The Effects of Short-Term PM2.5 Exposure on Pulmonary Function among Children with Asthma—A Panel Study in Shanghai, China
Abstract
:1. Introduction
2. Materials and Methods
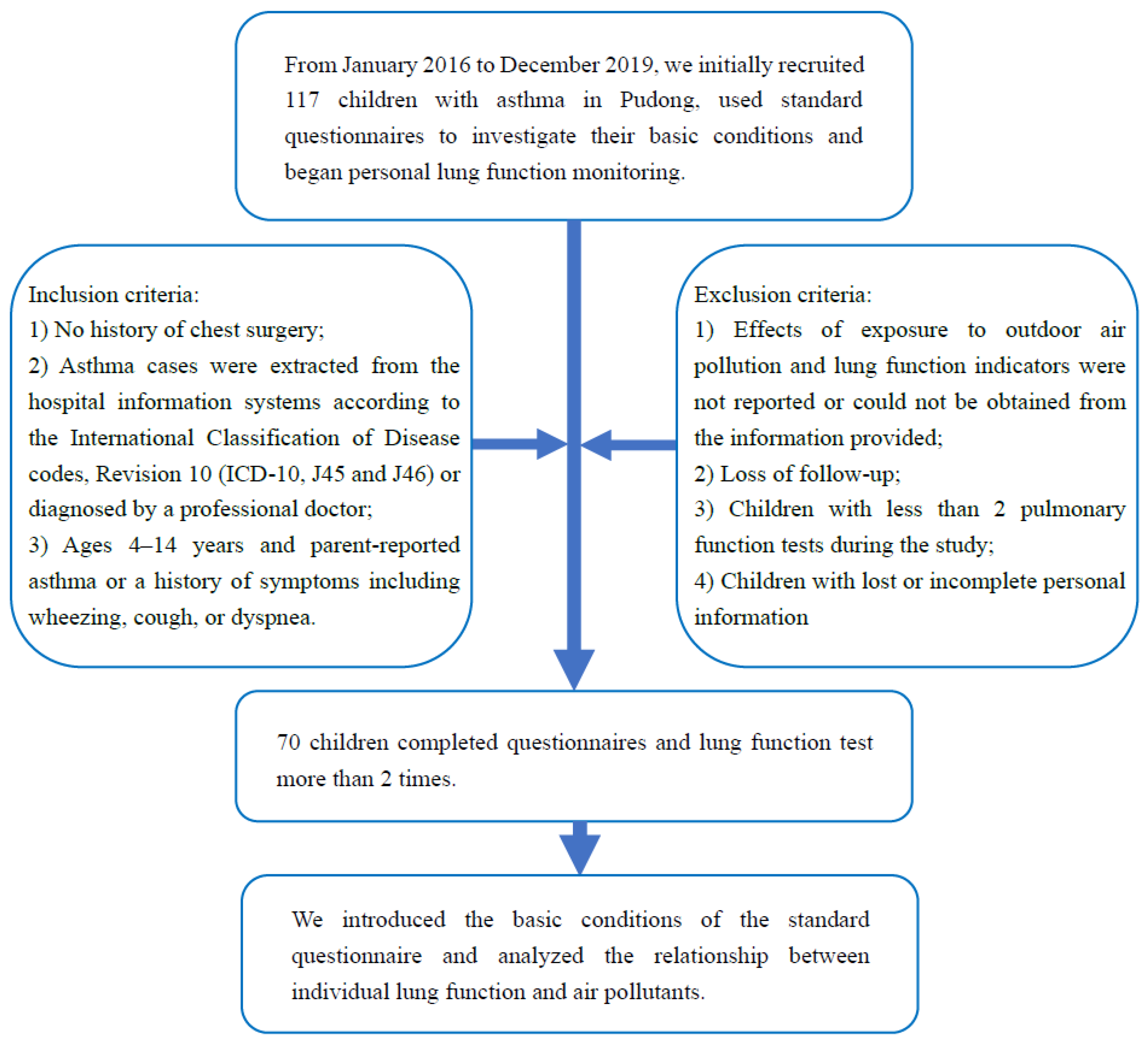
2.1. Design and Population
2.2. Lung Function Test and Questionnaire
2.3. Exposure
2.4. Statistical Analysis
3. Results
3.1. Descriptive Analysis
3.2. Regression Analysis
3.3. Subgroup Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maddox, L.; Schwartz, D.A. The pathophysiology of asthma. Annu. Rev. Med. 2002, 53, 477–498. [Google Scholar] [CrossRef] [PubMed]
- Holst, G.J.; Pedersen, C.B.; Thygesen, M.; Brandt, J.; Geels, C.; Bonlokke, J.H.; Sigsgaard, T. Air pollution and family related determinants of asthma onset and persistent wheezing in children: Nationwide case-control study. BMJ 2020, 370, m2791. [Google Scholar] [CrossRef] [PubMed]
- García-Marcos, L.; Asher, M.I.; Pearce, N.; Ellwood, E.; Bissell, K.; Chiang, C.-Y.; El Sony, A.; Ellwood, P.; Marks, G.B.; Mortimer, K.; et al. The burden of asthma, hay fever and eczema in children in 25 countries: GAN phase I study. Eur. Respir. J. 2022, 60, 2102866. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Abajobir, A.A.; Abate, K.H.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abdulkader, R.S.; Abdulle, A.M.; Abebo, T.A.; Abera, S.F.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Meghji, J.; Mortimer, K.; Agusti, A.; Allwood, B.W.; Asher, I.; Bateman, E.D.; Bissell, K.; Bolton, C.E.; Bush, A.; Celli, B.; et al. Improving lung health in low-income and middle-income countries: From challenges to solutions. Lancet 2021, 397, 928–940. [Google Scholar] [CrossRef]
- Chen, F.; Lin, Z.; Chen, R.; Norback, D.; Liu, C.; Kan, H.; Deng, Q.; Huang, C.; Hu, Y.; Zou, Z.; et al. The effects of PM2.5 on asthmatic and allergic diseases or symptoms in preschool children of six Chinese cities, based on China, children, homes and health (CCHH) project. Environ. Pollut. 2018, 232, 329–337. [Google Scholar] [CrossRef]
- Asher, M.I.; The ISAAC Phase One Study Group; Stewart, A.W.; Mallol, J.; Montefort, S.; Lai, C.K.W.; Aït-Khaled, N.; Odhiambo, J. Which population level environmental factors are associated with asthma, rhinoconjunctivitis and eczema? Review of the ecological analyses of ISAAC Phase One. Respir. Res. 2010, 11, 8. [Google Scholar] [CrossRef]
- Edginton, S.; O’Sullivan, D.E.; King, W.D.; Lougheed, M.D. The effect of acute outdoor air pollution on peak expiratory flow in individuals with asthma: A systematic review and meta-analysis. Environ. Res. 2021, 192, 110296. [Google Scholar] [CrossRef]
- Garcia, E.; Berhane, K.T.; Islam, T.; McConnell, R.; Urman, R.; Chen, Z.; Gilliland, F.D. Association of changes in air quality with incident asthma in children in California, 1993–2014. JAMA 2019, 321, 1906–1915. [Google Scholar] [CrossRef]
- He, L.; Cui, X.; Li, Z.; Teng, Y.; Barkjohn, K.J.; Norris, C.; Fang, L.; Lin, L.; Wang, Q.; Zhou, X.; et al. Malondialdehyde in nasal fluid: A biomarker for monitoring asthma control in relation to air pollution exposure. Environ. Sci. Technol. 2020, 54, 11405–11413. [Google Scholar] [CrossRef]
- Li, T.; Hu, R.; Chen, Z.; Li, Q.; Huang, S.; Zhu, Z.; Lin-Fu, Z. Fine particulate matter (PM2.5): The culprit for chronic lung diseases in China. Chronic Dis. Transl. Med. 2018, 4, 176–186. [Google Scholar] [CrossRef]
- Kuiper, I.N.; Svanes, C.; Markevych, I.; Accordini, S.; Bertelsen, R.J.; Bråbäck, L.; Christensen, J.H.; Forsberg, B.; Halvorsen, T.; Heinrich, J.; et al. Lifelong exposure to air pollution and greenness in relation to asthma, rhinitis and lung function in adulthood. Environ. Int. 2021, 146, 106219. [Google Scholar] [CrossRef]
- Branco, P.T.; Alvim-Ferraz, M.C.; Martins, F.G.; Ferraz, C.; Vaz, L.G.; Sousa, S.I. Impact of indoor air pollution in nursery and primary schools on childhood asthma. Sci. Total Environ. 2020, 745, 140982. [Google Scholar] [CrossRef]
- Cai, J.; Li, B.; Yu, W.; Yao, Y.; Wang, L.; Li, B.; Wang, Y.; Du, C.; Xiong, J. Associations of household dampness with asthma, allergies, and airway diseases among preschoolers in two cross-sectional studies in Chongqing, China: Repeated surveys in 2010 and 2019. Environ. Int. 2020, 140, 105752. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Li, B.; Zhao, Z.; Huang, C.; Zhang, X.; Deng, Q.; Lu, C.; Qian, H.; Yang, X.; et al. Asthma and allergic rhinitis among young parents in China in relation to outdoor air pollution, climate and home environment. Sci. Total Environ. 2021, 751, 141734. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Y.; Su, Z.; Pu, W.; Niu, M.; Song, S.; Wei, L.; Ding, Y.; Xu, L.; Tian, M.; et al. Respiratory exposure to PM2.5 soluble extract disrupts mucosal barrier function and promotes the development of experimental asthma. Sci. Total Environ. 2020, 730, 139145. [Google Scholar] [CrossRef]
- Gangwar, R.S.; Bevan, G.H.; Palanivel, R.; Das, L.; Rajagopalan, S. Oxidative stress pathways of air pollution mediated toxicity: Recent insights. Redox Biol. 2020, 34, 101545. [Google Scholar] [CrossRef]
- Anderson, H.R.; Butland, B.; Van Donkelaar, A.; Brauer, M.; Strachan, D.P.; Clayton, T.; Van Dingenen, R.; Amann, M.; Brunekreef, B.; Cohen, A.; et al. Satellite-based estimates of ambient air pollution and global variations in childhood asthma prevalence. Environ. Health Perspect. 2012, 120, 1333–1339. [Google Scholar] [CrossRef]
- Beasley, R. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. Lancet 1998, 351, 1225–1232. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, J.; Jiang, F.; Liu, S.; Li, S.; Tan, J.; Yin, Y.; Tong, S. Season-stratified effects of meteorological factors on childhood asthma in Shanghai, China. Environ. Res. 2020, 191, 110115. [Google Scholar] [CrossRef]
- Zheng, X.-Y.; Ding, H.; Jiang, L.-N.; Chen, S.-W.; Zheng, J.-P.; Qiu, M.; Zhou, Y.-X.; Chen, Q.; Guan, W.-J. Association between air pollutants and asthma emergency room visits and hospital admissions in time series studies: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0138146. [Google Scholar]
- Yamazaki, S.; Shima, M.; Ando, M.; Nitta, H.; Watanabe, H.; Nishimuta, T. Effect of hourly concentration of particulate matter on peak expiratory flow in hospitalized children: A panel study. Environ. Health 2011, 10, 15. [Google Scholar] [CrossRef]
- Jung, K.H.; Torrone, D.; Lovinsky-Desir, S.; Perzanowski, M.; Bautista, J.; Jezioro, J.R.; Hoepner, L.; Ross, J.; Perera, F.P.; Chillrud, S.N.; et al. Short-term exposure to PM2.5 and vanadium and changes in asthma gene DNA methylation and lung function decrements among urban children. Respir. Res. 2017, 18, 63. [Google Scholar] [CrossRef]
- Delfino, R.J.; Staimer, N.; Tjoa, T.; Gillen, D.; Kleinman, M.T.; Sioutas, C.; Cooper, D. Personal and ambient air pollution exposures and lung function decrements in children with asthma. Environ. Health Perspect. 2008, 116, 550–558. [Google Scholar] [CrossRef]
- Huang, C.; Liu, W.; Hu, Y.; Zou, Z.; Zhao, Z.; Shen, L.; Weschler, L.B.; Sundell, J. Updated prevalences of asthma, allergy, and airway symptoms, and a systematic review of trends over time for childhood asthma in Shanghai, China. PLoS ONE 2015, 10, e0121577. [Google Scholar]
- Zhang, Y.; Li, B.; Huang, C.; Yang, X.; Qian, H.; Deng, Q.; Zhao, Z.; Li, A.; Zhao, J.; Zhang, X.; et al. Ten cities cross-sectional questionnaire survey of children asthma and other allergies in China. Chin. Sci. Bull. 2013, 58, 4182–4189. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, Z.; Jiang, F.; Li, S.; Liu, S.; Wu, M.; Yan, C.; Tan, J.; Yu, G.; Hu, Y.; et al. Relative impact of meteorological factors and air pollutants on childhood allergic diseases in Shanghai, China. Sci. Total Environ. 2020, 706, 135975. [Google Scholar] [CrossRef]
- Zhou, X.; Hong, J. Pediatric asthma management in China: Current and future challenges. Pediatric Drugs 2018, 20, 105–110. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.M.; Zheng, J.; et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: The global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef]
- Wang, C.; Cai, J.; Chen, R.; Shi, J.; Yang, C.; Li, H.; Lin, Z.; Meng, X.; Liu, C.; Niu, Y.; et al. Personal exposure to fine particulate matter, lung function and serum club cell secretory protein (Clara). Environ. Pollut. 2017, 225, 450–455. [Google Scholar] [CrossRef]
- Baayen, R.H.; Davidson, D.J.; Bates, D.M. Mixed-effects modeling with crossed random effects for subjects and items. J. Mem. Lang. 2008, 59, 390–412. [Google Scholar] [CrossRef]
- Wu, S.; Ni, Y.; Li, H.; Pan, L.; Yang, D.; Baccarelli, A.A.; Deng, F.; Chen, Y.; Shima, M.; Guo, X. Short-term exposure to high ambient air pollution increases airway inflammation and respiratory symptoms in chronic obstructive pulmonary disease patients in Beijing, China. Environ. Int. 2016, 94, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Juarez, B.; Moraes, L.E.; Appuhamy, J.A.D.R.N.; Pellikaan, W.F.; Casper, D.P.; Tricarico, J.; Kebreab, E. Prediction and evaluation of enteric methane emissions from lactating dairy cows using different levels of covariate information. Anim. Prod. Sci. 2016, 56, 557–564. [Google Scholar] [CrossRef]
- Dales, R.; Chen, L.; Frescura, A.M.; Liu, L.; Villeneuve, P.J. Acute effects of outdoor air pollution on forced expiratory volume in 1 s: A panel study of schoolchildren with asthma. Eur. Respir. J. 2009, 34, 316. [Google Scholar] [CrossRef] [PubMed]
- Epton, M.; Dawson, R.D.; Brooks, W.M.; Kingham, S.; Aberkane, T.; Cavanagh, J.A.E.; Frampton, C.M.; Hewitt, T.; Cook, J.M.; McLeod, S.; et al. The effect of ambient air pollution on respiratory health of school children: A panel study. Environ. Health 2008, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhang, Y.; Zhou, L.; Li, T. Acute effects of PM2.5 on lung function parameters in schoolchildren in Nanjing, China: A panel study. Environ. Sci. Pollut. Res. 2018, 25, 14989–14995. [Google Scholar] [CrossRef] [PubMed]
- Zwozdziak, A.; Sówka, I.; Willak-Janc, E.; Zwozdziak, J.; Kwiecińska, K.; Balińska-Miśkiewicz, W. Influence of PM1 and PM2.5 on lung function parameters in healthy schoolchildren—A panel study. Environ. Sci. Pollut. Res. 2016, 23, 23892–23901. [Google Scholar] [CrossRef]
- Ward, D.J.; Ayres, J.G. Particulate air pollution and panel studies in children: A systematic review. Occup. Environ. Med. 2004, 61, e13. [Google Scholar] [CrossRef]
- He, L.; Norris, C.; Cui, X.; Li, Z.; Barkjohn, K.K.; Brehmer, C.; Teng, Y.; Fang, L.; Lin, L.; Wang, Q.; et al. Personal exposure to PM2.5 oxidative potential in association with pulmonary pathophysiologic outcomes in children with asthma. Environ. Sci. Technol. 2021, 55, 3101–3111. [Google Scholar] [CrossRef]
- Traina, G.; Bolzacchini, E.; Bonini, M.; Contini, D.; Mantecca, P.; Caimmi, S.M.E.; Licari, A. Role of air pollutants mediated oxidative stress in respiratory diseases. Pediatric Allergy Immunol. 2022, 33, 38–40. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, B.; Zhang, T.; Wang, H.; Peng, L.; Zhou, L. Effect of NF-kappaB signal pathway on mucus secretion induced by atmospheric PM2.5 in asthmatic rats. Cotoxicol. Environ. Saf. 2020, 190, 110094. [Google Scholar] [CrossRef]
- Chowdhury, P.H.; Okano, H.; Honda, A.; Kudou, H.; Kitamura, G.; Ito, S.; Ueda, K.; Takano, H. Aqueous and organic extract of PM2.5 collected in different seasons and cities of Japan differently affect respiratory and immune systems. Environ. Pollut. 2018, 235, 223–234. [Google Scholar] [CrossRef]
- Zhao, C.; Pu, W.; Wazir, J.; Jin, X.; Wei, L.; Song, S.; Su, Z.; Li, J.; Deng, Y.; Wang, H. Long-term exposure to PM2.5 aggravates pulmonary fibrosis and acute lung injury by disrupting Nrf2-mediated antioxidant function. Environ. Pollut. 2022, in press. [Google Scholar] [CrossRef]
- Liu, L.; Liu, C.; Chen, R.; Zhou, Y.; Meng, X.; Hong, J.; Cao, L.; Lu, Y.; Dong, X.; Xia, M.; et al. Associations of short-term exposure to air pollution and emergency department visits for pediatric asthma in Shanghai, China. Chemosphere 2021, 263, 127856. [Google Scholar] [CrossRef]
- Gauderman, W.J.; Urman, R.; Avol, E.; Berhane, K.; McConnell, R.; Rappaport, E.; Chang, R.; Lurmann, F.; Gilliland, F. Association of improved air quality with lung development in children. N. Engl. J. Med. 2015, 372, 905–913. [Google Scholar] [CrossRef]
- Gehring, U.; Gruzieva, O.; Agius, R.M.; Beelen, R.; Custovic, A.; Cyrys, J.; Eeftens, M.; Flexeder, C.; Fuertes, E.; Heinrich, J.; et al. Air pollution exposure and lung function in children: The ESCAPE Project. Environ. Health Perspect. 2013, 121, 1357–1364. [Google Scholar] [CrossRef]
- Kasamatsu, J.; Shima, M.; Yamazaki, S.; Tamura, K.; Sun, G. Effects of winter air pollution on pulmonary function of school children in Shenyang, China. Int. J. Hyg. Environ. Health 2006, 209, 435–444. [Google Scholar] [CrossRef]
- Erfinanda, L.; Ravindran, K.; Kohse, F.; Gallo, K.; Preissner, R.; Walther, T.; Kuebler, W.M. Oestrogen-mediated upregulation of the Mas receptor contributes to sex differences in acute lung injury and lung vascular barrier regulation. Eur. Respir. J. 2021, 57, 2000921. [Google Scholar] [CrossRef]
- Nahavandi, S.; Ahmadi, S.; Sobhani, S.A.; Abbasi, T.; Dehghani, A. A high dose of estrogen can improve renal ischemia-reperfusion-induced pulmonary injury in ovariectomized female rats. Can. J. Physiol. Pharmacol. 2021, 99, 1241–1252. [Google Scholar] [CrossRef]
- Beibei Wang, X.D. Exposure Factors Handbook of Chinese Population (6–17 Years); China Environmental Press: Beijing, China, 2016; Volume 455. [Google Scholar]
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef]
- Shah, R.; Newcomb, D.C. Sex bias in asthma prevalence and pathogenesis. Front. Immunol. 2018, 9, 2997. [Google Scholar] [CrossRef] [PubMed]
- Dunea, D.; Iordache, S.; Pohoata, A. Fine particulate matter in urban environments: A trigger of respiratory symptoms in sensitive children. Int. J. Environ. Res. Public Health 2016, 13, 1246. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, R.; Wang, Y.; Jiang, N.; Zhang, Y.; Li, T. The relationship between particulate matter and lung function of children: A systematic review and meta-analysis. Environ. Pollut. 2022, 309, 119735. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.-F.; Chen, Y.-H.; Lin, Y.-T.; Wu, X.-T.; Lee, Y.L. Relationship between exposure to fine particulates and ozone and reduced lung function in children. Environ. Res. 2015, 137, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Tortajada, J.I.; Castell, J.I.; Andreu, J.A.L.; Dominguez, F.I.; Garcia, J.A.O.; Tornero, O.B.; Garcia, V.I.; Conesa, A.C. Diseases associated to atmospheric pollution from fossil fuels. Pediatrics aspects. Rev. Esp. Pediatr. 2001, 57, 213–249. [Google Scholar]
- Hua, J.; Yin, Y.; Peng, L.; Du, L.; Geng, F.; Zhu, L. Acute effects of black carbon and PM2.5 on children asthma admissions: A time-series study in a Chinese city. Sci. Total Environ. 2014, 481, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ni, H.; Bai, L.; Cheng, Q.; Zhang, H.; Wang, S.; Xie, M.; Zhao, D.; Su, H. The short-term association between air pollution and childhood asthma hospital admissions in urban areas of Hefei City in China: A time-series study. Environ. Res. 2019, 169, 510–516. [Google Scholar] [CrossRef]
- Gleason, J.A.; Bielory, L.; Fagliano, J.A. Associations between ozone, PM2.5, and four pollen types on emergency department pediatric asthma events during the warm season in New Jersey: A case-crossover study. Environ. Res. 2014, 132, 421–429. [Google Scholar] [CrossRef]
- Yamazaki, S.; Shima, M.; Yoda, Y.; Kurosaka, F.; Isokawa, T.; Shimizu, S.; Ogawa, T.; Kamiyoshi, N.; Terada, K.; Nishikawa, J.; et al. Association between chemical components of PM2.5 and children’s primary care night-time visits due to asthma attacks: A case-crossover study. Allergol. Int. 2019, 68, 329–334. [Google Scholar] [CrossRef]
- Yu, Z.; Ma, Y.; Zhang, Y.; Cheng, B.; Feng, F.; Ma, B.; Jiao, H.; Zhou, J. A study on the short-term effect of particulate matter pollution on hospital visits for asthma in children in Shanghai, China. Environ. Geochem. Health 2021, 43, 4123–4138. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, B.; Fu, Q.; Zhao, Q.; Zhang, Q.; Chen, J.; Yang, X.; Duan, Y.; Li, J. Intense secondary aerosol formation due to strong atmospheric photochemical reactions in summer: Observations at a rural site in eastern Yangtze River Delta of China. Sci. Total Environ. 2016, 571, 1454–1466. [Google Scholar] [CrossRef]
- Huang, W.; Schinasi, L.H.; Kenyon, C.C.; Moore, K.; Melly, S.; Hubbard, R.A.; Zhao, Y.; Roux, A.V.D.; Forrest, C.B.; Maltenfort, M.; et al. Effects of ambient air pollution on childhood asthma exacerbation in the Philadelphia metropolitan Region, 2011–2014. Environ. Res. 2021, 197, 110955. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, Z.; Xiang, Q.; Wang, W.; Huang, L.; Mao, F. Short-term effects of ambient PM1 and PM2.5 air pollution on hospital admission for respiratory diseases: Case-crossover evidence from Shenzhen, China. Int. J. Hyg. Environ. Health 2020, 224, 113418. [Google Scholar] [CrossRef]
- Bell, M.L.; Ebisu, K.; Peng, R.D.; Walker, J.; Samet, J.M.; Zeger, S.L.; Dominici, F. Seasonal and regional short-term effects of fine particles on hospital admissions in 202 US counties, 1999–2005. Am. J. Epidemiol. 2008, 168, 1301–1310. [Google Scholar] [CrossRef]
- Chen, R.; Peng, R.D.; Meng, X.; Zhou, Z.; Chen, B.; Kan, H. Seasonal variation in the acute effect of particulate air pollution on mortality in the China Air Pollution and Health Effects Study (CAPES). Sci. Total Environ. 2013, 450, 259–265. [Google Scholar] [CrossRef]
- Huang, C.; Wang, X.; Liu, W.; Cai, J.; Shen, L.; Zou, Z.; Lu, R.; Chang, J.; Wei, X.; Sun, C.; et al. Household indoor air quality and its associations with childhood asthma in Shanghai, China: On-site inspected methods and preliminary results. Environ. Res. 2016, 151, 154–167. [Google Scholar] [CrossRef]
- Zhou, X.; Cai, J.; Zhao, Y.; Chen, R.; Wang, C.; Zhao, A.; Yang, C.; Li, H.; Liu, S.; Cao, J.; et al. Estimation of residential fine particulate matter infiltration in Shanghai, China. Environ. Pollut. 2018, 233, 494–500. [Google Scholar] [CrossRef]





| n | % | |
|---|---|---|
| Sex | ||
| boy | 39 | 55.71 |
| girl | 31 | 44.29 |
| Age | ||
| <6 | 40 | 57.14 |
| ≥6 | 30 | 42.86 |
| BMI,Mean ± SD | 16.55 ± 2.82 | |
| Atopic dermatitis | ||
| Yes | 44 | 62.86 |
| No | 26 | 37.14 |
| Allergic rhinitis | ||
| Yes | 61 | 87.14 |
| No | 9 | 12.86 |
| Food or drug allergies | ||
| Yes | 20 | 28.57 |
| No | 50 | 71.43 |
| Mother’s history of allergies | ||
| Yes | 52 | 74.29 |
| No | 18 | 25.71 |
| Father’s allergy history | ||
| Yes | 52 | 74.29 |
| No | 18 | 25.71 |
| Frequency of smoking in places where their children often had activities or rest | ||
| 0 units/day | 61 | 87.14 |
| <1 unit/day | 2 | 2.86 |
| 1~5 units/day | 6 | 8.57 |
| >5 units/day | 1 | 1.43 |
| Frequency of contact between smokers and children | ||
| 0 h/day | 43 | 61.43 |
| <1 h/day | 9 | 12.86 |
| 1–4 h/day | 11 | 15.71 |
| 4–8 h/day | 3 | 4.29 |
| >8 h/day | 4 | 5.71 |
| Mean | SD | Min | Max | IQR | |
|---|---|---|---|---|---|
| Daily temperature (℃) | 19.17 | 9.03 | −0.10 | 32.20 | 16.40 |
| Relative humidity (%) | 74.86 | 11.73 | 37.00 | 97.50 | 18.30 |
| PM2.5 (μg/m3) | 34.15 | 25.13 | 9.00 | 173.00 | 28.00 |
| PM10 (μg/m3) | 50.69 | 26.46 | 13.00 | 166.00 | 34.00 |
| O3 (μg/m3) | 107.34 | 48.04 | 32.00 | 251.00 | 67.00 |
| SO2 (μg/m3) | 9.92 | 4.30 | 5.00 | 40.00 | 5.00 |
| NO2 (μg/m3) | 40.38 | 19.84 | 13.00 | 118.00 | 26.00 |
| CO (μg/m3) | 663.23 | 236.13 | 400.00 | 1800.00 | 200.00 |
| FVC (L) | 1.63 | 0.52 | 0.57 | 3.85 | 0.62 |
| FEV1 (L) | 1.36 | 0.42 | 0.54 | 3.45 | 0.51 |
| PEF (L/s) | 3.03 | 1.61 | 1.05 | 21.80 | 1.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Lei, R.; Xu, J.; Peng, L.; Ye, X.; Yang, D.; Yang, S.; Yin, Y.; Zhang, R. The Effects of Short-Term PM2.5 Exposure on Pulmonary Function among Children with Asthma—A Panel Study in Shanghai, China. Int. J. Environ. Res. Public Health 2022, 19, 11385. https://doi.org/10.3390/ijerph191811385
Zhou J, Lei R, Xu J, Peng L, Ye X, Yang D, Yang S, Yin Y, Zhang R. The Effects of Short-Term PM2.5 Exposure on Pulmonary Function among Children with Asthma—A Panel Study in Shanghai, China. International Journal of Environmental Research and Public Health. 2022; 19(18):11385. https://doi.org/10.3390/ijerph191811385
Chicago/Turabian StyleZhou, Ji, Ruoyi Lei, Jianming Xu, Li Peng, Xiaofang Ye, Dandan Yang, Sixu Yang, Yong Yin, and Renhe Zhang. 2022. "The Effects of Short-Term PM2.5 Exposure on Pulmonary Function among Children with Asthma—A Panel Study in Shanghai, China" International Journal of Environmental Research and Public Health 19, no. 18: 11385. https://doi.org/10.3390/ijerph191811385





