Evaluating the Efficacy and Safety of Different Pterygium Surgeries: A Review of the Literature
Abstract
:1. Introduction
2. Materials and Methods
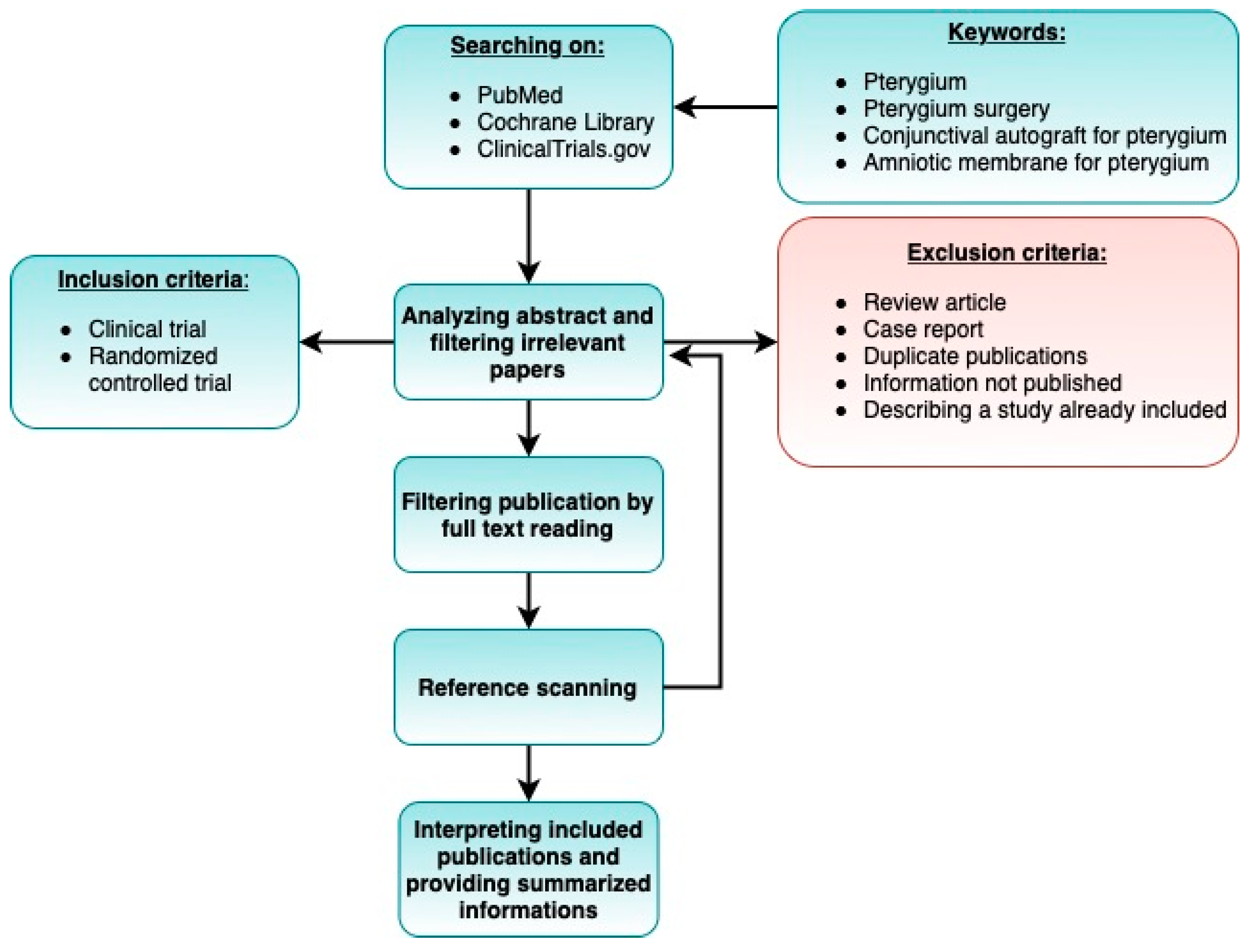
2.1. Literature Search
2.2. Study Selection
2.3. Inclusion Criteria
- The study was a clinical trial or randomized controlled trial.
- The study involved patients diagnosed with pterygium.
- At least one of the surgical procedures under consideration was used.
- The study analyzed variables such as rate of recurrence and complications.
- The study included an adequate follow-up period.
- The publication date: 2008–2021.
2.4. Exclusion Criteria
- Case reports.
- Studies describing partial results (if two or more articles were based on the same cohort, we selected the paper published most recently).
- Duplicate publications.
- Review-type papers.
- Sufficient information was not published (e.g., full text not accessible, or full text did not contain raw data).
- Follow-up period shorter than 6 months.
- Number of participants less than 20.
2.5. Risk of Bias Assessment
2.6. Data Extraction
3. Results
3.1. Bare Sclera Technique
3.2. Removal of Pterygium with Conjunctival Autograft (CAU)
3.3. Removal of Pterygium with Amniotic Membrane Transplantation (AMT)
3.4. Removal of Pterygium with Limbal Conjunctival Autograft (LCAG)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shahraki, T.; Arabi, A.; Feizi, S. Pterygium: An update on pathophysiology, clinical features, and management. Ther. Adv. Ophthalmol. 2021, 13, 25158414211020152. [Google Scholar] [CrossRef] [PubMed]
- Rezvan, F.; Khabazkhoob, M.; Hooshmand, E.; Yekta, A.; Saatchi, M.; Hashemi, H. Prevalence and risk factors of pterygium: A systematic review and meta-analysis. Surv. Ophthalmol. 2018, 63, 719–735. [Google Scholar] [CrossRef] [PubMed]
- Cajucom-Uy, H.; Tong, L.; Wong, T.Y.; Tay, W.T.; Saw, S.M. The Prevalence of and Risk Factors for Pterygium in an Urban Malay Population: The Singapore Malay Eye Study (SiMES). Br. J. Ophthalmol. 2010, 94, 977–981. [Google Scholar] [CrossRef] [PubMed]
- Luthra, R.; Nemesure, B.B.; Wu, S.Y.; Xie, S.H.; Leske, M.C. Barbados Eye Studies Group. Frequency and Risk Factors for Pterygium in the Barbados Eye Study. Arch. Ophthalmol. 2001, 119, 1827–1832. [Google Scholar] [CrossRef]
- Tananuvat, N.; Martin, T. The Results of Amniotic Membrane Transplantation for Primary Pterygium Compared with Conjunctival Autograft. Cornea 2004, 23, 458–463. [Google Scholar] [CrossRef]
- West, S.; Munoz, B. Prevalence of pterygium in Latinos: Proyecto VER. Br. J. Ophthalmol. 2009, 93, 1287–1290. [Google Scholar] [CrossRef]
- Bachelor, M.A.; Bowden, G. UVA-mediated activation of signaling pathways involved in skin tumor promotion and progression. Semin. Cancer Biol. 2004, 14, 131–138. [Google Scholar] [CrossRef]
- Chao, S.-C.; Hu, D.-N.; Yang, P.-Y.; Lin, C.-Y.; Nien, C.-W.; Yang, S.-F.; Roberts, J.E. Ultraviolet-A Irradiation Upregulated Urokinase-Type Plasminogen Activator in Pterygium Fibroblasts through ERK and JNK Pathways. Investig. Ophtalmol. Vis. Sci. 2013, 54, 999–1007. [Google Scholar] [CrossRef]
- Di Girolamo, N.; Chui, J.; Coroneo, M.T.; Wakefield, D. Pathogenesis of pterygia: Role of cytokines, growth factors, and matrix metalloproteinases. Prog. Retin. Eye Res. 2004, 23, 195–228. [Google Scholar] [CrossRef]
- Murube, J. Pterygium: Evolution of Medical and Surgical Treatments. Ocul. Surf. 2008, 6, 155–161. [Google Scholar] [CrossRef]
- Islam, S.I.; Wagoner, M.D. Pterygium in Young Members of One Family. Cornea 2001, 20, 708–710. [Google Scholar] [CrossRef] [PubMed]
- Hilgers, J. Pterygium: Its Incidence, Heredity and Etiology. Am. J. Ophthalmol. 1960, 50, 635–644. [Google Scholar] [CrossRef]
- Al Fayez, M.F. Limbal versus conjunctival autograft transplantation for advanced and recurrent pterygium. Ophthalmology 2002, 109, 1752–1755. [Google Scholar] [CrossRef]
- Bilge, A.D. Comparison of conjunctival autograft and conjunctival transposition flap techniques in primary pterygium surgery. Saudi J. Ophthalmol. 2018, 32, 110–113. [Google Scholar] [CrossRef]
- Fernandes, M.; Sangwan, V.; Bansal, A.K.; Gangopadhyay, N.; Sridhar, M.S.; Garg, P.; Aasuri, M.K.; Nutheti, R.; Rao, G.N. Outcome of pterygium surgery: Analysis over 14 years. Eye 2004, 19, 1182–1190. [Google Scholar] [CrossRef]
- Hall, R.C.; Logan, A.J.; Wells, A.P. Comparison of fibrin glue with sutures for pterygium excision surgery with conjunctival autografts. Clin. Exp. Ophthalmol. 2009, 37, 584–589. [Google Scholar] [CrossRef]
- Koranyi, G.; Seregard, S.; Kopp, E.D. The cut-and-paste method for primary pterygium surgery: Long-term follow-up. Acta Ophthalmol. Scand. 2005, 83, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.H.-K.; See, L.-C.; Liau, S.-B.; Tsai, R.J.-F. Amniotic membrane graft for primary pterygium: Comparison with conjunctival autograft and topical mitomycin C treatment. Br. J. Ophthalmol. 2000, 84, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Sati, A.; Shankar, S.; Jha, A.; Kalra, D.; Mishra, S.; Gurunadh, V.S. Comparison of efficacy of three surgical methods of conjunctival autograft fixation in the treatment of pterygium. Int. Ophthalmol. 2014, 34, 1233–1239. [Google Scholar] [CrossRef]
- Kaufman, S.C.; Jacobs, D.S.; Lee, W.B.; Deng, S.X.; Rosenblatt, M.I.; Shtein, R.M. Options and Adjuvants in Surgery for Pterygium: A Report by the American Academy of Ophthalmology. Ophthalmology 2013, 120, 201–208. [Google Scholar] [CrossRef]
- Sánchez-Thorin, J.C.; Rocha, G.; Yelin, J.B. Meta-analysis on the recurrence rates after bare sclera resection with and without mitomycin C use and conjunctival autograft placement in surgery for primary pterygium. Br. J. Ophthalmol. 1998, 82, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 74, 790–799. [Google Scholar] [CrossRef]
- Jha, K. Conjunctival-Limbal Autograft for Primary and Recurrent Pterygium. Med. J. Armed Forces India 2008, 64, 337–339. [Google Scholar] [CrossRef]
- Liang, W.; Li, R.; Deng, X. Comparison of the efficacy of pterygium resection combined with conjunctival autograft versus pterygium resection combined with amniotic membrane transplantation. Eye Sci. 2012, 27, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Bekibele, C.O.; Ashaye, A.; Olusanya, B.; Baiyeroju, A.; Fasina, O.; Ibrahim, A.O.; Ogun, O.; Ogun, O. 5-Fluorouracil versus mitomycin C as adjuncts to conjunctival autograft in preventing pterygium recurrence. Int. Ophthalmol. 2012, 32, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, Y.M.; Zaky, K.S. Efficacy of preoperative injection versus intraoperative application of mitomycin in recurrent pterygium surgery. Indian J. Ophthalmol. 2012, 60, 273. [Google Scholar] [CrossRef]
- Koranyi, G.; Artzén, D.; Seregard, S.; Kopp, E.D. Intraoperative mitomycin C versus autologous conjunctival autograft in surgery of primary pterygium with four-year follow-up. Acta Ophthalmol. 2012, 90, 266–270. [Google Scholar] [CrossRef]
- Kheirkhah, A.; Nazari, R.; Safi, H.; Ghassemi, H.; Behrouz, M.J.; Raju, V.K. Effects of intraoperative steroid injection on the outcome of pterygium surgery. Eye 2013, 27, 906–914. [Google Scholar] [CrossRef]
- Olusanya, B.A.; Ogun, O.; Bekibele, C.O.; Ashaye, A.O.; Baiyeroju, A.M.; Fasina, O.; Ogundipe, A.O.; Ibrahim, A.O. Risk factors for pterygium recurrence after surgical excision with combined conjunctival autograft (CAG) and intraoperative antimetabolite use. Afr. J. Med. Med. Sci. 2014, 43, 35–40. [Google Scholar]
- Promesberger, J.; Kohli, S.; Busse, H.; Uhlig, C.E. Pterygium Recurrence, Astigmatism and Visual Acuity following Bare-Sclera Excision and Conjunctival Autograft with or without Additional Phototherapeutic Keratectomy. Ophtalmic Res. 2014, 51, 52–58. [Google Scholar] [CrossRef]
- Hwang, S.; Choi, S. A Comparative Study of Topical Mitomycin C, Cyclosporine, and Bevacizumab after Primary Pterygium Surgery. Korean J. Ophthalmol. 2015, 29, 375–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasetsuwan, N.; Reinprayoon, U.; Satitpitakul, V. Prevention of Recurrent Pterygium with Topical Bevacizumab 0.05% Eye Drops: A Randomized Controlled Trial. Clin. Ther. 2015, 37, 2347–2351. [Google Scholar] [CrossRef] [PubMed]
- Katırcıoglu, Y.A.; Altiparmak, U.; Goktas, S.E.; Cakir, B.; Singar, E.; Ornek, F. Comparison of Two Techniques for the Treatment of Recurrent Pterygium: Amniotic Membrane vs. Conjunctival Autograft Combined with Mitomycin C. Semin. Ophthalmol. 2015, 30, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Huang, G.; Liu, S.; Ma, W.; Yin, X.; Zhou, S. Limbal conjunctival versus amniotic membrane in the intraoperative application of mitomycin C for recurrent pterygium: A randomized controlled trial. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 375–385. [Google Scholar] [CrossRef]
- Nadarajah, G.; Ratnalingam, V.H.; Isa, H.M. Autologous Blood Versus Fibrin Glue in Pterygium Excision with Conjunctival Autograft Surgery. Cornea 2017, 36, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Akbari, M.; Soltani-Moghadam, R.; Elmi, R.; Kazemnejad, E. Comparison of free conjunctival autograft versus amniotic membrane transplantation for pterygium surgery. J. Curr. Ophthalmol. 2017, 29, 282–286. [Google Scholar] [CrossRef]
- Lotfy, A.; Gad, A.A.M.; Abdelrahman, A.; Samir, A.; Abdulhalim, B.-E.H. Conjunctival Autograft Combined with Either Preoperative Mitomycin C Injection or Intraoperative Local Mitomycin C over the Medial Rectus Muscle Tendon in Primary Pterygium Surgery. Eye Contact Lens 2018, 44, 192–195. [Google Scholar] [CrossRef]
- Nassar, M.K.; Khairy, H.A.; Shalaby, A.M. Limbal conjunctival autograft versus simple excision with intraoperative mitomycin C in pterygium surgery. Menoufia Med. J. 2018, 31, 1350. [Google Scholar]
- Young, A.L.; Kam, K.W. Pterygium: Surgical Techniques and Choices. Asia-Pac. J. Ophthalmol. 2019, 8, 422–423. [Google Scholar] [CrossRef]
- Yu, J.; Feng, J.; Jin, T.; Tian, L.; Zhu, L.; Cao, K.; Li, S.; Jie, Y. The Effect of a Novel Strategy in Treating Primary Pterygium: A Prospective Randomized Clinical Study. Am. J. Ophthalmol. 2021, 225, 108–116. [Google Scholar] [CrossRef]
- Alsarhani, W.; Alshahrani, S.; Showail, M.; Alhabdan, N.; Alsumari, O.; Almalki, A.; Alsarhani, A.; Alluhaidan, A.; Alqahtani, B. Characteristics and recurrence of pterygium in Saudi Arabia: A single center study with a long follow-up. BMC Ophthalmol. 2021, 21, 207. [Google Scholar] [CrossRef] [PubMed]
- Janson, B.J.; Sikder, S. Surgical Management of Pterygium. Ocul. Surf. 2014, 12, 112–119. [Google Scholar] [CrossRef]
- Cardillo, J.A.; Alves, M.R.; Ambrosio, L.E.; Poterio, M.B.; Jose, N.K. Single Intraoperative Application versus Postoperative Mitomycin C Eye Drops in Pterygium Surgery. Ophthalmology 1995, 102, 1949–1952. [Google Scholar] [CrossRef]
- Kheirkhah, A.; Izadi, A.; Kiarudi, M.Y.; Nazari, R.; Hashemian, H.; Behrouz, M.J. Effects of Mitomycin C on Corneal Endothelial Cell Counts in Pterygium Surgery: Role of Application Location. Am. J. Ophthalmol. 2011, 151, 488–493. [Google Scholar] [CrossRef]
- Bekibele, C.O.; Baiyeroju, M.A.; Olusanya, B.A.; Ashaye, A.O.; Oluleye, T.S. Pterygium treatment using 5-FU as adjuvant treatment compared to conjunctiva autograft. Eye 2008, 22, 31–34. [Google Scholar] [CrossRef]
- Fallah, M.; Khosravi, K.; Hashemian, M.N.; Beheshtnezhad, A.H.; Rajabi, M.T.; Gohari, M. Efficacy of Topical Bevacizumab for Inhibiting Growth of Impending Recurrent Pterygium. Curr. Eye Res. 2010, 35, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Mourits, M.; Wyrdeman, H.; Jürgenliemk-Schulz, I.; Bidlot, E. Favorable Long-Term Results of Primary Pterygium Removal by Bare Sclera Extirpation Followed by a Single 90Strontium Application. Eur. J. Ophthalmol. 2008, 18, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.T.H.; Chee, S.-P.; Dear, K.; Lim, A.S.M. Effect of Pterygium Morphology on Pterygium Recurrence in a Controlled Trial Comparing Conjunctival Autografting with Bare Sclera Excision. Arch. Ophthalmol. 1997, 115, 1235–1240, Erratum in Arch. Ophthalmol. 1998, 116, 552. [Google Scholar] [CrossRef] [PubMed]
- Frucht-Pery, J.; Raiskup, F.; Ilsar, M.; Landau, D.; Orucov, F.; Solomon, A. Conjunctival Autografting Combined with Low-Dose Mitomycin C for Prevention of Primary Pterygium Recurrence. Am. J. Ophthalmol. 2006, 141, 1044–1050. [Google Scholar] [CrossRef]
- Zein, H.; Ismail, A.; Abdelmongy, M.; Elsherif, S.; Hassanen, A.; Muhammad, B.; Assaf, F.; Elsehili, A.; Negida, A.; Yamane, S.; et al. Autologous Blood for Conjunctival Autograft Fixation in Primary Pterygium Surgery: A Systematic Review and Meta-analysis. Curr. Pharm. Des. 2018, 24, 4197–4204. [Google Scholar] [CrossRef]
- McLaren, J.; Malak, T.; Bell, S. Structural characteristics of term human fetal membranes prior to labour: Identification of an area of altered morphology overlying the cervix. Hum. Reprod. 1999, 14, 237–241. [Google Scholar] [CrossRef]
- Nuzzi, R.; Tridico, F. How to minimize pterygium recurrence rates: Clinical perspectives. Clin. Ophthalmol. 2018, 12, 2347–2362. [Google Scholar] [CrossRef]
- Shimazaki, J.; Yang, H.-Y.; Tsubota, K. Limbal Autograft Transplantation for Recurrent and Advanced Pterygia. Ophthalmic Surg. Lasers 1996, 27, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Arain, M.A.; Yaqub, M.A.; Ameen, S.S.; Iqbal, Z.; Naqvi, A.H.; Niazi, M.K. Amniotic membrane transplantation in primary pterygium compared with bare sclera technique. J. Coll. Physicians Surg. Pak. 2012, 22, 440–443. [Google Scholar] [PubMed]
- Besharati, M.R.; Miratashi, S.A.M.; Ahmadi, A.B. Pterygium surgery: Amniotic membrane or conjunctival autograft transplantation. Int. J. Ophthalmol. 2008, 1, 326–362. [Google Scholar]
- Fernandes, L.; Paes, J.; de Morais, B.; da Costa, C.M.G.; de Oliveira, E.M.; Felix, F.S.; Ono, T., Jr.; Angotti, H.; de Morais, M.P.; de Queiroz, S.T. Surgical Treatment of Primary Pterygium: Comparison between Tecniques of Autologous Conjunctive Transplant and Transplantation of Amniotic Membrane. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5296. [Google Scholar]
- Katırcıoğlu, Y.A.; Altıparmak, U.E.; Duman, S.; Altiparmak, U.E. Comparison of Three Methods for the Treatment of Pterygium: Amniotic Membrane Graft, Conjunctival Autograft and Conjunctival Autograft plus Mitomycin C. Orbit 2007, 26, 5–13. [Google Scholar] [CrossRef]
- Keklikci, U.; Celik, Y.; Cakmak, S.S.; Unlu, M.K.; Bilek, B. Conjunctival-limbal autograft, amniotic membrane transplantation, and intraoperative mitomycin C for primary pterygium. Ann. Ophthalmol. 2007, 39, 296–301. [Google Scholar] [CrossRef]
- Kheirkhah, A.; Nazari, R.; Nikdel, M.; Ghassemi, H.; Hashemi, H.; Behrouz, M.J. Postoperative Conjunctival Inflammation after Pterygium Surgery with Amniotic Membrane Transplantation Versus Conjunctival Autograft. Am. J. Ophthalmol. 2011, 152, 733–738. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, S.-Y. The comparative study of clinical results in surgically treated pterygium patients with amniotic membrane transplantation versus limbal autograft. Investig. Ophthalmol. Vis. Sci. 2008, 49, 6031. [Google Scholar]
- Parra, Z.P.; Pérez, A.C.; Leyva, E.E.; Hernández, S.L.; Villalón, S.M. Conjunctival autograft versus amniotic membrane graft in primary pterygium surgery [Autoinjerto conjuntival versus injerto de membrana amniótica en la cirugía del pterigión primario]. Rev. Cuba Oftalmol. 2008, 21, 1–5. [Google Scholar]
- Prabhasawat, P.; Barton, K.; Burkett, G.; Tseng, S.C. Comparison of Conjunctival Autografts, Amniotic Membrane Grafts, and Primary Closure for Pterygium Excision. Ophthalmology 1997, 104, 974–985. [Google Scholar] [CrossRef]
- Küçükerdönmez, C.; Akova, Y.A.; Altnörs, D.D. Comparison of Conjunctival Autograft with Amniotic Membrane Transplantation for Pterygium Surgery: Surgical and Cosmetic Outcome. Cornea 2007, 26, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.H.-K.; See, L.-C.; Hwang, Y.-S.; Wang, S.-F. Comparison of Amniotic Membrane Graft Alone or Combined with Intraoperative Mitomycin C to Prevent Recurrence After Excision of Recurrent Pterygia. Cornea 2005, 24, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Keivyon, K.R.; Tseng, S.C. Limbal Autograft Transplantation for Ocular Surface Disorders. Ophthalmology 1989, 96, 709–723. [Google Scholar] [CrossRef]
- Koch, J.M.; Mellin, K.B.; Waubke, T.N. The pterygium, autologous conjunctiva-limbus transplantation as treatment. Ophthalmologe 1992, 89, 143–146. (In German) [Google Scholar]
- Özer, A.; Yildirim, N.; Erol, N.; Yurdakul, S. Long-Term Results of Bare Sclera, Limbal-Conjunctival Autograft and Amniotic Membrane Graft Techniques in Primary Pterygium Excisions. Ophthalmologica 2009, 223, 269–273. [Google Scholar] [CrossRef]
- Mutlu, F.M.; Sobaci, G.; Tatar, T.; Yildirim, E. A comparative study of recurrent pterygium surgery Limbal conjunctival autograft transplantation versus mitomycin C with conjunctival flap. Ophthalmology 1999, 106, 817–821. [Google Scholar] [CrossRef]
- Rao, S.K.; Lekha, T.; Mukesh, B.N.; Sitalakshmi, G.; Padmanabhan, P. Conjunctival-limbal autografts for primary and recurrent pterygia: Technique and results. Indian J. Ophthalmol. 1998, 46, 203–209. [Google Scholar]
- Kheirkhah, A.; Hashemi, H.; Adelpour, M.; Nikdel, M.; Rajabi, M.B.; Behrouz, M.J. Randomized Trial of Pterygium Surgery with Mitomycin C Application Using Conjunctival Autograft versus Conjunctival-Limbal Autograft. Ophthalmology 2012, 119, 227–232. [Google Scholar] [CrossRef]
- Aydin, A.; Karadayi, K.; Aykan, U.; Can, G.; Colakoglu, K.; Bilge, A. Efficacité du traitement topique de ciclosporine A après excision du ptérygion primaire et autogreffe conjonctivo-limbique [Effectiveness of topical ciclosporin A treatment after excision of primary pterygium and limbal conjunctival autograft]. J. Fr. Ophtalmol. 2008, 31, 699–704. Erratum in J. Fr. Ophtalmol. 2010, 33, 435(In French) [Google Scholar] [CrossRef]
- Young, A.L.; Leung, G.Y.S.; Wong, A.K.K.; Cheng, L.L.; Lam, D.S.C. A randomised trial comparing 0.02% mitomycin C and limbal conjunctival autograft after excision of primary pterygium. Br. J. Ophthalmol. 2004, 88, 995–997. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, F.D.; Hirst, L.W.; Battistutta, D.; Green, A. Risk Analysis in the Development of Pterygia. Ophthalmology 1992, 99, 1056–1061. [Google Scholar] [CrossRef]
- Moran, D.J.; Hollows, F.C. Pterygium and ultraviolet radiation: A positive correlation. Br. J. Ophthalmol. 1984, 68, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Chen, X.; Kang, Y.; Ke, L.; Wei, X.; Zhang, W. Pterygium in Tibetans: A population-based study in China. Clin. Exp. Ophthalmol. 2007, 35, 828–833. [Google Scholar] [CrossRef]
- McCarty, C.A.; Fu, C.L.; Taylor, H.R. Epidemiology of pterygium in Victoria, Australia. Br. J. Ophthalmol. 2000, 84, 289–292. [Google Scholar] [CrossRef]
- Walter, W.L. Another Look at Pterygium Surgery with Postoperative Beta Radiation. Ophthalmic Plast. Reconstr. Surg. 1994, 10, 247–252. [Google Scholar] [CrossRef]
- Murube, J. Pterygium: Its Treatment with Beta Therapy. Ocul. Surf. 2009, 7, 3–10. [Google Scholar] [CrossRef]
- Yamada, T.; Mochizuki, H.; Ue, T.; Kiuchi, Y.; Takahashi, Y.; Oinaka, M. Comparative Study of Different β-Radiation Doses for Preventing Pterygium Recurrence. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1394–1398. [Google Scholar] [CrossRef]

| Reference and Date | Participants (Number) | Aim of Study | Follow Up Period (Months) | Preoperative Assessment 1 | Postoperative Treatment | Recurrence Rate (%) 2 | Safety |
|---|---|---|---|---|---|---|---|
| Jha KN (2008) [23] | 32 | LCAG: primary vs. recurrent pterygium | 18 | L: 93.75% N/6.25% T M: no data S: 2–4 mm | Gutt ATB + GCs qid, 2 w. Sutures removal: YES (14 d) | Primary pterygium—0% Recurrent pterygium—0% |
|
| Reece C Hall Andre J Logan (2009) [16] | 50 | CAU + 8/0 Vicryl vs. CAU + fibrin glue | 12 | L: 100% N M: no data S: >4 mm | Gutt ATB qid 1 w; gutt DEX qid 1 m. Sutures removal: NO | Vicryl group—8.7% Fibrin glue group—0% | Fibrin glue group:
|
| Wanhong Liang Rongxu Li (2012) [24] | 133 | CAU vs. AMT | 12 | L: 100% N M: no data S: pterygium invaded pupil | Gutt ATB qid 1 w, ung ATB od 1 w. Sutures removal: YES (12 d) | CAU—7.4% AMT—19.2% |
|
| Charles O Bekibele Adeyinka Ashaye (2012) [25] | 88 | CAU + 5-FU vs. CAU + MMC | 8 | L: 5-FU: 95.65% N/4.35% T MMC: 95.6% N/4.4% T M: (T1/T2/T3) 5-FU:2.2%/6.5%/91.3% MMC:0%/0%/100% S: 5-FU: 3.4 ± 1.3 MMC: 3.3 ± 1.2 | Ung ATB tid, gutt DEX qid 6–10 w. Wearing dark glasses advised. | CAU + 5-FU—8.7% CAU + MMC—11.8% |
|
| Khaled A Zaky, Yasser M Khalifa (2012) [26] | 50 | BS + subconj. injection MMC (0.1 mL of 0.15 mg/mL) the day before surgery vs. BS + intraoperative topical application of MMC (0.1 mL of 0.15 mg/mL) | 12 | L: no data M: no data S: 3 to 5 mm | Gutt ATB qid 4 w, gutt DEX qid 4 w, ung ATB + DEX od 4 w. | 0% in both groups |
|
| Gabor Koranyi Ditte Artze’n (2012) [27] | 115 | BS + intraoperative 0.04% MMC vs. CAU + 7/0 Vicryl | 48 | L: no data M: no data Area: MMC group: 6.5 mm 2 ± 4.0 CAU: 7.5 mm 2 ± 5.5 | Gutt DEX 6/d 1 w tapered-off over the next 5 w, ung ATB tid 1 w. Sutures removal: no data. | MMC group—38% CAU group—15%, (p < 0.05). |
|
| Kheirkhah A, Nazari R (2013) [28] | 54 | BS + intraoperative MMC and TMC vs. BS + intraoperative MMC | 12 | L: 100% N M: TMC group: 17.4%/56.5%/26.1% Control group: 20%/56%/24% Size: no data | Gutt ATB 2 w, gutt GCs (BTM→FML) 3 m | Conjunctival recurrence rate: TMC group—8.7% Control group—4.0%, (p < 0.05). Corneal recurrence rate: 0% in both groups. |
|
| Alok Sati, Sandeep Shankar (2014) [19] | 90 | CAU + 8/0 Vicryl vs. CAU + fibrin glue vs. CAU + autologous blood | 12 | L: no data M: Vicryl: 20%/53.33%26.67% Fibrin glue: 20%/53.33%26.67% Autologous blood: 20%/53.33%26.67% S: Vicryl: 3.73 ± 0.41 Fibrin glue: 3.93 ± 0.5 Autologous blood: 3.85 ± 0.42 | Gutt DEX qid 1 m, ung ATB qid 1 w. Sutures removal—no data | Vicryl group—10% Fibrin glue group—6.67% Autologous blood group—3.33% | Autologous blood group:
|
| Olusanya BA, Ogun OA (2014) [29] | 80 | CAU + MMC vs. CAU + 5-FU | 8 | L: 92.4% N/1.3% T/6.3% D M: 1.3%/3.7%/95% S: no data | Ung ATB qid, gutt DEX qid, 6–10 w. | MMC group—11.8% 5-FU group—8.7% |
|
| Julia Promesberger,Sharmila Kohli (2014) [30] | 81 | Primary pterygium: CAU (P1) vs. CAU + PTK (P2) Recurrent pterygium: CAU (R1) vs. CAU + PTK(R2) | >24 | Primary pterygium: L (P1 + P2): 87.7% N/3.5% T/8.8% D M: no data S: no data Recurrent pterygium: L (R1 + R2): 87.1% N/3.2% T/9.7% D M: no data S: no data | Gutt ATB qid, P1 + R1: dexpanthenol cream qid P2 + R2 gutt NSAID 5x/d. | P1—32.5% P2—7.1% R1—26.3% R2—23.1% |
|
| Shinyoung Hwang, Sangkyoung Choi (2015) [31] | 132 | BS + POV vs. BS + 0.02% MMC vs. BS + 0.05% CYP vs. BS + 2.5% BEV | 6 | L: no data M: POV: 9.1%/36.4%/54.5% MMC: 13.8%/37.9%/48.3% CYP: 14.7%/26.5%/58.8% BEV: 11.1%/27.8%/61.1% S: POV: 3.7 ± 0.5 MMC: 3.8 ± 0.7 CYP: 4.1 ± 0.3 BEV: 3.4 ± 0.9 | Gutt ATB qid 1 w, FML qid 1 m utt: POV, 0.02%MMC, 0.05% CYP, 2.5% BEV qid 3 m. | POV—45.5% MMC—10.3% CYP—20.6% BEV—41.7% |
|
| Ngamjit Kasetsuwan, Usanee Reinprayoon (2015) [32] | 22 | BS vs. BS + 0.05% BEV | 6 | L: no data M: BS: 0%/75%/25% BS + BEV: 10%/70%/20% S: no data | Gutt ATB + DEX qid 1 m, Gutt FML qid 2 m. BS: gutt AT qid 3 m BS + BEV: gutt 0.05% BEV qid 3 m. | BS—90% BS + BEV—33.33% |
|
| Yasemin Arslan Katircioglu, Ugur Altiparmak (2015) [33] | 60 | AMT + MMC vs. CAU + MMC | 27 | L: no data M: no data Size: no data | Gutt ATB qid 1 w, gutt AT qid 1 w, gutt PRED qid 1 m. Sutures removal: YES (within 14 d) | AMT + MMC—8% CAU + MMC—13.3% |
|
| Rongxin Chen, Goufu Huang (2016) [34] | 96 | LCAG + intraoperative 0.02% MMC vs. AMT + intraoperative 0.02% MMC | 12 | L: LCAG group: 89.4% N/10.6% T AMT group 86.9% N/13.1% T M: no data S: LCAG: 3.77 ± 1.38 AMT: 4.19 ± 1.88 (p < 0.05) | Gutt ATB, gutt AT, ung ATB; gutt NSAID at the end of the first week, gutt + ung ATB + DEX 1 m. Sutures removal: YES (14 d). | LCAG group—2.1% AMG group—10.9% |
|
| Gaayathri Nadarajah, Vanithe H Ratnalingam (2017) [35] | 120 | CAU + autologous blood vs. CAU + fibrin glue | 12 | L: no data M: Autologous blood group: 11.7%/25%/15% Fibrin glue group: 10.8%/25.8%.11.7% S: no data | Gutt ATB + DEX q 2 h 1 w, qid 3 w, bds 2 w. Ung ATB od 6 w. | Autologous blood group—10.6% Fibrin glue group—3.4% |
|
| Mitra Akbari, Reza Soltani-Moghadam (2017) [36] | 60 | AMT vs. CAU | 12 | L: no data M: no data S: no data | Gutt ATB 2 w Gutt BTM 3 m | AMT group—6.7% CAU group—3.3% |
|
| Ayman Lotfy, Ahmed A. M. Gad (2018) [37] | 108 | CAU + preoperative MMC injection vs. CAU + intraoperative MMC Group I—injection of 0.1 mL of 0.15 mg/mL MMC into the body of pterygium 1 day before surgery Group II—local application of 0.2 mg/mL MMC for 2 min over the medial rectus tendon during surgery. | 23 | L: no data M: no data S: Group I: 3.93 ± 0.65 Group II: 3.91 ± 0.6 | Gutt PRED qid 1.5 m, gutt ATB qid 1 w. Sutures removal: YES (3–4 w) | Group I—3.92% Group II—1.85% |
|
| Moustafa K Nassar, Hany A Khairy (2018) [38] | 100 | LCAG vs. BS + intraoperative 0.02% MMC | 12 | L: no data M: no data S: no data | Gutt ATB 1 w, gutt AT 1 w, gutt DEX 6 w. | LCAG group—2% MMC group—16% |
|
| Alvin L Young, Ka Wai Kam (2019) [39] | 40 | LCAG vs. BS + intraoperative 0.02% MMC vs. LCAG + intraoperative 0.02% MMC | 180 | L: no data M: no data S: no data | Ung ATB tid 4 w, gutt PRED qid 4 w. | LCAG—5.9% BS + MMC—0% LCAG + MMC—0% |
|
| Jing Yu, Jun Feng (2021) [40] | 85 | CAU vs. AMT + IFN alfa-2b vs. mCAU + AMT + IFN alfa-2b | 12 | L: no data M: CAU:20%/53.3%/26.7% AMT + IFN:16%/64%/20% mCAU + AMT + IFN: 16.7%/56.7%26.7% S: CAU:3.0 ± 1.0 AMT + IFN: 3.0 ± 1.0 mCAU + AMT + IFN: 3.0 ± 1.0 | Gutt ATB qid 2 w; gutt AT qid 2 w, gutt FML 3 m (qid→od) AMT + IFN, mCAU + AMT + IFN: gutt IFN alfa-2b: tid 3 m. Sutures removal: YES (14 d) | Conjunctival recurrence rate: CAU: 6.7% AMT + IFN: 12.0% mCAU + AMT + IFN: 6.7% Corneal recurrence rate: 0% in all groups |
|
| Waleed Alsarhani, Saeed Alshahrani (2021) [41] | 94 | CAU vs. CAU + intraoperative 0.02% MMC vs. AMT | 14 | L: 92.6% N/7.4% T M: no data S: no data | Ung ATB + DEX bid 2 w, gutt DEX 4 w. | CAU—15.6% CAU + MMC—15.8% AMT—27% | No data |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palewski, M.; Budnik, A.; Konopińska, J. Evaluating the Efficacy and Safety of Different Pterygium Surgeries: A Review of the Literature. Int. J. Environ. Res. Public Health 2022, 19, 11357. https://doi.org/10.3390/ijerph191811357
Palewski M, Budnik A, Konopińska J. Evaluating the Efficacy and Safety of Different Pterygium Surgeries: A Review of the Literature. International Journal of Environmental Research and Public Health. 2022; 19(18):11357. https://doi.org/10.3390/ijerph191811357
Chicago/Turabian StylePalewski, Marcin, Agnieszka Budnik, and Joanna Konopińska. 2022. "Evaluating the Efficacy and Safety of Different Pterygium Surgeries: A Review of the Literature" International Journal of Environmental Research and Public Health 19, no. 18: 11357. https://doi.org/10.3390/ijerph191811357




