activity guidelines. They will therefore benefit from both muscular strength and aerobic training. Patients may start with neuromuscular adaptations.
- Group C: Patients do not meet the ACSM physical activity guidelines, which are impaired by other causes other than lack of function (i.e., walking impaired by neuropathy or lack of motivation). Aerobic training will therefore be prioritized to enhance cardiovascular adaptations.
Appendix A.2. Neuromuscular Adaptations
Individualization: The intervention was preceded by a physical assessment of the musculoskeletal system.
Progression:
| Week | Objective | Method |
| 1–2 | Learn proper exercise technique | Patients carried out 3 sets of 15 repetitions (reps) with a load that would guarantee proper execution [55]. In the case of bone metastasis, adaptations were made whenever there were increased symptoms during performance. Furthermore, in patients with lower levels of function (subgroup B and C), the TE program started with isometric exercises [56]. |
| 2–4 | Target exercise dose | Patients carried out 4 sets of 10 repetitions (10-RM), with an estimated intensity of 75% 1-RM [57]. Repetitions were set at a speed of 24 bpm, controlled by a metronome. If the patient could perform more than 12 reps in 30 s while maintaining proper execution, the weight to be lifted was increased [55]. Patients learned both deceleration and loss of motor control as a sign of muscle fatigue [58]. |
| 12–14 | Ensure learning and behavior change | Patients were informed about changes and progression since starting, to make them aware of their improvement. Personal adaptations and self-perceptions were discussed to empower patients for positive long-term exercise behavior. |
In order to ensure proper technique and adequate progression, all exercises and changes were incorporated and supervised by a physical therapist.
Specificity: Muscular strength training was carried out in order to obtain adaptation in the musculoskeletal system and improve function. Exercises targeted the major muscle groups according to the literature [30]. Muscle isolation was carried out as determined by muscle weakness and patient priorities during physical assessment.
Recovery: To optimize physiological adaptations, sessions were held on Tuesdays and Thursdays, with a minimum of 48 h for recovery between sessions. Furthermore, patients were asked to report any symptom days following exercise to the physical therapist in order to avoid overload.
Appendix A.3. Cardiorespiratory Adaptations
Individualization: TE cardiovascular intensity was established based on submaximal oncology ergometry protocol tested in BC survivors [29]. This submaximal test was based on heart rate (HR) [59] and Borg Perceived Exertion (BPE) with the Borg Scale (0–10) [60]. This allowed the aerobic–anaerobic transition zone to be determined [55].
Progression:
| Week | Objective | Method |
| 1–2 | Adapt to the experience of fatigue during exercise. | Low-intensity adaptation (under 60% HR) [61]. Patients from group C (who did not meet the ACSM guidelines but have function > 50%) were recommended to increase physical activity, e.g., brisk walking at low intensity guided by BPE during pre-assessment. In patients from group B (low physical activity and functional status), training was gradually increased from 5 to 15 min. Intensity was modified based on PE. |
| 2–14 | Maintain constant intensity to achieve the prescribed aerobic HR thresholds based on an individualized test [62]. | Patients were told to maintain both speed and BPE corresponding to a range between 60% and 80% of their maximum HR. Patients reduced intensity if they experienced any symptoms while exercising. Every two weeks, HR and BPE at a selected speed were measured in order to increase intensity in cases of improvement. In patients from group B, intensity was kept at 60% HR during 20 min during the first 3 weeks and increased to 60–80% maximum HR if tolerated. |
| 12–14 | Ensure learning and behavior change | These were the same methods as those of the neuromuscular adaptations. |
Specificity: Endurance with aerobic training was carried out at moderate intensity. During the assessment, 60% and 80% of HR was correlated with treadmill speed and BPE. This allowed feedback between the physical therapist and the patient during the program to achieve the proper exercise dose.
Recovery: The same method as the recovery method for neuromuscular adaptations was used.
References
- Lahart, I.M.; Metsios, G.S.; Nevill, A.M.; Carmichael, A.R. Physical Activity, Risk of Death and Recurrence in Breast Cancer Survivors: A Systematic Review and Meta-Analysis of Epidemiological Studies. Acta Oncol. Stockh. Swed. 2015, 54, 635–654. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Jiang, T.; Ma, T.; Zhang, X.; Tang, J.; Chen, W.; Lv, M.; Zhao, J. Association between Physical Activity and Mortality in Breast Cancer: A Meta-Analysis of Cohort Studies. Eur. J. Epidemiol. 2014, 29, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.D.; Chen, W.Y.; Feskanich, D.; Kroenke, C.H.; Colditz, G.A. Physical Activity and Survival after Breast Cancer Diagnosis. J. Am. Med. Assoc. 2005, 293, 2479–2486. [Google Scholar] [CrossRef] [PubMed]
- Ashcraft, K.A.; Peace, R.M.; Betof, A.S.; Dewhirst, M.W.; Jones, L.W. Efficacy and Mechanisms of Aerobic Exercise on Cancer Initiation, Progression, and Metastasis: A Critical Systematic Review of In Vivo Preclinical Data. Cancer Res. 2016, 76, 4032–4050. [Google Scholar] [CrossRef]
- Giovannucci, E. An Integrative Approach for Deciphering the Causal Associations of Physical Activity and Cancer Risk: The Role of Adiposity. J. Natl. Cancer Inst. 2018, 110, 935–941. [Google Scholar] [CrossRef]
- De Groef, A.; Geraerts, I.; Demeyer, H.; Van der Gucht, E.; Dams, L.; de Kinkelder, C.; Dukers-van Althuis, S.; Van Kampen, M.; Devoogdt, N. Physical Activity Levels after Treatment for Breast Cancer: Two-Year Follow-Up. Breast 2018, 40, 23–28. [Google Scholar] [CrossRef]
- Sasso, J.P.; Eves, N.D.; Christensen, J.F.; Koelwyn, G.J.; Scott, J.; Jones, L.W. A Framework for Prescription in Exercise-Oncology Research. J. Cachexia Sarcopenia Muscle 2015, 6, 115–124. [Google Scholar] [CrossRef]
- Gerritsen, J.K.W.; Vincent, A.J.P.E. Exercise Improves Quality of Life in Patients with Cancer: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Br. J. Sports Med. 2016, 50, 796–803. [Google Scholar] [CrossRef]
- Dennett, A.M.; Peiris, C.L.; Shields, N.; Prendergast, L.A.; Taylor, N.F. Moderate-Intensity Exercise Reduces Fatigue and Improves Mobility in Cancer Survivors: A Systematic Review and Meta-Regression. J. Physiother. 2016, 62, 68–82. [Google Scholar] [CrossRef]
- Heywood, R.; McCarthy, A.L.; Skinner, T.L. Efficacy of Exercise Interventions in Patients With Advanced Cancer: A Systematic Review. Arch. Phys. Med. Rehabil. 2018, 99, 2595–2620. [Google Scholar] [CrossRef]
- Ligibel, J.A.; Giobbie-Hurder, A.; Shockro, L.; Campbell, N.; Partridge, A.H.; Tolaney, S.M.; Lin, N.U.; Winer, E.P. Randomized Trial of a Physical Activity Intervention in Women with Metastatic Breast Cancer. Cancer 2016, 122, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Headley, J.A.; Ownby, K.K.; John, L.D. The Effect of Seated Exercise on Fatigue and Quality of Life in Women with Advanced Breast Cancer. Oncol. Nurs. Forum 2004, 31, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Palesh, O.; Kamen, C.; Sharp, S.; Golden, A.; Neri, E.; Spiegel, D.; Koopman, C. Physical Activity and Survival in Women with Advanced Breast Cancer. Cancer Nurs. 2018, 41, E31–E38. [Google Scholar] [CrossRef] [PubMed]
- Kokkonen, K.; Saarto, T.; Mäkinen, T.; Pohjola, L.; Kautio, H.; Järvenpää, S.; Puustjärvi-Sunabacka, K. The Functional Capacity and Quality of Life of Women with Advanced Breast Cancer. Breast Cancer 2017, 24, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Groen, W.G.; van Harten, W.H.; Vallance, J.K. Systematic Review and Meta-Analysis of Distance-Based Physical Activity Interventions for Cancer Survivors (2013–2018): We Still Haven’t Found What We’re Looking For. Cancer Treat. Rev. 2018, 69, 188–203. [Google Scholar] [CrossRef]
- Mina, D.S.; Sabiston, C.M.; Au, D.; Fong, A.J.; Capozzi, L.C.; Langelier, D.; Chasen, M.; Chiarotto, J.; Tomasone, J.R.; Jones, J.M.; et al. Connecting People with Cancer to Physical Activity and Exercise Programs: A Pathway to Create Accessibility and Engagement. Curr. Oncol. 2018, 25, 149–162. [Google Scholar] [CrossRef]
- Results of the September 2019 CCS/CIHR Cancer Survivorship Team Grants, in Partnership with ACF Competition. Available online: https://www.cancer.ca/en/research/funding-results/recent-competition-results/sept-2019-cstg-results/ (accessed on 18 September 2020).
- Dennett, A.M.; Zappa, B.; Wong, R.; Ting, S.B.; Williams, K.; Peiris, C.L. Bridging the Gap: A Pre-Post Feasibility Study of Embedding Exercise Therapy into a Co-Located Cancer Unit. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2021, 29, 6701–6711. [Google Scholar] [CrossRef]
- Czosnek, L.; Richards, J.; Zopf, E.; Cormie, P.; Rosenbaum, S.; Rankin, N.M. Exercise Interventions for People Diagnosed with Cancer: A Systematic Review of Implementation Outcomes. BMC Cancer 2021, 21, 643. [Google Scholar] [CrossRef]
- Purdy, G.M.; Sobierajski, F.M.; Dolgoy, N.D.; McNeely, M.L. Evaluating Implementation and Pragmatism of Cancer-Specific Exercise Programs: A Scoping Review. J. Cancer Surviv. Res. Pract. 2021, 16, 374–387. [Google Scholar] [CrossRef]
- Roldán-Jiménez, C.; Pajares, B.; Ruiz-Medina, S.; Trinidad-Fernández, M.; González-Sánchez, M.; Ribelles, N.; García-Almeida, J.M.; Ríos-López, M.J.; Alba, E.; Cuesta-Vargas, A.I. Design and Implementation of a Standard Care Programme of Therapeutic Exercise and Education for Breast Cancer Survivors. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2021, 30, 1243–1251. [Google Scholar] [CrossRef]
- Mina, D.S.; Langelier, D.; Adams, S.C.; Alibhai, S.M.H.; Chasen, M.; Campbell, K.L.; Oh, P.; Jones, J.M.; Chang, E. Exercise as Part of Routine Cancer Care. Lancet Oncol. 2018, 19, e433–e436. [Google Scholar] [CrossRef]
- Maltser, S.; Cristian, A.; Silver, J.K.; Morris, G.S.; Stout, N.L. A Focused Review of Safety Considerations in Cancer Rehabilitation. PM R 2017, 9, S415–S428. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.W.; Eves, N.D.; Peppercorn, J. Pre-Exercise Screening and Prescription Guidelines for Cancer Patients. Lancet Oncol. 2010, 11, 914–916. [Google Scholar] [CrossRef]
- Hartman, S.J.; Rosen, R.K. Breast Cancer Relatives’ Physical Activity Intervention Needs and Preferences: Qualitative Results. BMC Womens Health 2017, 17, 36. [Google Scholar] [CrossRef]
- LeVasseur, N.; Stober, C.; Ibrahim, M.; Gertler, S.; Hilton, J.; Robinson, A.; McDiarmid, S.; Fergusson, D.; Mazzarello, S.; Hutton, B.; et al. Perceptions of Vascular Access for Intravenous Systemic Therapy and Risk Factors for Lymphedema in Early-Stage Breast Cancer-a Patient Survey. Curr. Oncol. 2018, 25, e305–e310. [Google Scholar] [CrossRef]
- Cheema, B.S.; Kilbreath, S.L.; Fahey, P.P.; Delaney, G.P.; Atlantis, E. Safety and Efficacy of Progressive Resistance Training in Breast Cancer: A Systematic Review and Meta-Analysis. Breast Cancer Res. Treat. 2014, 148, 249–268. [Google Scholar] [CrossRef]
- Singh, B.; Spence, R.R.; Steele, M.L.; Sandler, C.X.; Peake, J.M.; Hayes, S.C. A Systematic Review and Meta-Analysis of the Safety, Feasibility, and Effect of Exercise in Women With Stage II+ Breast Cancer. Arch. Phys. Med. Rehabil. 2018, 99, 2621–2636. [Google Scholar] [CrossRef]
- Cuesta-Vargas, A.I.; Buchan, J.; Pajares, B.; Alba, E.; Trinidad-Fernández, M.; Ruíz-Medina, S.; García-Almeida, J.M.; Ríos-López, M.J.; Roldán-Jiménez, C. Energy System Assessment in Survivors of Breast Cancer. Phys. Ther. J. 2020, 100, 438–446. [Google Scholar] [CrossRef]
- Cormie, P.; Atkinson, M.; Bucci, L.; Cust, A.; Eakin, E.; Hayes, S.; McCarthy, S.; Murnane, A.; Patchell, S.; Adams, D. Clinical Oncology Society of Australia Position Statement on Exercise in Cancer Care. Med. J. Aust. 2018, 209, 184–187. [Google Scholar] [CrossRef]
- Feigenbaum, M.S.; Pollock, M.L. Prescription of Resistance Training for Health and Disease. Med. Sci. Sports Exerc. 1999, 31, 38–45. [Google Scholar] [CrossRef]
- Pollock Michael, L.; Franklin Barry, A.; Balady Gary, J.; Chaitman Bernard, L.; Fleg Jerome, L.; Barbara, F.; Marian, L.; Piña Ileana, L.; Stein Richard, A.; Williams, M.; et al. Resistance Exercise in Individuals With and Without Cardiovascular Disease. Circulation 2000, 101, 828–833. [Google Scholar] [CrossRef]
- Roldán-Jiménez, C.; Bennett, P.; Cuesta-Vargas, A.I. Muscular Activity and Fatigue in Lower-Limb and Trunk Muscles during Different Sit-To-Stand Tests. PLoS ONE 2015, 10, e0141675. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A Review of the Measurement of Grip Strength in Clinical and Epidemiological Studies: Towards a Standardised Approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Cuesta-Vargas, A.; Buchan, J.; Alba, E.; Iglesias Campos, M.; Roldán-Jiménez, C.; Pajares, B. Development of a Functional Assessment Task in Metastatic Breast Cancer Patients: The 30-Second Lie-to-Sit Test. Disabil. Rehabil. 2022, 1–8. [Google Scholar] [CrossRef]
- Cantarero-Villanueva, I.; Fernández-Lao, C.; Díaz-Rodríguez, L.; Cuesta-Vargas, A.I.; Fernández-de-las-Peñas, C.; Piper, B.F.; Arroyo-Morales, M. The Piper Fatigue Scale-Revised: Translation and Psychometric Evaluation in Spanish-Speaking Breast Cancer Survivors. Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil. 2014, 23, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Cuesta-Vargas, A.I.; Gabel, P.C. Cross-Cultural Adaptation, Reliability and Validity of the Spanish Version of the Upper Limb Functional Index. Health Qual. Life Outcomes 2013, 11, 126. [Google Scholar] [CrossRef] [PubMed]
- Cuesta-Vargas, A.I.; Gabel, C.P.; Bennett, P. Cross Cultural Adaptation and Validation of a Spanish Version of the Lower Limb Functional Index. Health Qual. Life Outcomes 2014, 12, 75. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Arraras, J.I.; Arias, F.; Tejedor, M.; Pruja, E.; Marcos, M.; Martínez, E.; Valerdi, J. The EORTC QLQ-C30 (Version 3.0) Quality of Life Questionnaire: Validation Study for Spain with Head and Neck Cancer Patients. Psychooncology 2002, 11, 249–256. [Google Scholar] [CrossRef]
- Sprangers, M.A.; Groenvold, M.; Arraras, J.I.; Franklin, J.; te Velde, A.; Muller, M.; Franzini, L.; Williams, A.; de Haes, H.C.; Hopwood, P.; et al. The European Organization for Research and Treatment of Cancer Breast Cancer-Specific Quality-of-Life Questionnaire Module: First Results from a Three-Country Field Study. J. Clin. Oncol. 1996, 14, 2756–2768. [Google Scholar] [CrossRef]
- Oldervoll, L.M.; Loge, J.H.; Lydersen, S.; Paltiel, H.; Asp, M.B.; Nygaard, U.V.; Oredalen, E.; Frantzen, T.L.; Lesteberg, I.; Amundsen, L.; et al. Physical Exercise for Cancer Patients with Advanced Disease: A Randomized Controlled Trial. Oncologist 2011, 16, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, K.H.; Campbell, A.M.; Stuiver, M.M.; Pinto, B.M.; Schwartz, A.L.; Morris, G.S.; Ligibel, J.A.; Cheville, A.; Galvão, D.A.; Alfano, C.M.; et al. Exercise Is Medicine in Oncology: Engaging Clinicians to Help Patients Move through Cancer. CA Cancer J. Clin. 2019, 69, 468–484. [Google Scholar] [CrossRef] [PubMed]
- Santa Mina, D.; Au, D.; Auger, L.E.; Alibhai, S.M.H.; Matthew, A.G.; Sabiston, C.M.; Oh, P.; Ritvo, P.G.; Chang, E.B.; Jones, J.M. Development, Implementation, and Effects of a Cancer Center’s Exercise-Oncology Program. Cancer 2019, 125, 3437–3447. [Google Scholar] [CrossRef] [PubMed]
- Yee, J.; Davis, G.M.; Beith, J.M.; Wilcken, N.; Currow, D.; Emery, J.; Phillips, J.; Martin, A.; Hui, R.; Harrison, M.; et al. Physical Activity and Fitness in Women with Metastatic Breast Cancer. J. Cancer Surviv. Res. Pract. 2014, 8, 647–656. [Google Scholar] [CrossRef]
- Dudzińska-Griszek, J.; Szuster, K.; Szewieczek, J. Grip Strength as a Frailty Diagnostic Component in Geriatric Inpatients. Clin. Interv. Aging 2017, 12, 1151–1157. [Google Scholar] [CrossRef]
- Vasconcelos, K.S.d.S.; Dias, J.M.D.; Bastone, A. de C.; Vieira, R.A.; Andrade, A.C. de S.; Perracini, M.R.; Guerra, R.O.; Dias, R.C. Handgrip Strength Cutoff Points to Identify Mobility Limitation in Community-Dwelling Older People and Associated Factors. J. Nutr. Health Aging 2016, 20, 306–315. [Google Scholar] [CrossRef]
- Millor, N.; Lecumberri, P.; Gómez, M.; Martínez-Ramírez, A.; Izquierdo, M. An Evaluation of the 30-s Chair Stand Test in Older Adults: Frailty Detection Based on Kinematic Parameters from a Single Inertial Unit. J. Neuroeng. Rehabil. 2013, 10, 86. [Google Scholar] [CrossRef]
- Rief, H.; Omlor, G.; Akbar, M.; Welzel, T.; Bruckner, T.; Rieken, S.; Haefner, M.F.; Schlampp, I.; Gioules, A.; Habermehl, D.; et al. Feasibility of Isometric Spinal Muscle Training in Patients with Bone Metastases under Radiation Therapy-First Results of a Randomized Pilot Trial. BMC Cancer 2014, 14, 67. [Google Scholar] [CrossRef]
- Jones, C.J.; Rikli, R.E.; Beam, W.C. A 30-s Chair-Stand Test as a Measure of Lower Body Strength in Community-Residing Older Adults. Res. Q. Exerc. Sport 1999, 70, 113–119. [Google Scholar] [CrossRef]
- Guinan, E.M.; Devenney, K.; Quinn, C.; Sheill, G.; Eochagáin, C.M.; Kennedy, M.J.; McDermott, R.; Balding, L. Associations Among Physical Activity, Skeletal Related Events, and Patient Reported Outcomes in Patients with Bone Metastases. Semin. Oncol. Nurs. 2022, 38, 151274. [Google Scholar] [CrossRef]
- Schmitz, K.H.; Courneya, K.S.; Matthews, C.; Demark-Wahnefried, W.; Galvão, D.A.; Pinto, B.M.; Irwin, M.L.; Wolin, K.Y.; Segal, R.J.; Lucia, A.; et al. American College of Sports Medicine Roundtable on Exercise Guidelines for Cancer Survivors. Med. Sci. Sports Exerc. 2010, 42, 1409–1426. [Google Scholar] [CrossRef] [PubMed]
- Alley, D.E.; Shardell, M.D.; Peters, K.W.; McLean, R.R.; Dam, T.-T.L.; Kenny, A.M.; Fragala, M.S.; Harris, T.B.; Kiel, D.P.; Guralnik, J.M.; et al. Grip Strength Cutpoints for the Identification of Clinically Relevant Weakness. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Dodds, R.M.; Syddall, H.E.; Cooper, R.; Kuh, D.; Cooper, C.; Sayer, A.A. Global Variation in Grip Strength: A Systematic Review and Meta-Analysis of Normative Data. Age Ageing 2016, 45, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Cuesta-Vargas, A.I.; Carabantes, F.; Caracuel, Z.; Conejo, I.; Alba, E. Effectiveness of an Individualized Program of Muscular Strength and Endurance with Aerobic Training for Improving Germ Cell Cancer-Related Fatigue in Men Undergoing Chemotherapy: EFICATEST Study Protocol for a Randomized Controlled Trial. Trials 2016, 17, 8. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, G.H. A Re-Evaluation of Isometric Strength Training. Br. J. Sports Med. 1971, 6, 20–21. [Google Scholar] [CrossRef]
- Vivian, H.H.; Ann, G. Assessing Muscular Fitness. In Advanced Fitness Assessment and Exercise Prescription, 7th ed.; Human Kinetics: Champaign, IL, USA, 2014; pp. 155–160. ISBN 978-1-4504-6600-4. [Google Scholar]
- González Badillo, J.J.; Ribas Serna, J. Bases de la Programación del Entrenamiento de Fuerza, 1st ed.; INDE: Barcelona, Spain, 2002; ISBN 978-84-9729-013-5. [Google Scholar]
- Conconi, F.; Grazzi, G.; Casoni, I.; Guglielmini, C.; Borsetto, C.; Ballarin, E.; Mazzoni, G.; Patracchini, M.; Manfredini, F. The Conconi Test: Methodology After 12 Years of Application. Int. J. Sports Med. 1996, 17, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.A. Psychophysical Bases of Perceived Exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Tian, X.; Zhang, H.; Huang, R.; Li, N.; Chen, P.; Wang, R. Exercise as a Prescription for Patients with Various Diseases. J. Sport Health Sci. 2019, 8, 422–441. [Google Scholar] [CrossRef]
- Cuesta-Vargas, A.I.; Buchan, J.; Arroyo-Morales, M. A Multimodal Physiotherapy Programme plus Deep Water Running for Improving Cancer-Related Fatigue and Quality of Life in Breast Cancer Survivors. Eur. J. Cancer Care 2014, 23, 15–21. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).