The Prevalence of Oral Mucosa Lesions in Pediatric Patients
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hong, C.H.L.; Dean, D.R.; Hull, K.; Hu, S.J.; Sim, Y.F.; Nadeau, C.; Gonçalves, S.; Lodi, G.; Hodgson, T.A. World Workshop on Oral Medicine VII: Relative frequency of oral mucosal lesions in children, a scoping review. Oral Dis. 2019, 25, 193–203. [Google Scholar] [CrossRef]
- Shulman, J.D. Prevalence of oral mucosal lesions in children and youths in the USA. Int. J. Paediatr. Dent. 2005, 15, 89–97. [Google Scholar] [CrossRef]
- Yáñez, M.; Escobar, E.; Oviedo, C.; Stillfried, A.; Pennacchiotti, G. Prevalence of Oral Mucosal Lesions in Children Prevalencia de Lesiones de la Mucosa Oral en Niños. Int. J. Odontostomat 2016, 10, 463–468. [Google Scholar] [CrossRef]
- Colaci, R.; Sfasciott, G. Most Common Oral Mucosal Lesions in Children: Prevalence and Differential Diagnosis. WedmedCentral DENTISTRY, 4. 2013. Available online: https://pdfs.semanticscholar.org/4f-d7/4cf18648a9168da47dc95b0ef6e96202b466.pdf (accessed on 12 May 2022).
- Yilmaz, A.E.; Gorpelioglu, C.; Sarifakioglu, E.; Dogan, D.G.; Bilici, M.; Celik, N. Prevalence of oral mucosal lesions from birth to two years. Niger. J. Clin. Pract. 2011, 14, 349–353. [Google Scholar] [CrossRef]
- Jahanbani, J.; Morse, D.E.; Alinejad, H. Prevalence of oral lesions and normal variants of the oral mucosa in 12 to 15-year-old students in Tehran, Iran. Arch. Iran. Med. 2012, 15, 142–145. [Google Scholar]
- Mumcu, G.; Cimilli, H.; Sur, H.; Hayran, O.; Atalay, T. Prevalence and distribution of oral lesions: A cross-sectional study in Turkey. Oral Dis. 2005, 11, 81–87. [Google Scholar] [CrossRef]
- Vieira-Andrade, R.G.; Martins-Júnior, P.A.; Corrêa-Faria, P.; Stella, P.E.M.; Marinho, S.A.; Marques, L.S.; Ramos-Jorge, M.L. Oral mucosal conditions in preschool children of low socioeconomic status: Prevalence and determinant factors. Eur. J. Pediatr. 2013, 172, 675–681. [Google Scholar] [CrossRef]
- Amadori, F.; Bardellini, E.; Conti, G.; Majorana, A. Oral mucosal lesions in teenagers: A cross-sectional study. Ital. J. Pediatr. 2017, 43, 50. [Google Scholar] [CrossRef]
- Majorana, A.; Bardellini, E.; Flocchini, P.; Amadori, F.; Conti, G.; Campus, G. Oral mucosal lesions in children from 0 to 12 years old: Ten years’ experience. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2010, 110, e13–e18. [Google Scholar] [CrossRef]
- Natah, S.S.; Konttinen, Y.T.; Enattah, N.S.; Ashammakhi, N.; Sharkey, K.A.; Häyrinen-Immonen, R. Recurrent aphthous ulcers today: A review of the growing knowledge. Int. J. Oral Maxillofac. Surg. 2004, 33, 221–234. [Google Scholar] [CrossRef]
- Edgar, N.R.; Saleh, D.; Miller, R.A. Recurrent Aphthous Stomatitis: A review. J. Clin. Aesthet. Derm. 2017, 10, 26–36. [Google Scholar]
- Owczarek-Drabińska, J.E.; Radwan-Oczko, M. A Case of Lingua Villosa Nigra (Black Hairy Tongue) in a 3-Month-Old Infant. Am. J. Case Rep. 2020, 21, e926362-1–e926362-4. [Google Scholar] [CrossRef]
- Radwan-Oczko, M.; Sokół, I.; Babuśka, K.; Owczarek-Drabińska, J.E. Prevalence and Characteristic of Oral Mucosa Lesions. Symmetry 2022, 14, 307. [Google Scholar] [CrossRef]
- Vučićević Boras, V.; Andabak Rogulj, A.; Alajbeg, I.; Skrinjar, I.; Brzak, B.L.; Brailo, V.; Vidović Juras, D.; Verzak, Z. The prevalence of oral mucosal lesions in Croatian children. Paediatr. Croat. 2013, 57, 235–238. [Google Scholar]
- Williams, K.; Thomson, D.; Seto, I.; Contopoulos-Ioannidis, D.G.; Ioannidis, J.P.A.; Curtis, S.; Constantin, E.; Batmanabane, G.; Hartling, L.; Klassen, T. Standard 6: Age Groups for Pediatric Trials. Pediatrics 2012, 129, S153–S160. [Google Scholar] [CrossRef]
- Kleinman, D.V.; Swango, P.A.; Pindborg, J.J. Epidemiology of oral mucosal lesions in United States schoolchildren: 1986-87. Community Dent. Oral Epidemiol. 1994, 22, 243–253. [Google Scholar] [CrossRef]
- Lima, G.d.S.; Fontes, S.T.; de Araújo, L.M.A.; Etges, A.; Tarquinio, S.B.C.; Gomes, A.P.N. A survey of oral and maxillofacial biopsies in children: A single-center retrospective study of 20 years in Pelotas-Brazil. J. Appl. Oral Sci. 2008, 16, 397–402. Available online: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1678-77572008000600008&lng=en&tlng=en (accessed on 1 May 2022). [CrossRef]
- Espinosa-Zapata, M.; Loza-Hernández, G.; Mondragón-Ballesteros, R. Prevalencia de lesiones de la mucosa bucal en pacien_tes pediátricos. Informe prelimina. Cir. Ciruj 2006, 74, 153–157. [Google Scholar]
- Garcia-Pola, M.J.; Garcia-Martin, J.M.; Gonzalez-Garcia, M. Prevalence of oral lesions in the 6-year-old pediatric population of Oviedo (Spain). Med. Oral 2002, 7, 184–191. Available online: http://www.ncbi.nlm.nih.gov/pubmed/11984500 (accessed on 1 May 2022).
- Furlanetto, D.L.C.; Crighton, A.; Topping, G.V.A. Differences in methodologies of measuring the prevalence of oral mucosal lesions in children and adolescents. Int. J. Paediatr. Dent. 2006, 16, 31–39. [Google Scholar] [CrossRef]
- Ata-Ali, J.; Carrillo, C.; Bonet, C.; Balaguer, J.; Penarrocha, M.; Penarrocha, M. Oral mucocele: Review of the literature. J. Clin. Exp. Dent. 2010, 2, e18–e21. [Google Scholar] [CrossRef]
- Sousa, F.B.; Etges, A.; Corrêa, L.; Mesquita, R.A.; Soares de Araújo, N. Pediatric oral lesions: A 15-year review from São Paulo, Brazil. J. Clin. Pediatr. Dent. 2002, 26, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Bentley, J.M.; Barankin, B.; Guenther, L.C. A Review of Common Pediatric Lip Lesions: Herpes Simplex/Recurrent Herpes Labialis, Impetigo, Mucoceles, and Hemangiomas. Clin. Pediatr. 2003, 42, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, M.S.; Hebling, J.; Filho, V.A.P.; Santos, L.L.; Vita, T.M.; Costa, C.A.S. Extravasation mucocele involving the ventral surface of the tongue (glands of Blandin? Nuhn). Int. J. Paediatr. Dent. 2006, 16, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Vale, E.B.; Ramos-Perez, F.M.; Rodrigues, G.L.; Carvalho, E.J.; Castro, J.F.; Perez, D.E. A review of oral biopsies in children and adolescents: A clinicopathological study of a case series. J. Clin. Exp. Dent. 2013, 5, e144-9. [Google Scholar] [CrossRef]
- Bessa, C.F.N.; Santos, P.J.B.; Aguiar, M.C.F.; do Carmo, M.A.V. Prevalence of oral mucosal alterations in children from 0 to 12 years old. J. Oral Pathol. Med. 2004, 33, 17–22. [Google Scholar] [CrossRef]
- Jiménez Palacios, C.; Kkilikan, R.; Ramírez, R.; Ortiz, V.; Benítez, A.; Virgüez, Y. Levantamiento epidemiológico de lesiones patológicas en los tejidos blandos de la cavidad bucal de los niños y adolescente del centro odontopediátrico de carapa, parroquia antímano, caracas, distrito capital -venezuela. Período mayo-noviembre 2005. Acta Odontol. Venez 2007, 45, 540–545. [Google Scholar]
- Owczarek, J.E.; Lion, K.M.; Radwan-Oczko, M. Manifestation of stress and anxiety in the stomatognathic system of undergraduate dentistry students. J. Int. Med. Res. 2020, 48, 0300060519889487. [Google Scholar] [CrossRef]
- Owczarek, J.E.; Lion, K.M.; Radwan-Oczko, M. The impact of stress, anxiety and depression on stomatognathic system of physiotherapy and dentistry first-year students. Brain Behav. 2020, 10, e01797. [Google Scholar] [CrossRef]
- Moritz, S.; Müller, K.; Schmotz, S. Escaping the mouth-trap: Recovery from long-term pathological lip/cheek biting (morsicatio buccarum, cavitadaxia) using decoupling. J. Obs. Compuls. Relat. Disord. 2020, 25, 100530. [Google Scholar] [CrossRef]
- Wang, Y.L.; Chang, H.H.; Chang, J.Y.F.; Huang, G.F.; Guo, M.K. Retrospective Survey of Biopsied Oral Lesions in Pediatric Patients. J. Formos. Med. Assoc. 2009, 108, 862–871. [Google Scholar] [CrossRef]
- Jones, A.V.; Franklin, C.D. An analysis of oral and maxillofacial pathology found in children over a 30-year period. Int. J. Paediatr. Dent. 2006, 16, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Ünür, M.; Bektaş Kayhan, K.; Altop, M.S.; Boy Metin, Z.; Keskin, Y. The prevalence of oral mucosal lesions in children: A single center study. J. Istanbul Univ. Fac. Dent. 2015, 49, 29. [Google Scholar] [CrossRef] [PubMed]
- Sedano, H.O.; Freyre, I.C.; de la Garza, M.L.G.; Franco, C.M.G.; Hernandez, C.G.; Montoya, M.E.H.; Hipp, C.; Keenan, K.M.; Bravo, J.M.; López, J.A.M.; et al. Clinical orodental abnormalities in Mexican children. Oral Surg. Oral Med. Oral Pathol. 1989, 68, 300–311. [Google Scholar] [CrossRef]
- Gültelkin, S.E.; Tokman, B.; Türkseven, M.R. A review of paediatric oral biopsies in Turkey. Int. Dent. J. 2003, 53, 26–32. [Google Scholar] [CrossRef]
- Sato, M.; Tanaka, N.; Sato, T.; Amagasa, T. Oral and maxillofacial tumours in children: A review. Br. J. Oral Maxillofac. Surg. 1997, 35, 92–95. [Google Scholar] [CrossRef]
- Skinner, R.L.; Davenport, W.D.; Weir, J.C.; Carr, R.F. A survey of biopsied oral lesions in pediatric dental patients. Pediatr. Dent. 1986, 8, 163–167. [Google Scholar]
- Sixto-Requeijo, R.; Diniz-Freitas, M.; Torreira-Lorenzo, J.C.; García-García, A.; Gándara-Rey, J.M. An analysis of oral biopsies extracted from 1995 to 2009, in an oral medicine and surgery unit in Galicia (Spain). Med. Oral Patol. Oral Cir. Bucal 2012, 17, 1–7. [Google Scholar] [CrossRef]
- Sklavounou-Andrikopoulou, A.; Piperi, E.; Papanikolaou, V.; Karakoulakis, I. Oral soft tissue lesions in Greek children and adolescents: A retrospective analysis over a 32-year period. J. Clin. Pediatr. Dent. 2005, 29, 175–178. [Google Scholar] [CrossRef]
- Sȩkowska, A.; Chałas, R. Diastema size and type of upper lip midline frenulum attachment. Folia Morphol. 2017, 76, 501–505. [Google Scholar] [CrossRef]
- Mirko, P.; Miroslav, S.; Lubor, M. Significance of the Labial Frenum Attachment in Periodontal Disease in Man. Part 1. Classification and Epidemiology of the Labial Frenum Attachment. J. Periodontol. 1974, 45, 891–894. [Google Scholar] [CrossRef] [PubMed]
- Thilander, B.; Pena, L.; Infante, C.; Parada, S.S.; De Mayorga, C. Prevalence of malocclusion and orthodontic treatment need in children and adolescents in Bogota, Colombia. Eur. J. Orthod. 2001, 23, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Bergese, F. Research on the development of the labial frenum in children of age 9–12. Minerva Stomatol. 1966, 15, 672–676. Available online: http://www.ncbi.nlm.nih.gov/pubmed/5225222 (accessed on 1 May 2022).
- Kaimenyi, J.T. Occurrence of midline diastema and frenum attachments amongst school children in Nairobi, Kenya. Indian J. Dent. Res. 1998, 9, 67–71. Available online: http://www.ncbi.nlm.nih.gov/pubmed/10530193 (accessed on 1 May 2022). [PubMed]


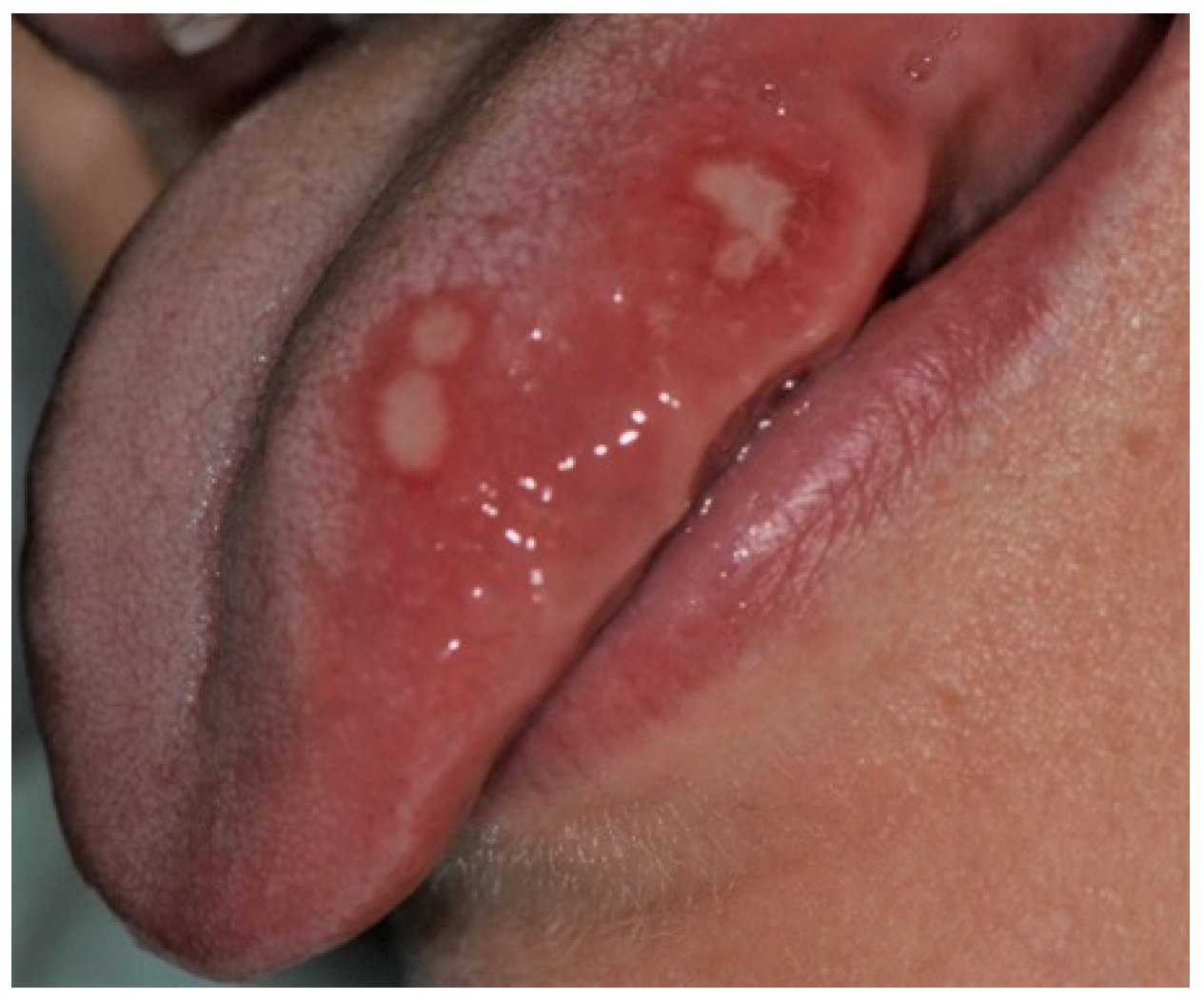
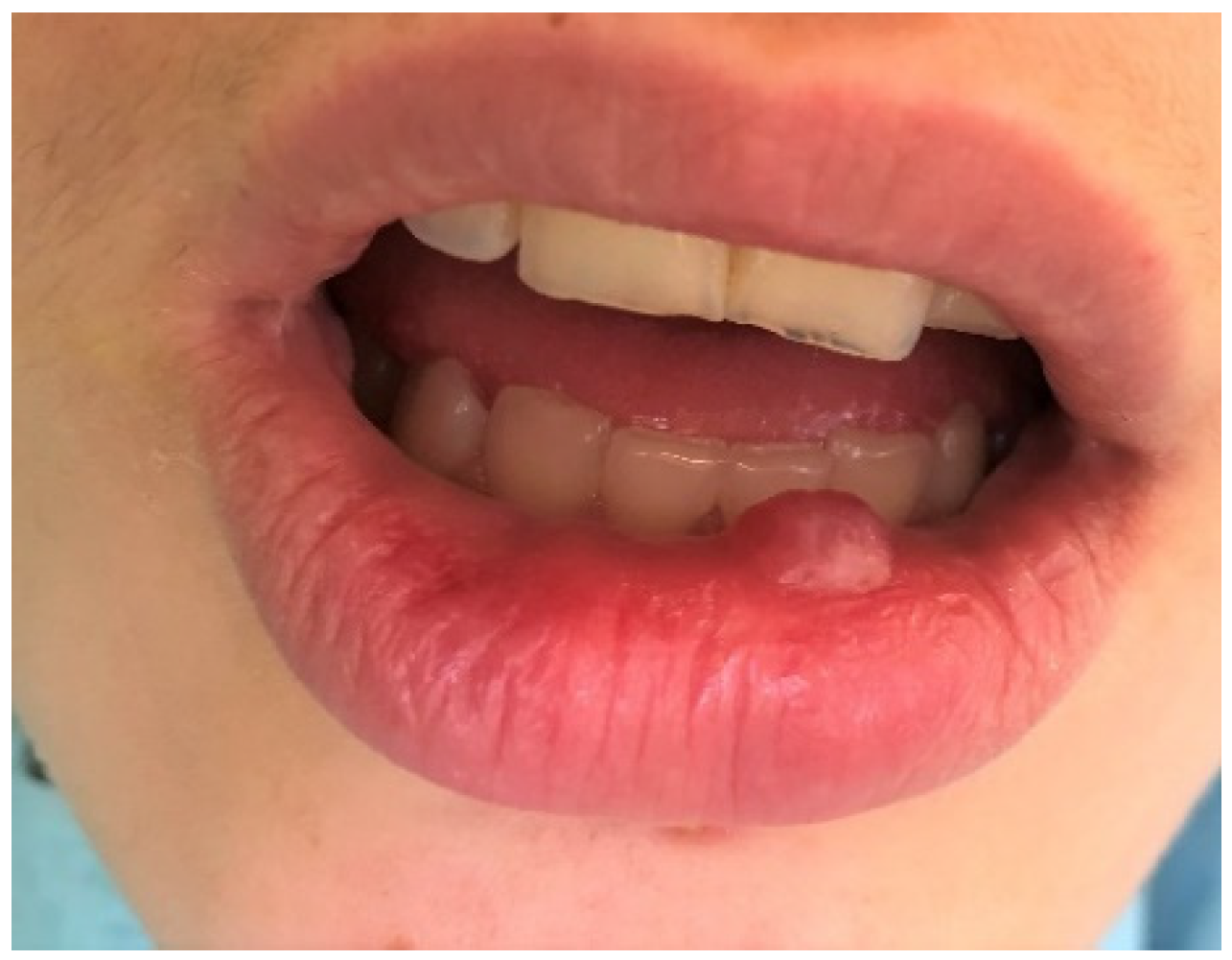
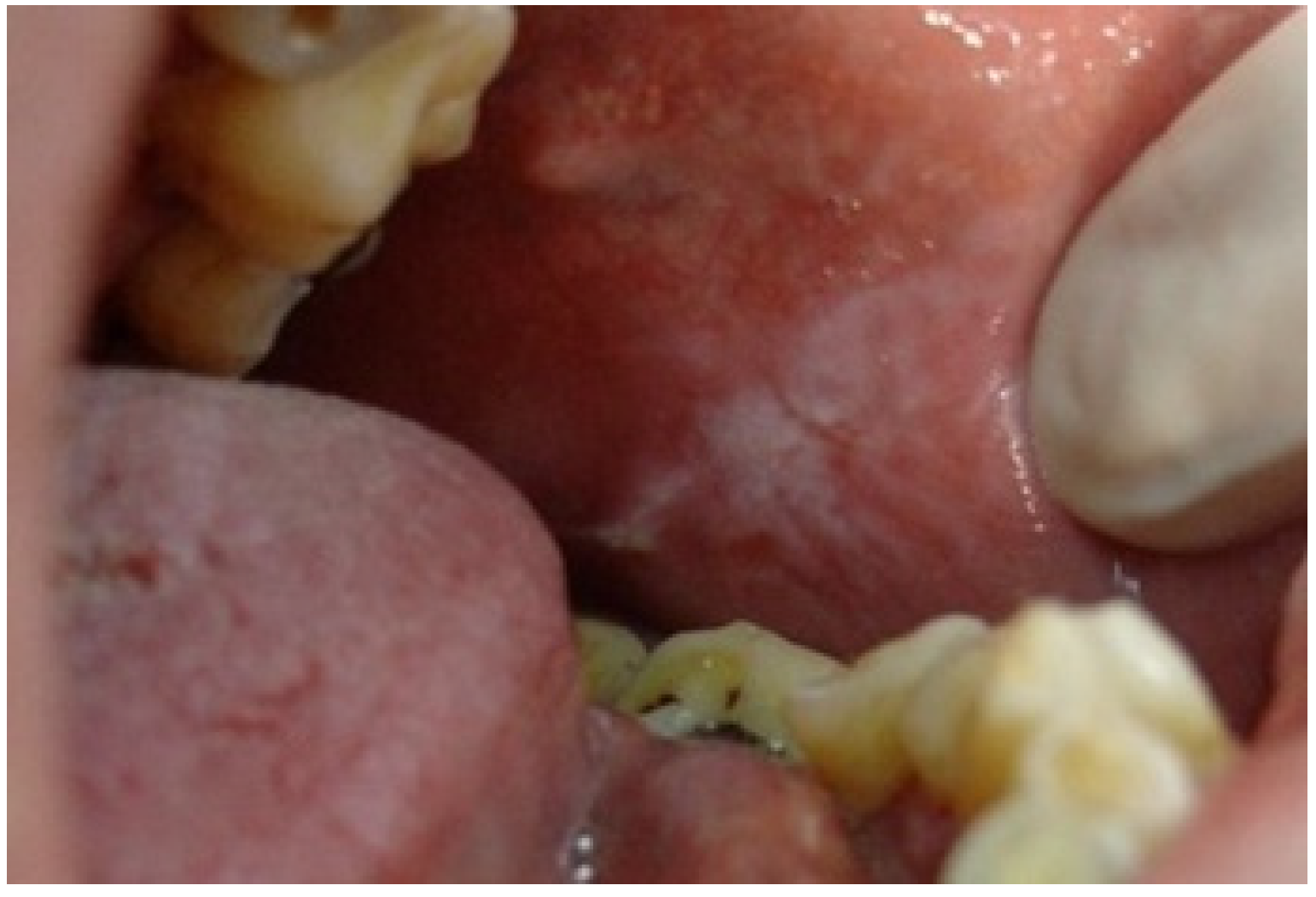


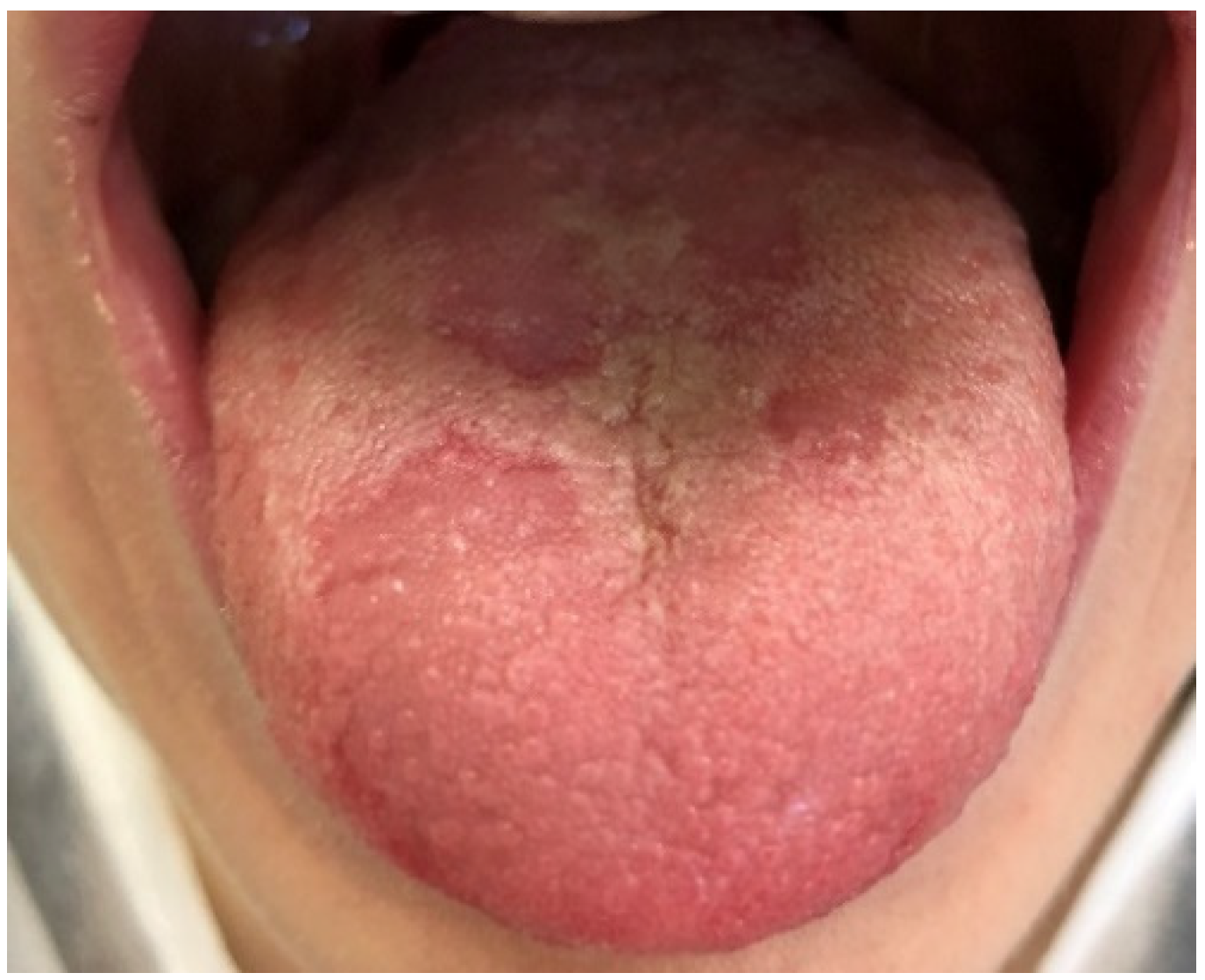




| Age Groups | Nr of Patients | Mean Age | |
|---|---|---|---|
| Entire Study Group | 0–17 | 143 100% | 8.4 (SD 5.2) |
| 0–6 Preschool | 55 38.46% | 2.8 (SD 1.9) | |
| 7–13 School | 58 40.56% | 10 (SD 1.8) | |
| 14–17 Adolescent | 30 20.98% | 15.5 (SD 1.2) | |
| Girls | 0–17 | 60 42% | 8.6 (SD 5.1) |
| 0–6 Preschool | 20 36.36% | 2.7 (SD 1.9) | |
| 7–13 School | 27 46.55% | 9.6 (SD 1.8) | |
| 14–17 Adolescent | 13 43.33% | 15.7 (SD 1.0) | |
| Boys | 0–17 | 83 58% | 8.2 (SD 5.3) |
| 0–6 Preschool | 35 63.64% | 2.9 (SD 1.9) | |
| 7–13 School | 31 53.45% | 10.5 (SD 1.7) | |
| 14–17 Adolescent | 17 56.67% | 15.4 (SD 1.2) |
| Entire Study Group | Girls | Boys | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis | 0–6 n = 55 | 7–13 n = 58 | 14–17 n = 30 | Σ n = 143 | 0–6 n = 20 | 7–13 n = 27 | 14–17 n = 13 | Σ n = 60 | 0–6 n = 35 | 7–13 n = 31 | 14–17 n = 17 | Σ n = 83 | |
| 1 | Aphthae | 7 | 15 | 5 | 27 | 1 | 5 | 3 | 9 | 6 | 10 | 2 | 18 |
| 2 | Mucocele | 2 | 9 | 4 | 15 | 1 | 5 | 2 | 8 | 1 | 4 | 2 | 7 |
| 3 | Morsicatio buccarum | 2 | 5 | 6 | 13 | 0 | 0 | 3 | 3 | 2 | 5 | 3 | 10 |
| 4 | Hairy tongue | 5 | 3 | 2 | 10 | 0 | 2 | 0 | 2 | 5 | 1 | 2 | 8 |
| 5 | Fibroma | 4 | 2 | 3 | 9 | 1 | 1 | 2 | 4 | 3 | 1 | 1 | 5 |
| 6 | Geographic tongue | 7 | 1 | 0 | 8 | 3 | 1 | 0 | 4 | 4 | 0 | 0 | 4 |
| 7 | Papilloma | 3 | 3 | 1 | 7 | 3 | 2 | 0 | 5 | 0 | 1 | 1 | 2 |
| 8 | Abnormal upper lip frenulum | 0 | 4 | 2 | 6 | 0 | 4 | 0 | 4 | 0 | 0 | 2 | 2 |
| 9 | Pyogenic granuloma | 1 | 5 | 0 | 6 | 0 | 3 | 0 | 3 | 1 | 2 | 0 | 3 |
| 10 | Traumatic erosions and ulcers | 2 | 2 | 2 | 6 | 0 | 0 | 0 | 0 | 2 | 2 | 2 | 6 |
| 11 | Gingival enlargement | 0 | 3 | 2 | 5 | 0 | 1 | 1 | 2 | 0 | 2 | 1 | 3 |
| 12 | Oral Candidiasis | 2 | 1 | 1 | 4 | 2 | 1 | 1 | 4 | 0 | 0 | 0 | 0 |
| 13 | Melanoplakia | 3 | 0 | 0 | 3 | 2 | 0 | 0 | 2 | 1 | 0 | 0 | 1 |
| 14 | Herpetic stomatitis | 3 | 0 | 0 | 3 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 2 |
| 15 | Gingival cyst | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 2 |
| 16 | Ankyloglossia | 2 | 0 | 0 | 2 | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| 17 | Frictional hyperkeratosis | 0 | 1 | 1 | 2 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 0 |
| 18 | Eruptive cyst | 2 | 0 | 0 | 2 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 |
| 19 | Linea alba | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| 20 | Hack’s disease | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| 21 | Granulomatous cheilitis | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| 22 | Gingivitis | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| 23 | Mucosal atrophy | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| 24 | Pigmented nevus | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| 25 | Contact allergic reaction | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| 26 | Leukoedema | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| 27 | Epstein pearls | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| 28 | Bohn nodules | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| 29 | Bone exostosis | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| 30 | Exfoliative cheilitis | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| 31 | Macroglossia | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Diagnosis | Entire Study Group | % | Girls n = 60 | Girls 42% | Boys n = 83 | Boys 58% | χ2 | p | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Aphthae | 27 | 18.9 | 9 | 15.0 | 18 | 21.7 | 1.02 | 0.31 |
| 2 | Mucocele | 15 | 10.5 | 8 | 13.3 | 7 | 8.4 | 0.89 | 0.34 |
| 3 | Morsicatio buccarum | 13 | 9.1 | 3 | 5.0 | 10 | 12.0 | 2.09 | 0.15 |
| 4 | Hairy tongue | 10 | 7.0 | 2 | 3.3 | 8 | 9.6 | 2.13 | 0.14 |
| 5 | Fibroma | 9 | 6.3 | 4 | 6.7 | 5 | 6.0 | 0.07 | 0.79 |
| 6 | Geographic tongue | 8 | 5.6 | 4 | 6.7 | 4 | 4.8 | 0.22 | 0.63 |
| 7 | Papilloma | 7 | 4.9 | 5 | 8.3 | 2 | 2.4 | 2.62 | 0.10 |
| 8 | Abnormal upper lip frenulum | 6 | 4.2 | 4 | 6.7 | 2 | 2.4 | 1.57 | 0.21 |
| 9 | Pyogenic granuloma | 6 | 4.2 | 3 | 5.0 | 3 | 3.6 | 0.00 | 0.96 |
| 10 | Traumatic erosions and ulcers | 6 | 4.2 | 0 | 0.0 | 6 | 7.2 | 4.53 | 0.03 |
| Diagnosis | 0–6 n = 55 | % 38.5% | 7–13 n = 58 | % 40.6% | 14–17 n = 30 | % 20.9% | Σ n = 143 | χ22 | p | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Aphthae | 7 | 12.7 | 15 | 25.9 | 5 | 16.7 | 27 | 3.30 | 0.19 |
| 2 | Mucocele | 2 | 3.6 | 9 | 15.5 | 4 | 13.3 | 15 | 4.57 | 0.10 |
| 3 | Morsicatio buccarum | 2 | 3.6 | 5 | 8.6 | 6 | 20.0 | 13 | 6.32 | 0.04 |
| 4 | Hairy tongue | 5 | 9.1 | 3 | 5.2 | 2 | 6.7 | 10 | 0.673 | 0.71 |
| 5 | Fibroma | 4 | 7.3 | 2 | 3.4 | 3 | 10.0 | 9 | 1.61 | 0.45 |
| 6 | Geographic tongue | 7 | 12.7 | 1 | 1.7 | 0 | 0.0 | 8 | 8.72 | 0.01 |
| 7 | Papilloma | 3 | 5.5 | 3 | 5.2 | 1 | 3.3 | 7 | 0.204 | 0.90 |
| 8 | Lip-tie | 0 | 0.0 | 4 | 6.9 | 2 | 6.7 | 6 | 3.92 | 0.14 |
| 9 | Pyogenic granuloma | 1 | 1.8 | 5 | 8.6 | 0 | 0.0 | 6 | 3.46 | 0.18 |
| 10 | Traumatic erosions and ulcers | 2 | 3.6 | 2 | 3.4 | 2 | 6.7 | 6 | 0.579 | 0.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owczarek-Drabińska, J.E.; Nowak, P.; Zimoląg-Dydak, M.; Radwan-Oczko, M. The Prevalence of Oral Mucosa Lesions in Pediatric Patients. Int. J. Environ. Res. Public Health 2022, 19, 11277. https://doi.org/10.3390/ijerph191811277
Owczarek-Drabińska JE, Nowak P, Zimoląg-Dydak M, Radwan-Oczko M. The Prevalence of Oral Mucosa Lesions in Pediatric Patients. International Journal of Environmental Research and Public Health. 2022; 19(18):11277. https://doi.org/10.3390/ijerph191811277
Chicago/Turabian StyleOwczarek-Drabińska, Joanna Elżbieta, Patrycja Nowak, Małgorzata Zimoląg-Dydak, and Małgorzata Radwan-Oczko. 2022. "The Prevalence of Oral Mucosa Lesions in Pediatric Patients" International Journal of Environmental Research and Public Health 19, no. 18: 11277. https://doi.org/10.3390/ijerph191811277
APA StyleOwczarek-Drabińska, J. E., Nowak, P., Zimoląg-Dydak, M., & Radwan-Oczko, M. (2022). The Prevalence of Oral Mucosa Lesions in Pediatric Patients. International Journal of Environmental Research and Public Health, 19(18), 11277. https://doi.org/10.3390/ijerph191811277








