Harmless Treatment of High Arsenic Tin Tailings and Environmental Durability Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Experimental Procedure
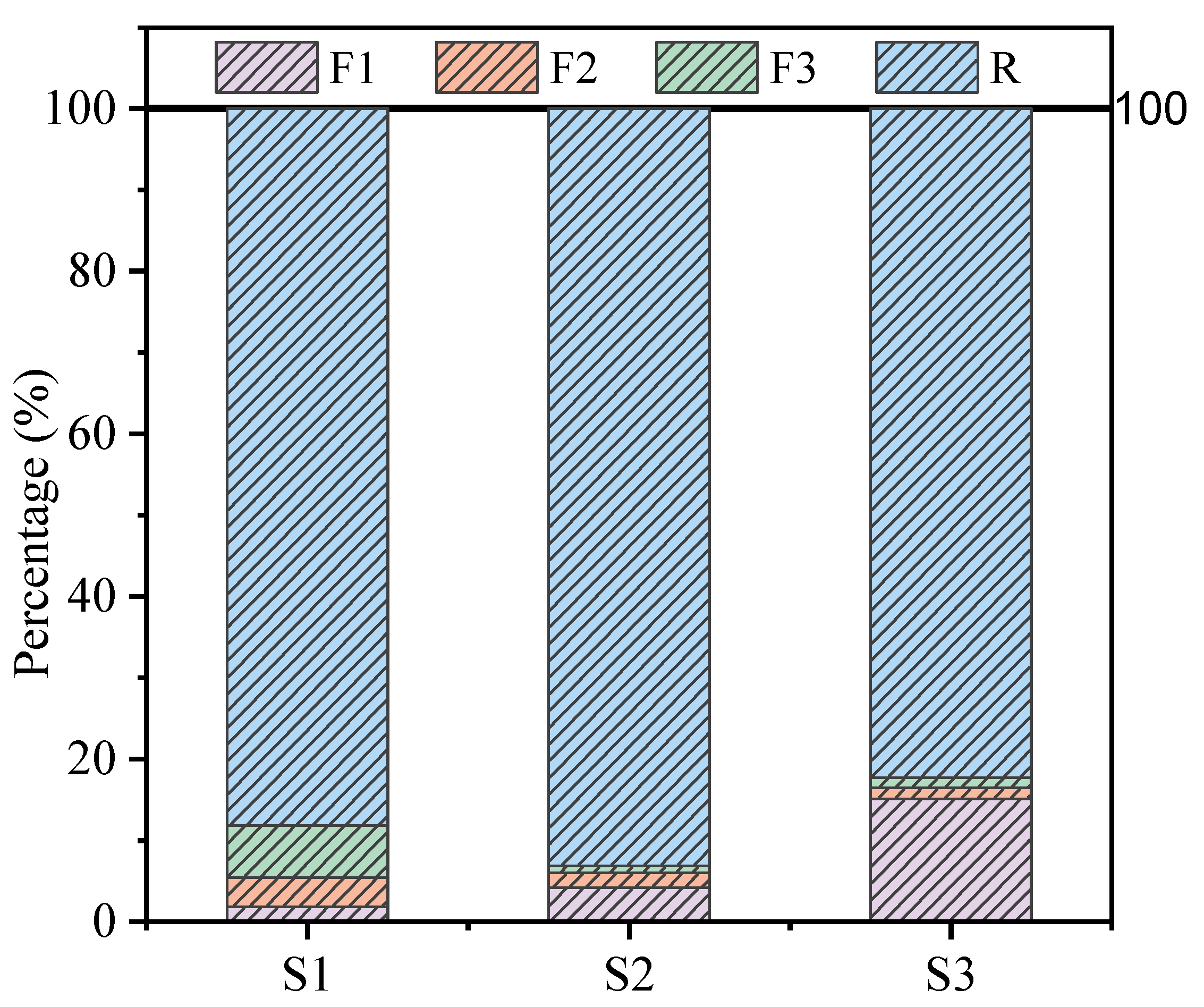
2.2. Leaching Tests
2.3. Analysis and Characterization
3. Results and Discussion
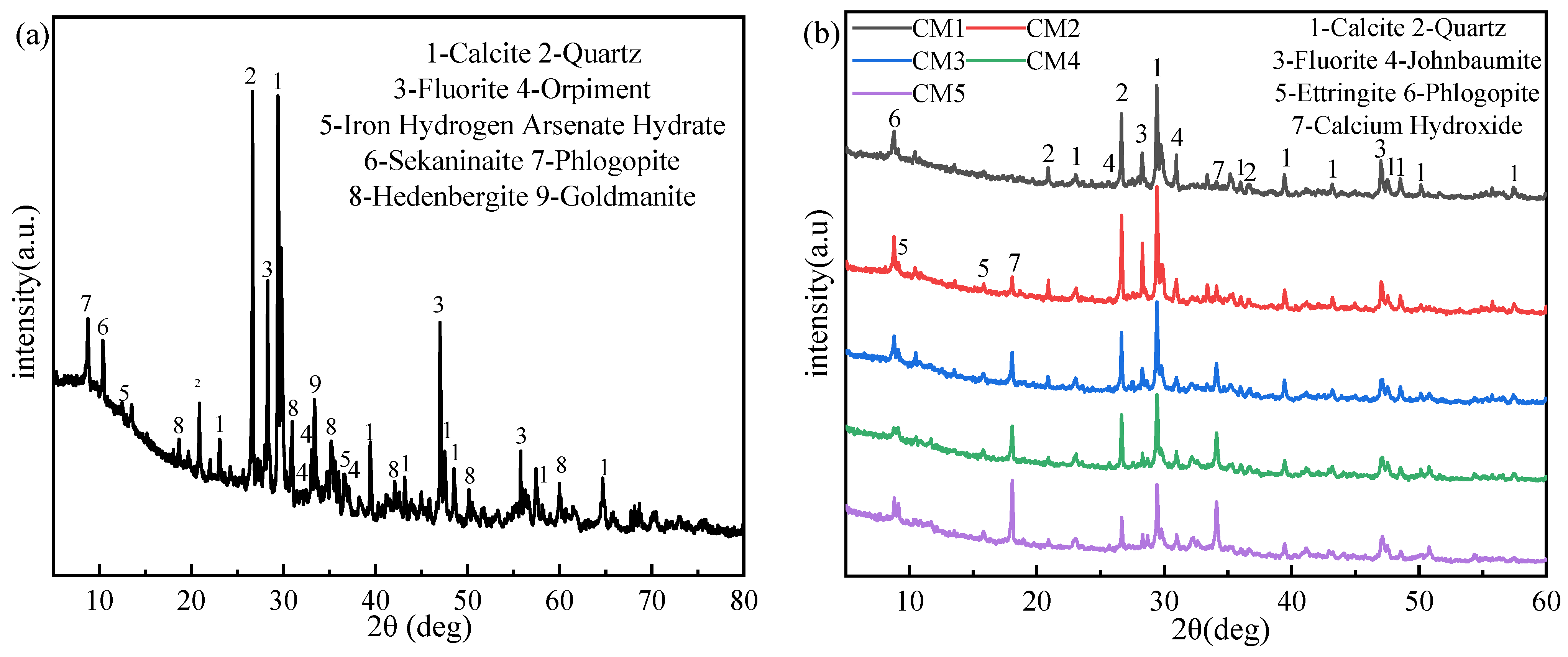
3.1. X-ray Diffraction Analysis of TT and Sample
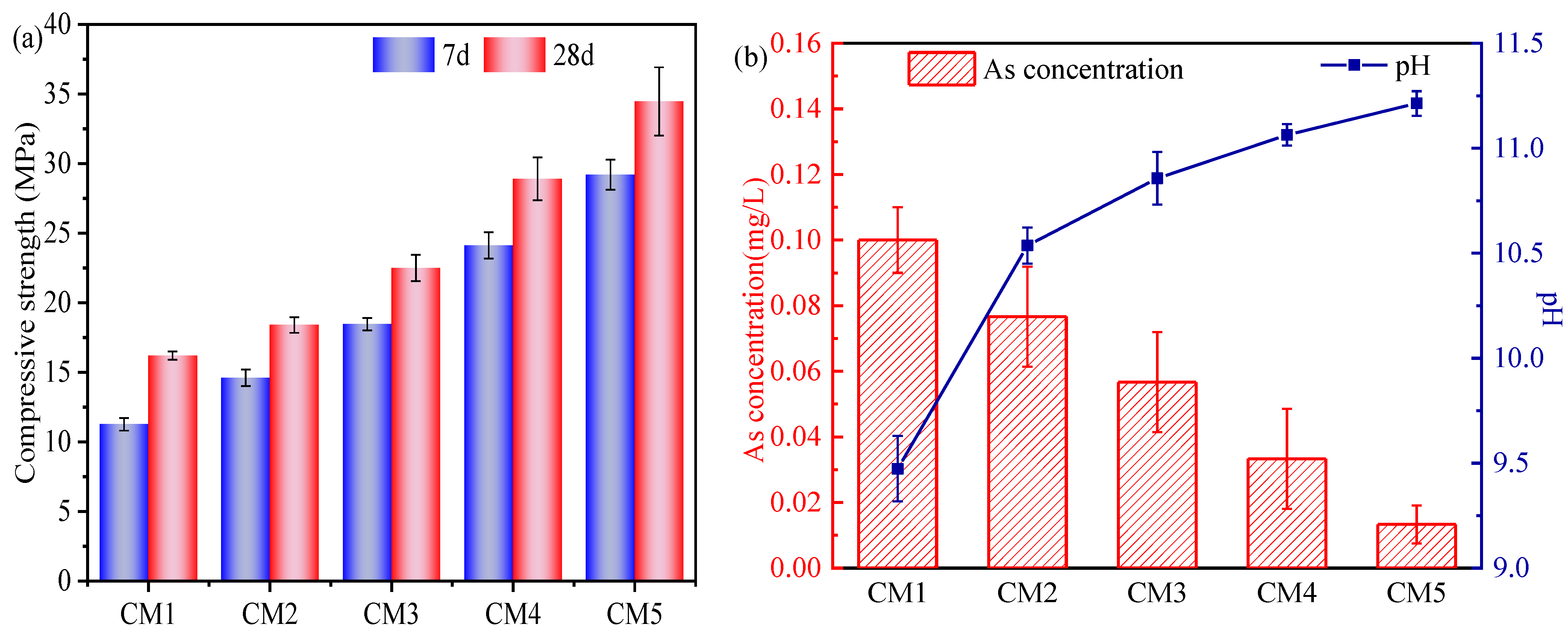
3.2. Compressive Strength and Leaching Test
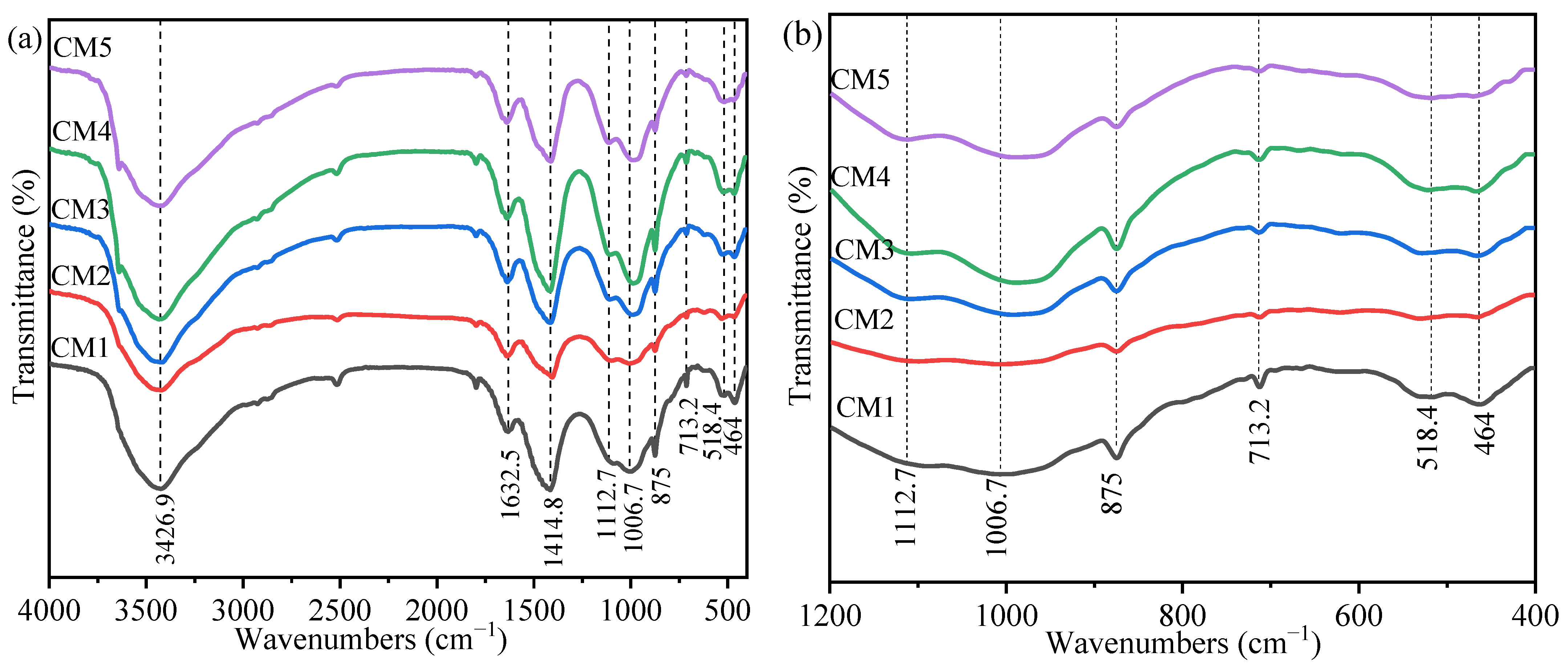
3.3. Fourier Transform Infrared Spectroscopy Analysis
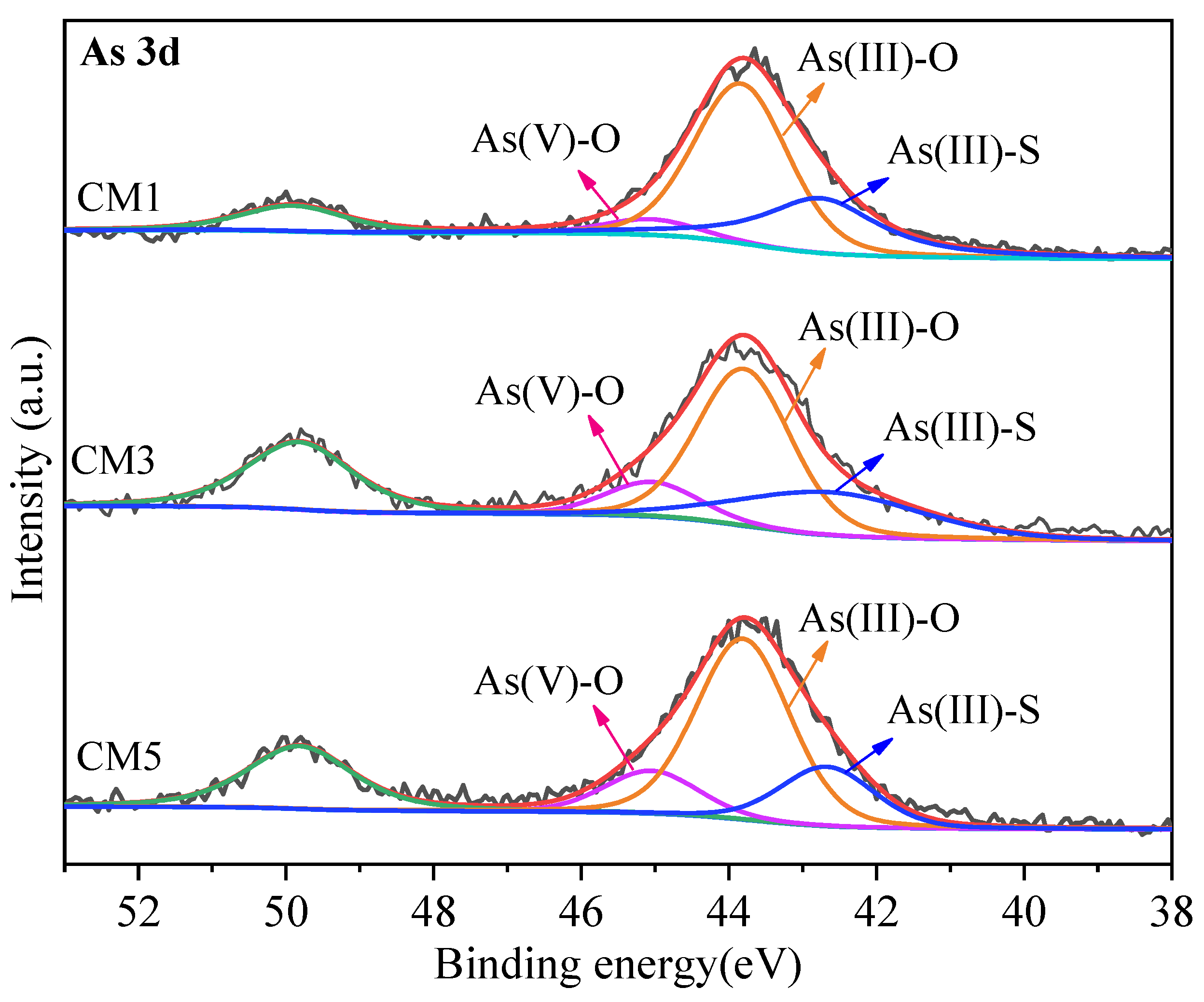
3.4. X-ray Photoelectron Spectroscopy Analysis
3.5. SEM-EDS Analysis
3.6. Environmental Durability Assessment
3.6.1. Effect of pH on the Leaching Behavior of As
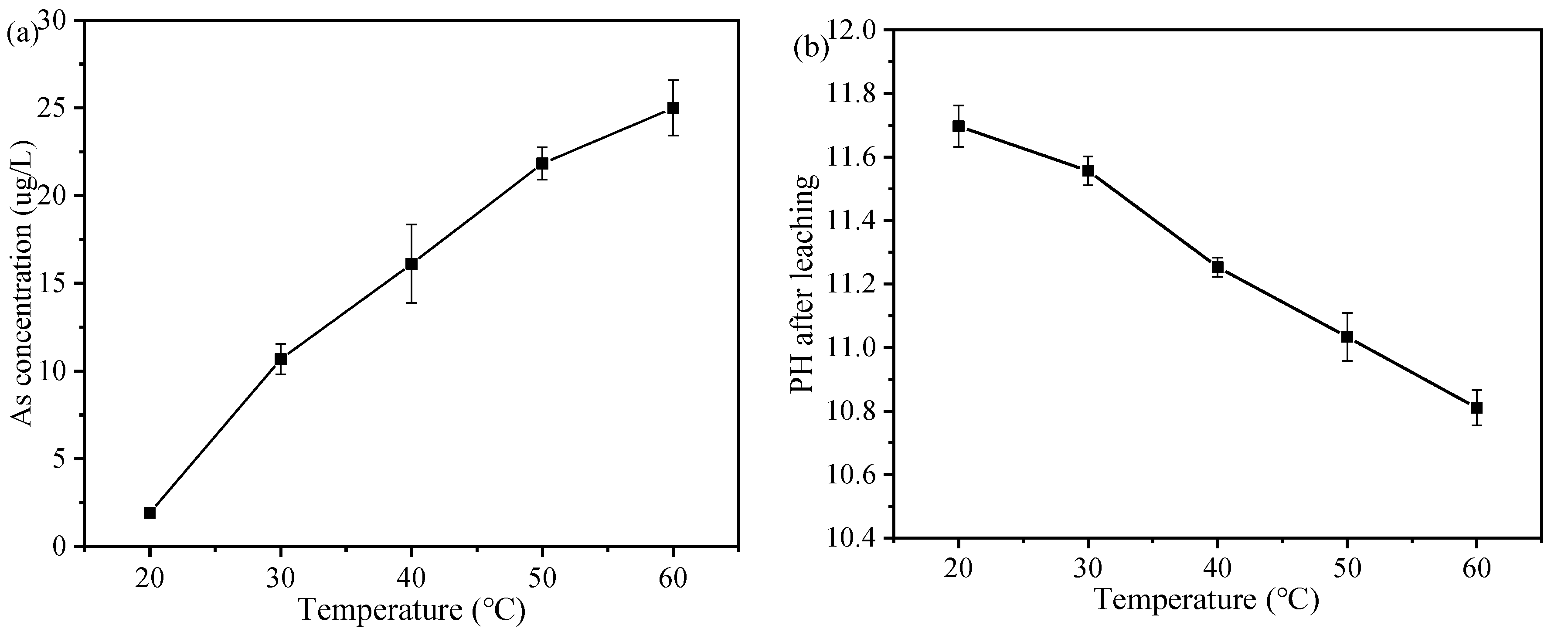
3.6.2. Effect of Temperature on the Leaching Behavior of As
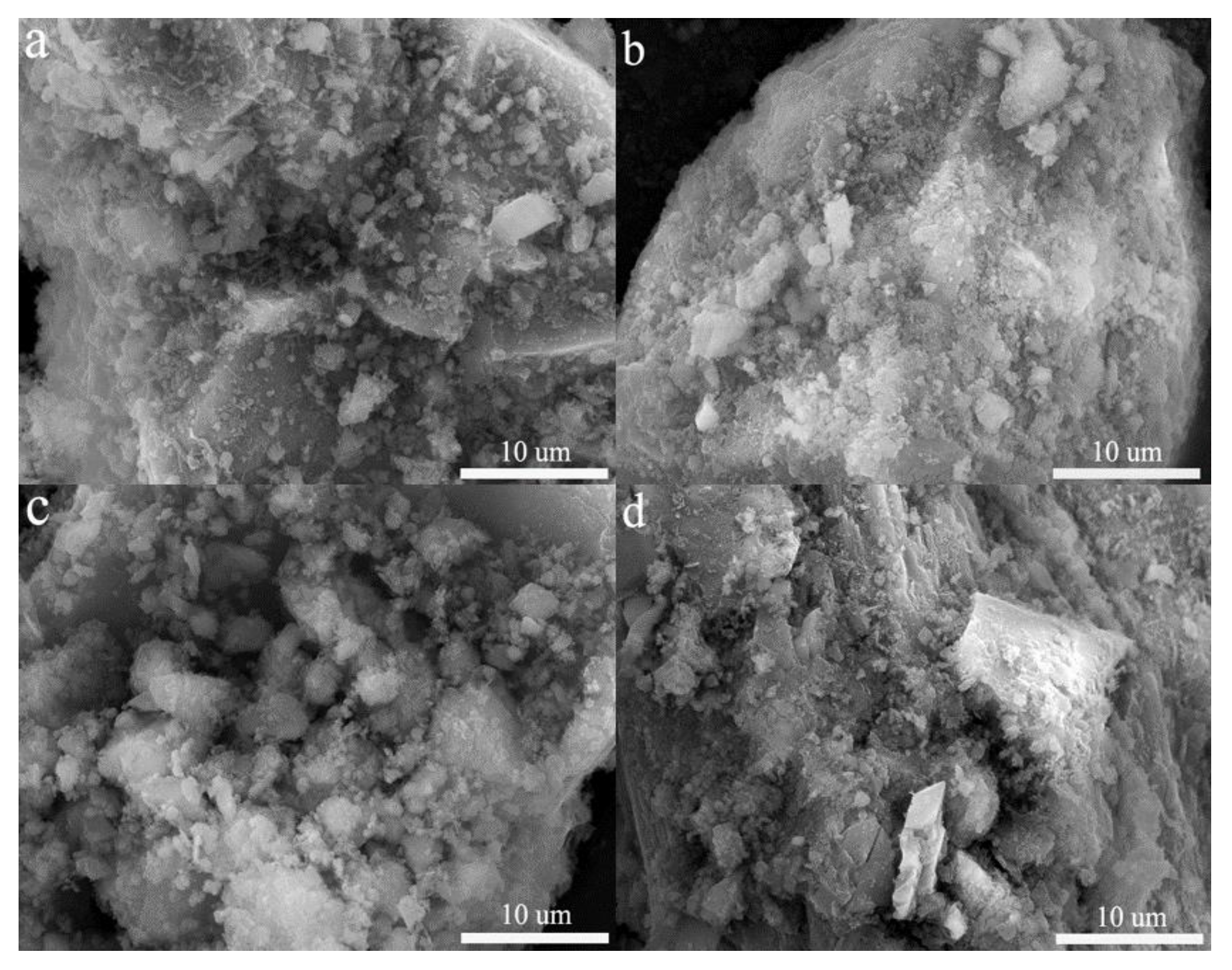
3.6.3. SEM Analysis
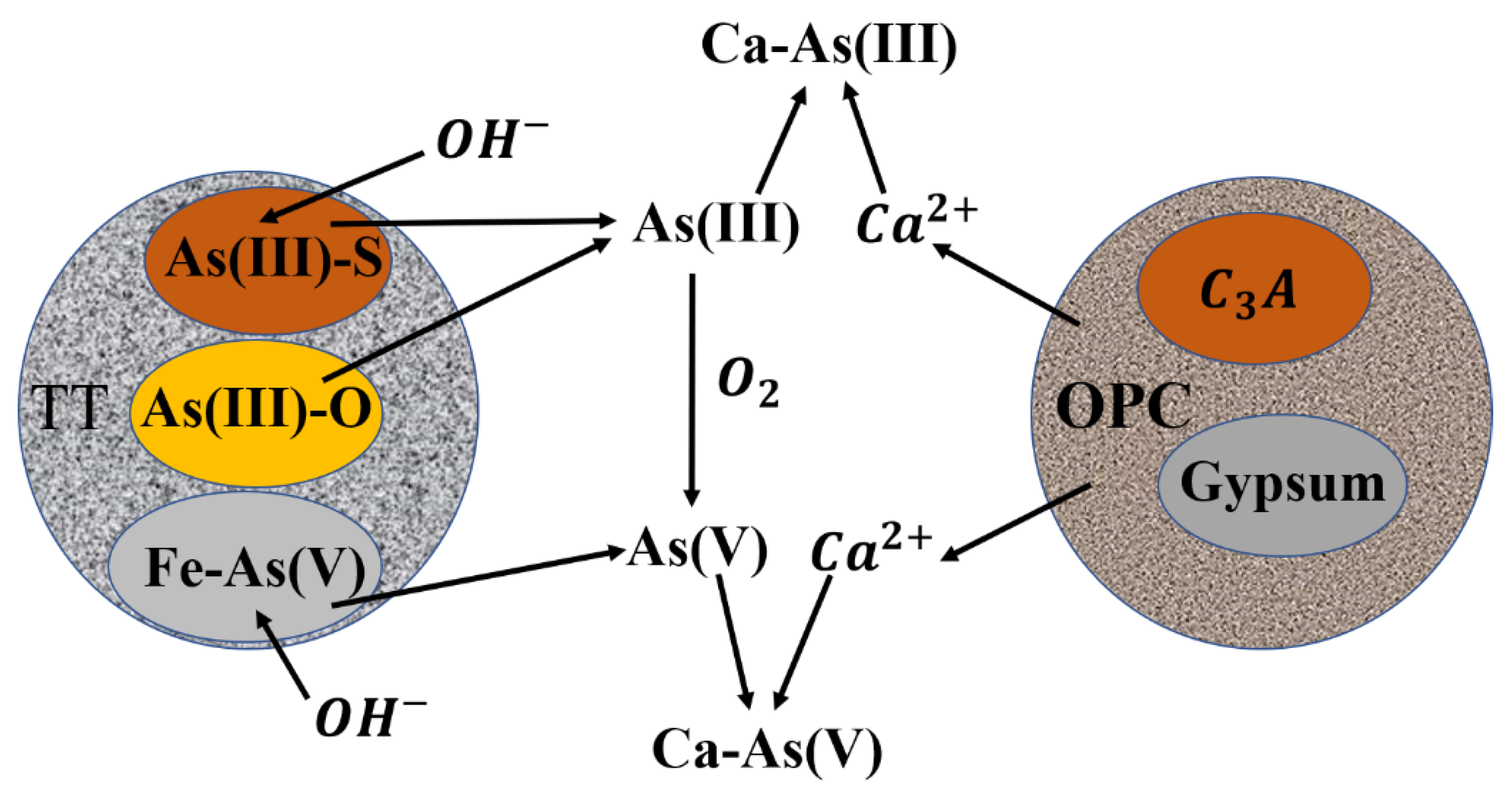
3.7. Mechanism of S/S Arsenic
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Xu, J.; Lv, Z.; Xie, R.; Huang, L.; Jiang, J. Impacts of biochar and oyster shells waste on the immobilization of arsenic in highly contaminated soils. J. Environ. Manag. 2018, 217, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Prommer, H.; Siade, A.J.; Chillrud, S.N.; Mailloux, B.J.; Bostick, B.C. Model-Based Analysis of Arsenic Immobilization via Iron Mineral Transformation under Advective Flows. Environ. Sci. Technol. 2018, 52, 9243–9253. [Google Scholar] [CrossRef] [PubMed]
- Nazari, A.M.; Radzinski, R.; Ghahreman, A. Review of arsenic metallurgy: Treatment of arsenical minerals and the immobilization of arsenic. Hydrometallurgy 2017, 174, 258–281. [Google Scholar] [CrossRef]
- Yoshiasa, A.; Tokuda, M.; Misawa, M.; Shimojo, F.; Momma, K.; Miyawaki, R.; Matsubara, S.; Nakatsuka, A.; Sugiyama, K. Natural arsenic with a unique order structure: Potential for new quantum materials. Sci. Rep. 2019, 9, 6275. [Google Scholar] [CrossRef]
- Shamsudduha, M.; Uddin, A.; Saunders, J.A.; Lee, M.-K. Quaternary stratigraphy, sediment characteristics and geochemistry of arsenic-contaminated alluvial aquifers in the Ganges-Brahmaputra floodplain in central Bangladesh. J. Contam. Hydrol. 2008, 99, 112–136. [Google Scholar] [CrossRef]
- Wen, Z.; Zhang, Y.; Zhou, X.; Chen, R. Effective As(III) and As(V) immobilization from aqueous solution by nascent ferrous hydroxide colloids (FHC). Sep. Purif. Technol. 2017, 176, 395–401. [Google Scholar] [CrossRef]
- Silva, R.A.; Park, J.; Ilyas, S.; Borja, D.; Zhao, H.; Urík, M.; Rastegar, S.O.; Kim, H. Biodegradation mechanism of arsenopyrite mine tailing with Acidithiobacillus ferrooxidans and influence of ferric supplements. Int. Biodeterior. Biodegrad. 2020, 153, 105042. [Google Scholar] [CrossRef]
- Wang, X.; Ding, J.; Wang, L.; Zhang, S.; Hou, H.; Zhang, J.; Chen, J.; Ma, M.; Tsang, D.C.W.; Wu, X. Stabilization treatment of arsenic-alkali residue (AAR): Effect of the coexisting soluble carbonate on arsenic stabilization. Environ. Int. 2020, 135, 105406. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Wang, L.; Chen, J.; Xu, S.; Hou, H.; Shi, Y.; Zhang, J.; Ma, M.; Tsang, D.C.W.; et al. Transformation of arsenic during realgar tailings stabilization using ferrous sulfate in a pilot-scale treatment. Sci. Total Environ. 2019, 668, 32–39. [Google Scholar] [CrossRef]
- Vermeulen, M.; Sanyova, J.; Janssens, K.; Nuyts, G.; De Meyer, S.; De Wael, K. The darkening of copper- or lead-based pigments explained by a structural modification of natural orpiment: A spectroscopic and electrochemical study. J. Anal. At. Spectrom. 2017, 32, 1331–1341. [Google Scholar] [CrossRef]
- Alvarez-Ayuso, E.; Murciego, A. Stabilization methods for the treatment of weathered arsenopyrite mine wastes: Arsenic immobilization under selective leaching conditions. J. Clean. Prod. 2021, 283, 125265. [Google Scholar] [CrossRef]
- Hammond, C.M.; Root, R.A.; Maier, R.M.; Chorover, J. Mechanisms of Arsenic Sequestration by Prosopis juliflora during the Phytostabilization of Metalliferous Mine Tailings. Environ. Sci. Technol. 2018, 52, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Hamberg, R.; Maurice, C.; Alakangas, L. The use of low binder proportions in cemented paste backfill—Effects on As-leaching. Miner. Eng. 2015, 78, 74–82. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, W.; Ni, W.; Zhang, S.; Li, Y.; Wang, K.; Huang, X.; Fu, P.; Hu, W. Influence of calcium hydroxide addition on arsenic leaching and solidification/stabilisation behaviour of metallurgical-slag-based green mining fill. J. Hazard. Mater. 2020, 390, 122161. [Google Scholar] [CrossRef]
- Wang, J.; Sun, X.; Xing, Y.; Xia, J.; Feng, X. Immobilization of mercury and arsenic in a mine tailing from a typical Carlin-type gold mining site in southwestern part of China. J. Clean. Prod. 2019, 240, 118171. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Ni, W.; Yan, Q.; Gao, W.; Li, Y. Immobilisation of high-arsenic-containing tailings by using metallurgical slag-cementing materials. Chemosphere 2019, 223, 117–123. [Google Scholar] [CrossRef]
- Long, W.J.; Ye, T.H.; Xing, F.; Khayat, K.H. Decalcification effect on stabilization/solidification performance of Pb-containing geopolymers. Cem. Concr. Compos. 2020, 114, 103803. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Tsang, D.C.W.; Zhou, Y.; Rinklebe, J.; Song, H.; Kwon, E.E.; Baek, K.; Ok, Y.S. Mechanistic insights into red mud, blast furnace slag, or metakaolin-assisted stabilization/solidification of arsenic-contaminated sediment. Environ. Int. 2019, 133, 105247. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, K.; Li, J.-S.; Tsang, D.C.W.; Poon, C.S.; Yoo, J.-C.; Baek, K.; Ding, S.; Hou, D.; Dai, J.-G. Low-carbon and low-alkalinity stabilization/solidification of high-Pb contaminated soil. Chem. Eng. J. 2018, 351, 418–427. [Google Scholar] [CrossRef]
- Wang, L.; Cho, D.W.; Tsang, D.C.W.; Cao, X.; Hou, D.; Shen, Z.; Alessi, D.S.; Ok, Y.S.; Poon, C.S. Green remediation of As and Pb contaminated soil using cement-free clay-based stabilization/solidification. Environ. Int. 2019, 126, 336–345. [Google Scholar] [CrossRef]
- Liu, D.G.; Min, X.B.; Ke, Y.; Chai, L.-Y.; Liang, Y.; Li, Y.; Yao, L.; Wang, Z. Co-treatment of flotation waste, neutralization sludge, and arsenic-containing gypsum sludge from copper smelting: Solidification/stabilization of arsenic and heavy metals with minimal cement clinker. Environ. Sci. Pollut. Res. 2018, 25, 7600–7607. [Google Scholar] [CrossRef] [PubMed]
- Kunther, W.; Lothenbach, B.; Skibsted, J. Influence of the Ca/Si ratio of the C-S-H phase on the interaction with sulfate ions and its impact on the ettringite crystallization pressure. Cem. Concr. Res. 2015, 69, 37–49. [Google Scholar] [CrossRef]
- Sasaki, T.; Iizuka, A.; Watanabe, M.; Hongo, T.; Yamasaki, A. Preparation and performance of arsenate (V) adsorbents derived from concrete wastes. Waste Manag. 2014, 34, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wei, W.; Tang, S.; Zhu, Z.; Yan, Q.; Zhang, L.; Deng, H. A comparative study on the dissolution and stability of beudantite and hidalgoite at pH 2–12 and 25–45 degrees C for the possible long-term simultaneous immobilization of arsenic and lead. Chemosphere 2021, 263, 128386. [Google Scholar]
- Bull, A.J.; Fall, M. Curing temperature dependency of the release of arsenic from cemented paste backfill made with Portland cement. J. Environ. Manag. 2020, 269, 110772. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, P.; Yuan, S.; Tong, M. Arsenic oxidation and immobilization in acid mine drainage in karst areas. Sci. Total Environ. 2020, 727, 138629. [Google Scholar] [CrossRef]
- Yang, D.Z.; Sasaki, A.; Endo, M. Reclamation of an arsenic-bearing gypsum via acid washing and CaO-As stabilization involving svabite formation in thermal treatment. J. Environ. Manag. 2019, 231, 811–818. [Google Scholar] [CrossRef]
- Ghosh, A.; Mukiibi, M.; Ela, W. TCLP underestimates leaching of arsenic from solid residuals under landfill conditions. Environ. Sci. Technol. 2004, 38, 4677–4682. [Google Scholar] [CrossRef]
- Wang, C.; Hu, X.; Chen, M.L.; Wu, Y.H. Total concentrations and fractions of Cd, Cr, Pb, Cu, Ni and Zn in sewage sludge from municipal and industrial wastewater treatment plants. J. Hazard. Mater. 2005, 119, 245–249. [Google Scholar] [CrossRef]
- Ji, Z.H.; Pei, Y.S. Immobilization efficiency and mechanism of metal cations (Cd2+, Pb2+ and Zn2+) and anions (AsO43− and Cr2O72−) in wastes-based geopolymer. J. Hazard. Mater. 2020, 384, 121290. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Min, X.; Ke, Y.; Fei, J.; Liu, D.; Tang, C. Immobilization potential and immobilization mechanism of arsenic in cemented paste backfill. Miner. Eng. 2019, 138, 101–107. [Google Scholar] [CrossRef]
- Askarian, M.; Tao, Z.; Samali, B.; Adam, G.; Shuaibu, R. Mix composition and characterisation of one-part geopolymers with different activators. Constr. Build. Mater. 2019, 225, 526–537. [Google Scholar] [CrossRef]
- Min, X.; Liu, D.; Chai, L.; Ke, Y.; Liang, Y.; Shi, M.; Li, Y.; Yang, C.; Wang, W.; Wang, Z. Comparison of arsenic immobilization properties among calcium silicate hydrate, ettringite, and friedel’s salt in a slag-based binder. Environ. Prog. Sustain. Energy 2019, 38, S422–S428. [Google Scholar] [CrossRef]
- Kim, B.J.; Jang, J.G.; Park, C.Y.; Han, O.H.; Kim, H.K. Recycling of arsenic-rich mine tailings in controlled low-strength materials. J. Clean. Prod. 2016, 118, 151–161. [Google Scholar] [CrossRef]
- Li, Y.; Min, X.; Ke, Y.; Chai, L.; Shi, M.; Tang, C.; Wang, Q.; Liang, Y.; Lei, J.; Liu, D. Utilization of red mud and Pb/Zn smelter waste for the synthesis of a red mud-based cementitious material. J. Hazard. Mater. 2018, 344, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tan, H.; He, X.; Yang, W.; Deng, X.; Su, Y.; Yang, J. Compressive strength and hydration process of ground granulated blast furnace slag-waste gypsum system managed by wet grinding. Constr. Build. Mater. 2019, 228, UNSP116777. [Google Scholar] [CrossRef]
- Lu, Q.; Xiao, H.; Du, Y.; Du, D. Using CaO to stabilize arsenic sulfide slag by moderate temperature calcination. Environ. Earth Sci. 2017, 76, 262. [Google Scholar] [CrossRef]
- Kurz, E.E.C.; Luong, V.T.; Hellriegel, U.; Leidinger, F.; Luu, T.L.; Bundsch, J.; Hoinkis, J. Iron-based subsurface arsenic removal (SAR): Results of a long-term pilot-scale test in Vietnam. Water Res. 2020, 181, 115929. [Google Scholar] [CrossRef]
- Zhong, D.; Zhao, Z.; Jiang, Y.; Yang, X.; Wang, L.; Chen, J.; Guan, C.; Zhang, Y.; Tsang, D.C.W.; Crittenden, J.C. Contrasting abiotic As(III) immobilization by undissolved and dissolved fractions of biochar in Ca2+-rich groundwater under anoxic conditions. Water Res. 2020, 183, 116106. [Google Scholar] [CrossRef]
- Elyamine, A.M.; Moussa, M.G.; Afzal, J.; Rana, M.S.; Imran, M.; Zhao, X.; Hu, C.X. Modified Rice Straw Enhanced Cadmium (II) Immobilization in Soil and Promoted the Degradation of Phenanthrene in Co-Contaminated Soil. Int. J. Mol. Sci. 2019, 20, 2189. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, C.; Zhao, M.; Yang, K.; Shen, R.; Zheng, Y. Immobilization potential of Cr(VI) in sodium hydroxide activated slag pastes. J. Hazard. Mater. 2017, 321, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hu, L.; Tang, L.; Ren, J. Utilisation of municipal solid waste incinerator (MSWI) fl y ash with metakaolin for preparation of alkali-activated cementitious material. J. Hazard. Mater. 2021, 402, 123451. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Yuen, T.F.; Shen, C.J. Conductivity and FTIR studies on PEO-LiX X: CF3SO3-, SO42- polymer electrolytes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 69, 670–675. [Google Scholar] [CrossRef] [PubMed]
- El-Moselhy, M.M.; Ates, A.; Celebi, A. Synthesis and characterization of hybrid iron oxide silicates for selective removal of arsenic oxyanions from contaminated water. J. Colloid Interface Sci. 2017, 488, 335–347. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, H.; Xia, J.; Nie, Z.; Fan, X.; Liu, H.; Zheng, L.; Zhang, L.; Yang, H. Humic acid promotes arsenopyrite bio-oxidation and arsenic immobilization. J. Hazard. Mater. 2020, 384, 121359. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Corpuz, R.D.; Iarashi, T.; Villacorte-Tabeline, M.; Ito, M.; Hiroyoshi, N. Hematite-catalysed scorodite formation as a novel arsenic immobilisation strategy under ambient conditions. Chemosphere 2019, 233, 946–953. [Google Scholar] [CrossRef]
- Zhao, Z.; Song, Y.; Min, X.; Liang, Y.; Chai, L.; Shi, M. XPS and FTIR studies of sodium arsenate vitrification by cullet. J. Non-Cryst. Solids 2018, 502, 254. [Google Scholar] [CrossRef]
- Opiso, E.M.; Tabelin, C.B.; Maestre, C.V.; Aseniero, J.P.J.; Park, I.; Villacorte-Tabelin, M. Synthesis and characterization of coal fly ash and palm oil fuel ash modified artisanal and small-scale gold mine (ASGM) tailings based geopolymer using sugar mill lime sludge as Ca-based activator. Heliyon 2021, 7, e06654. [Google Scholar] [CrossRef]
- Suess, E.; Planer-Friedrich, B. Thioarsenate formation upon dissolution of orpiment and arsenopyrite. Chemosphere 2012, 89, 1390–1398. [Google Scholar] [CrossRef]
- Phenrat, T.; Marhaba, T.F.; Rachakornkij, M. A SEM and X-ray study for investigation of solidified/stabilized arsenic-iron hydroxide sludge. J. Hazard. Mater. 2005, 118, 185–195. [Google Scholar] [CrossRef]
- Fei, J.; Ma, J.; Yang, J.; Liang, Y.; Ke, Y.; Yao, L.; Li, Y.; Liu, D.; Min, X. Effect of simulated acid rain on stability of arsenic calcium residue in residue field. Environ. Geochem. Health 2020, 42, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, D.; Li, X.; Zhang, G.; Wang, Y.; Wang, X.; Gomez, M.A.; Jia, Y. Arsenic associated with gypsum produced from Fe(III)-As(V) coprecipitation: Implications for the stability of industrial As-bearing waste. J. Hazard. Mater. 2018, 360, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yao, S.; Yuan, Z.; Bi, R.; Wu, X.; Zhang, J.; Wang, S.; Wang, X.; Jia, Y. Detoxification and reclamation of hydrometallurgical arsenic- and trace metals-bearing gypsum via hydrothermal recrystallization in acid solution. Chemosphere 2020, 250, 126290. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, L.; Mccabe, B.A.; Chen, Y.; Morrison, L. Dredged marine sediments stabilized/solidi fied with cement and GGBS: Factors affecting mechanical behaviour and leachability. Sci. Total Environ. 2020, 733, 138551. [Google Scholar] [CrossRef]
- Wang, P.; Mo, R.; Li, S.; Xu, J.; Jin, Z.; Zhao, T.; Wang, D. A chemo-damage-transport model for chloride ions diffusion n cement-based materials: Combined effects of sulfate attack and temperature. Constr. Build. Mater. 2021, 288, 123121. [Google Scholar] [CrossRef]
- Gacsi, A.; Kutus, B.; Konya, Z.; Kukovecz, A.; Pálinkó, I.; Sipos, P. Estimation of the solubility product of hydrocalumite-hydroxide, a layered double hydroxide with the formula of Ca2Al(OH)6OH·nH2O. J. Phys. Chem. Solids 2016, 98, 167–173. [Google Scholar] [CrossRef]
- Li, G.; Li, X.; Qi, X.; Zhang, A. Copper slag gel encapsulates sludge through encapsulation and precipitation in weakly acidic to strongly basic environments. J. Clean. Prod. 2021, 294, 126227. [Google Scholar] [CrossRef]
- Bull, A.J.; Fall, M. Thermally induced changes in metalloid leachability of cemented paste backfill that contains blast furnace slag. Miner. Eng. 2020, 156, 106520. [Google Scholar] [CrossRef]


| Material | Al2O3 | CaO | Fe2O3 | K2O | MgO | P2O5 | SiO2 | SO3 | LOI |
|---|---|---|---|---|---|---|---|---|---|
| OPC | 7.1 | 41.3 | 6.2 | 0.3 | 2.8 | 0.09 | 24.5 | 0.05 | 17.66 |
| TT | 6.2 | 28.5 | 16.4 | 1.1 | 0.08 | 0.06 | 31.7 | 2.4 | 13.56 |
| Sample | Mixture Ratio (wt%) | Water Cement Ratio (W/C) | |
|---|---|---|---|
| TT | OPC | ||
| CM1 | 75 | 25 | 0.28 |
| CM2 | 66.7 | 33.3 | |
| CM3 | 50 | 50 | |
| CM4 | 33.3 | 66.7 | |
| CM5 | 25 | 75 | |
| Wavenumbers (cm−1) | Functional Group | Types of Vibration | Reference |
|---|---|---|---|
| 3426.9 cm−1 | Free H2O | stretching vibration | [30] |
| 1632.5 cm−1 | H2O in C-S-H | bending vibration | [41] |
| 1414.8 cm−1 | O-C-O | stretching vibration | [42] |
| 1112.7 cm−1 | SO42− | v3 vibrations | [43] |
| 1006.7 cm−1 | SiO42− | v3 vibrations | [44] |
| 875.0 cm−1 | As-O | stretching vibration | [45,46] |
| 713.2 cm−1 | SO42− | v4 vibrations | [16] |
| 518.4 cm−1 | SiO42− | v4 vibrations | [47] |
| 464.0 cm−1 | Si-O | bending vibration | [48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Zhang, Z.; Yang, H.; Zhou, X.; Wang, J.; Li, C. Harmless Treatment of High Arsenic Tin Tailings and Environmental Durability Assessment. Int. J. Environ. Res. Public Health 2022, 19, 11247. https://doi.org/10.3390/ijerph191811247
Zhao W, Zhang Z, Yang H, Zhou X, Wang J, Li C. Harmless Treatment of High Arsenic Tin Tailings and Environmental Durability Assessment. International Journal of Environmental Research and Public Health. 2022; 19(18):11247. https://doi.org/10.3390/ijerph191811247
Chicago/Turabian StyleZhao, Weiwei, Zhengfu Zhang, Hui Yang, Xian Zhou, Jinsong Wang, and Chengping Li. 2022. "Harmless Treatment of High Arsenic Tin Tailings and Environmental Durability Assessment" International Journal of Environmental Research and Public Health 19, no. 18: 11247. https://doi.org/10.3390/ijerph191811247
APA StyleZhao, W., Zhang, Z., Yang, H., Zhou, X., Wang, J., & Li, C. (2022). Harmless Treatment of High Arsenic Tin Tailings and Environmental Durability Assessment. International Journal of Environmental Research and Public Health, 19(18), 11247. https://doi.org/10.3390/ijerph191811247





