Thermodynamics, Kinetics, and Mechanisms of the Co-Removal of Arsenate and Arsenite by Sepiolite-Supported Nanoscale Zero-Valent Iron in Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Materials
2.3. Characterization of Samples
2.4. Batch Sorption Experiments
2.4.1. Optimization of the Adsorbent Preparation Conditions
2.4.2. Adsorption Kinetics
2.4.3. Adsorption Isotherm
2.4.4. Effect of Initial pH, Adsorbent Dosage and Coexisting Ions
3. Results
3.1. Adsorption Kinetics of As(III)/As(V) by S-nZVI
3.2. Isotherm Adsorption Study of As(III)/As(V) by S-nZVI
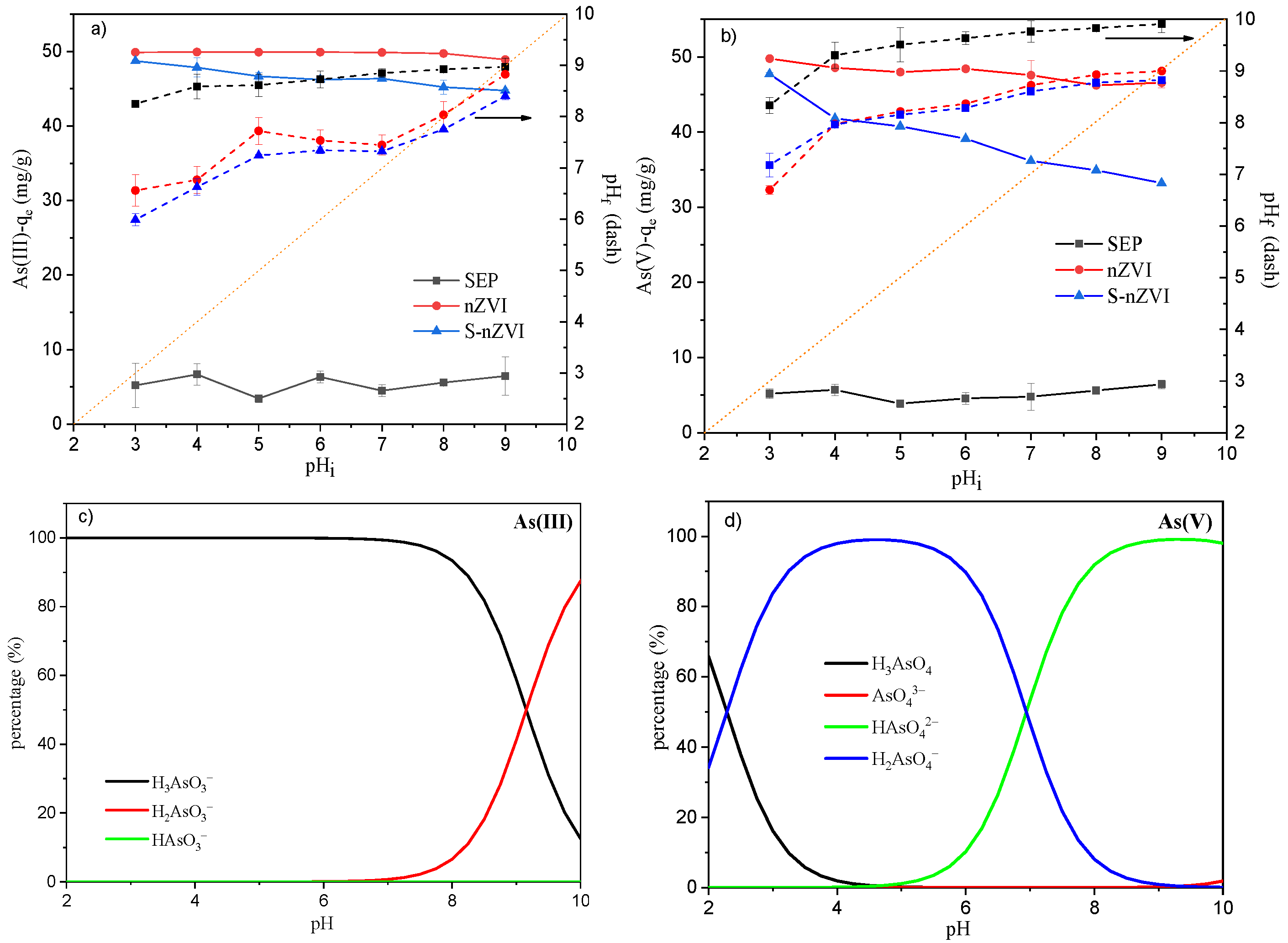
3.3. Influence of Initial pH on As(III)/As(V) Adsorption
3.4. Influence of Adsorbent Dosage
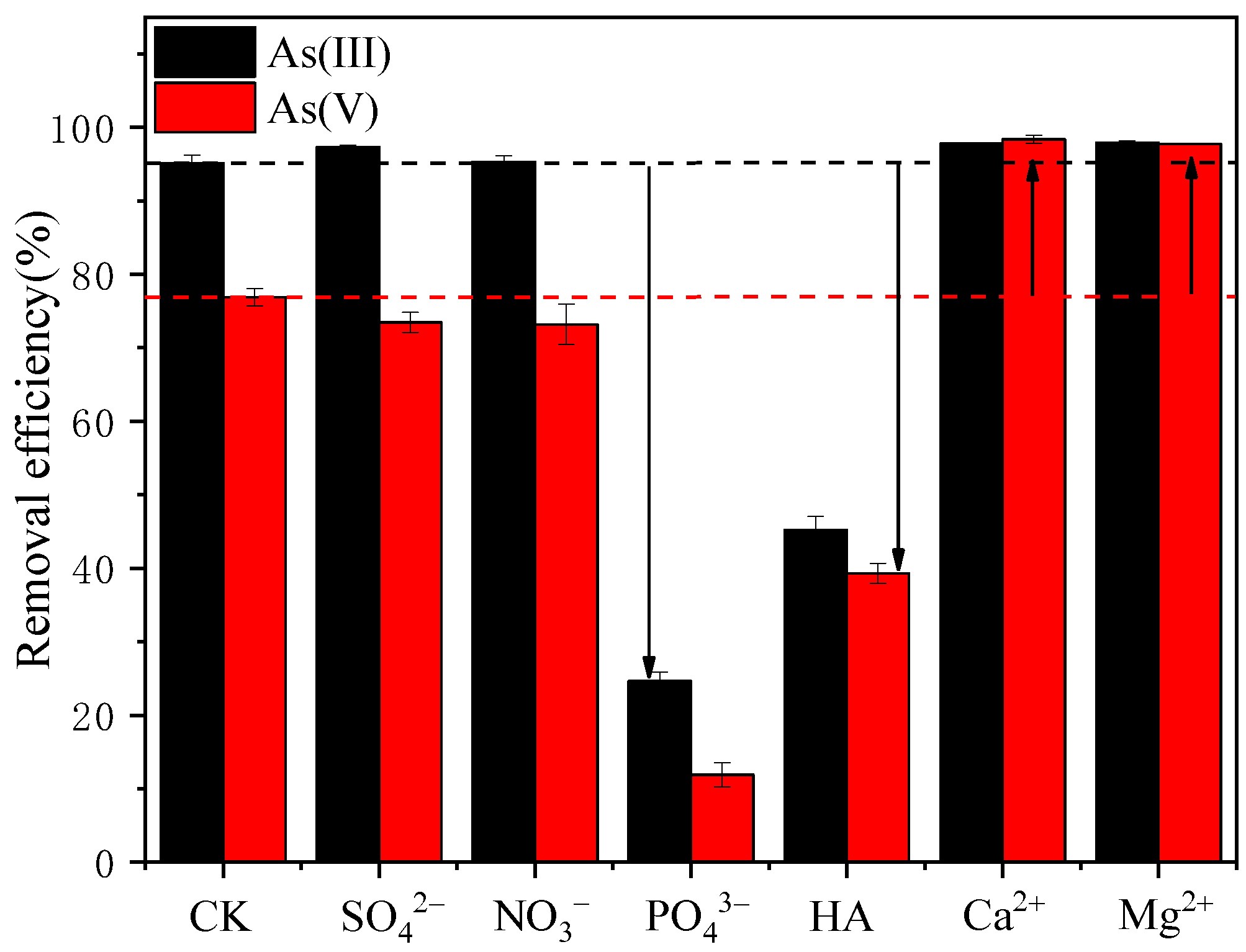
3.5. Influence of Coexisting Ions/Substances on As(III)/As(V) Adsorption
3.6. S-nZVI Characterization and Sorption Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, B.; Zhu, Q.H.; Zhang, Q.; Zhu, H.H.; Huang, D.Y.; Su, S.M.; Wang, Y.N.; Zeng, X.B. Cadmium and arsenic availability in soil under submerged incubation: The influence of humic substances on iron speciation. Ecotoxicol. Environ. Saf. 2021, 225, 112773. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-M.; Tang, D.-D.; Yuan, X.-Y.; Uchimiya, M.; Li, J.-Z.; Li, Z.-Y.; Luo, Z.-C.; Xu, Z.-W.; Sun, S.-G. Effect of amendments on soil Cd sorption and trophic transfer of Cd and mineral nutrition along the food chain. Ecotoxicol. Environ. Saf. 2020, 189, 110045. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.; Li, B.; Li, J.; Chen, W.; Xu, L. Heavy metals in paddy soil-rice systems of industrial and township areas from subtropical China: Levels, transfer and health risks. J. Geochem. Explor. 2018, 194, 210–217. [Google Scholar] [CrossRef]
- Jing, M.; Chen, W.; Zheng, T.; Liao, Y.; Ellis Burnet, J.; Xu, M.; Yang, C.; Shen, L.; Liang, M. Water geochemical characteristic variations in and around a karst-dominated natural reserve area, southwestern China. Environ. Earth Sci. 2011, 64, 1051–1058. [Google Scholar] [CrossRef]
- Mäki-Paakkanen, J.; Kurttio, P.; Paldy, A.; Pekkanen, J. Association between the clastogenic effect in peripheral lymphocytes and human exposure to arsenic through drinking water. Environ. Mol. Mutagen. 1998, 32, 301–313. [Google Scholar] [CrossRef]
- Rahman, M.A.; Hasegawa, H.; Rahman, M.M.; Miah, M.M.; Tasmin, A. Arsenic accumulation in rice (Oryza sativa L.): Human exposure through food chain. Ecotoxicol. Environ. Saf. 2008, 69, 317–324. [Google Scholar] [CrossRef]
- Cubadda, F.; Jackson, B.P.; Cottingham, K.L.; Van Horne, Y.O.; Kurzius-Spencer, M. Human exposure to dietary inorganic arsenic and other arsenic species: State of knowledge, gaps and uncertainties. Sci. Total Environ. 2017, 579, 1228–1239. [Google Scholar] [CrossRef]
- Abdul, K.S.M.; Jayasinghe, S.S.; Chandana, E.P.; Jayasumana, C.; De Silva, P.M.C. Arsenic and human health effects: A review. Environ. Toxicol. Pharmacol. 2015, 40, 828–846. [Google Scholar] [CrossRef]
- Rahman, M.M.; Dong, Z.; Naidu, R. Concentrations of arsenic and other elements in groundwater of Bangladesh and West Bengal, India: Potential cancer risk. Chemosphere 2015, 139, 54–64. [Google Scholar] [CrossRef]
- Tang, J.; Liao, Y.; Yang, Z.; Chai, L.; Yang, W. Characterization of arsenic serious-contaminated soils from Shimen realgar mine area, the Asian largest realgar deposit in China. J. Soils Sediments 2016, 16, 1519–1528. [Google Scholar] [CrossRef]
- Adlassnig, W.; Schmidt, B.; Jirsa, F.; Gradwohl, A.; Ivesic, C.; Koller-Peroutka, M. The Arsenic–Antimony Creek at Sauerbrunn/Burgenland, Austria: A Toxic Habitat for Amphibians. Int. J. Environ. Res. Public Health 2022, 19, 6010. [Google Scholar] [CrossRef] [PubMed]
- Cuong, D.V.; Wu, P.C.; Chen, L.I.; Hou, C.H. Active MnO2/biochar composite for efficient As (III) removal: Insight into the mechanisms of redox transformation and adsorption. Water Res. 2021, 188, 116495. [Google Scholar] [CrossRef] [PubMed]
- Jain, C.K.; Ali, I. Arsenic: Occurrence, toxicity and speciation techniques. Water Res. 2000, 34, 4304–4312. [Google Scholar] [CrossRef]
- Petrick, J.S.; Ayala-Fierro, F.; Cullen, W.R.; Carter, D.E.; Vasken Aposhian, H. Monomethylarsonous acid (MMA(III)) is more toxic than arsenite in Chang human hepatocytes. Toxicol. Appl. Pharmacol. 2000, 163, 203–207. [Google Scholar] [CrossRef] [PubMed]
- An, B.; Zhao, D. Immobilization of As(III) in soil and groundwater using a new class of polysaccharide stabilized Fe-Mn oxide nanoparticles. J. Hazard. Mater. 2012, 211–212, 332–341. [Google Scholar] [CrossRef]
- Ding, Z.; Fu, F.; Cheng, Z.; Lu, J.; Tang, B. Novel mesoporous Fe[sbnd]Al bimetal oxides for As(III) removal: Performance and mechanism. Chemosphere 2017, 169, 297–307. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef]
- Fu, F.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef]
- Shrestha, R.; Ban, S.; Devkota, S.; Sharma, S.; Joshi, R.; Tiwari, A.P.; Kim, H.Y.; Joshi, M.K. Technological trends in heavy metals removal from industrial wastewater: A review. J. Environ. Chem. Eng. 2021, 9, 105688. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhou, S.; Zhou, X.; Mo, S.; Zhu, Y.; Zhang, L.; Tang, S.; Fang, Z.; Fan, Y. Effective Remediation of Arsenic-Contaminated Soils by EK-PRB of Fe/Mn/C-LDH: Performance, Characteristics, and Mechanism. Int. J. Environ. Res. Public Health 2022, 19, 4389. [Google Scholar] [CrossRef]
- Ali, A.; Siddique, M.; Chen, W.; Han, Z.; Khan, R.; Bilal, M.; Waheed, U.; Shahzadi, I. Promising Low-Cost Adsorbent from Waste Green Tea Leaves for Phenol Removal in Aqueous Solution. Int. J. Environ. Res. Public Health 2022, 19, 6396. [Google Scholar] [CrossRef]
- Gil-Díaz, M.; Diez-Pascual, S.; González, A.; Alonso, J.; Rodríguez-Valdés, E.; Gallego, J.R.; Lobo, M.C. A nanoremediation strategy for the recovery of an As-polluted soil. Chemosphere 2016, 149, 137–145. [Google Scholar] [CrossRef]
- Wadhawan, S.; Jain, A.; Nayyar, J.; Mehta, S.K. Role of nanomaterials as adsorbents in heavy metal ion removal from waste water: A review. J. Water Process Eng. 2020, 33, 101038. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Meng, J.; Liu, X.; Xu, J.; Wang, F.; Brookes, P. Zeolite-supported nanoscale zero-valent iron: New findings on simultaneous adsorption of Cd(II), Pb(II), and As(III) in aqueous solution and soil. J. Hazard. Mater. 2018, 344, 1–11. [Google Scholar] [CrossRef]
- Zhu, H.; Jia, Y.; Wu, X.; Wang, H. Removal of arsenic from water by supported nano zero-valent iron on activated carbon. J. Hazard. Mater. 2009, 172, 1591–1596. [Google Scholar] [CrossRef]
- Du, Y.; Zhen, S.; Wang, J.; Ma, Y.; Wu, J.; Dai, H. FeOOH-MnO2/Sepiolite and Fe2O3-MnO2/Diatomite: Highly efficient adsorbents for the removal of as (V). Appl. Clay Sci. 2022, 222, 106491. [Google Scholar] [CrossRef]
- Zhou, F.; Ye, G.; Gao, Y.; Wang, H.; Zhou, S.; Liu, Y.; Yan, C. Cadmium adsorption by thermal-activated sepiolite: Application to in-situ remediation of artificially contaminated soil. J. Hazard. Mater. 2022, 423, 127104. [Google Scholar] [CrossRef]
- Daneshkhah, M.; Hossaini, H.; Malakootian, M. Removal of metoprolol from water by sepiolite-supported nanoscale zero-valent iron. J. Environ. Chem. Eng. 2017, 5, 3490–3499. [Google Scholar] [CrossRef]
- Fu, R.; Yang, Y.; Xu, Z.; Zhang, X.; Guo, X.; Bi, D. The removal of chromium (VI) and lead (II) from groundwater using sepiolite-supported nanoscale zero-valent iron (S-NZVI). Chemosphere 2015, 138, 726–734. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, Z.; Ouyang, J.; Yang, H.; Chen, D. Highly dispersed sepiolite-based organic modified nanofibers for enhanced adsorption of Congo red. Appl. Clay Sci. 2018, 157, 76–85. [Google Scholar] [CrossRef]
- Yang, D.; Wang, L.; Li, Z.; Tang, X.; He, M.; Yang, S.; Liu, X.; Xu, J. Simultaneous adsorption of Cd(II) and As(III) by a novel biochar-supported nanoscale zero-valent iron in aqueous systems. Sci. Total Environ. 2020, 708, 134823. [Google Scholar] [CrossRef] [PubMed]
- Malana, M.A.; Qureshi, R.B.; Ashiq, M.N. Adsorption studies of arsenic on nano aluminium doped manganese copper ferrite polymer (MA, VA, AA) composite: Kinetics and mechanism. Chem. Eng. J. 2011, 172, 721–727. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Astakhov, V.A. Development of the concepts of volume filling of micropores in the adsorption of gases and vapors by microporous adsorbents Communication 2. General bases of the theory of adsorption of gases and vapors on zeolites. Bull. Acad. Sci. Ussr Div. Chem. Sci. 1971, 20, 8–12. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Radushkevich, L.V. The equation of the characteristic curve of the activated charcoal. Proc. Acad. Sci. USSR Phys. Chem. Sect. 1947, 55, 331–337. [Google Scholar]
- Wang, J.; Guo, X. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- Kanematsu, M.; Young, T.M.; Fukushi, K.; Green, P.G.; Darby, J.L. Arsenic(III, V) adsorption on a goethite-based adsorbent in the presence of major co-existing ions: Modeling competitive adsorption consistent with spectroscopic and molecular evidence. Geochim. Cosmochim. Acta 2013, 106, 404–428. [Google Scholar] [CrossRef]
- Chen, S.G.; Yang, R.T. Theoretical Basis for the Potential Theory Adsorption Isotherms. The Dubinin-Radushkevich and Dubinin-Astakhov Equations. Langmuir 1994, 10, 4244–4249. [Google Scholar] [CrossRef]
- Kanel, S.R.; Greneche, J.M.; Choi, H. Arsenic(V) removal from groundwater using nano scale zero-valent iron as a colloidal reactive barrier material. Environ. Sci. Technol. 2006, 40, 2045–2050. [Google Scholar] [CrossRef]
- Kanel, S.R.; Manning, B.; Charlet, L.; Choi, H. Removal of arsenic(III) from groundwater by nanoscale zero-valent iron. Environ. Sci. Technol. 2005, 39, 1291–1298. [Google Scholar] [CrossRef]
- Stachowicz, M.; Hiemstra, T.; van Riemsdijk, W.H. Multi-competitive interaction of As(III) and As(V) oxyanions with Ca2+, Mg2+, PO43−, and CO32− ions on goethite. J. Colloid Interface Sci. 2008, 320, 400–414. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wei, S.; Liu, C.; Chen, T.; Tang, Y.; Ma, J.; Yin, K.; Luo, S. Efficient removal of arsenic from groundwater using iron oxide nanoneedle array-decorated biochar fibers with high Fe utilization and fast adsorption kinetics. Water Res. 2019, 167, 115107. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Sparks, D.L. ATR-FTIR spectroscopic investigation on phosphate adsorption mechanisms at the ferrihydrite-water interface. J. Colloid Interface Sci. 2001, 241, 317–326. [Google Scholar] [CrossRef]
- Khare, N.; Hesterberg, D.; Martin, J.D. XANES investigation of phosphate sorption in single and binary systems of iron and aluminum oxide minerals. Environ. Sci. Technol. 2005, 39, 2152–2160. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, B.; Liu, H.; Qu, J. Simultaneous removal of arsenite and fluoride via an integrated electro-oxidation and electrocoagulation process. Chemosphere 2011, 83, 726–729. [Google Scholar] [CrossRef]
- Jones, R.G.; Loeppert, R.H. Calcite Surface Adsorption of As(V), As(III), MMAs(V), and DMAs(V) and the Impact of Calcium and Phosphate. Soil Sci. Soc. Am. J. 2013, 77, 83–93. [Google Scholar] [CrossRef]
- Naushad, M.; Ahamad, T.; Al-Maswari, B.M.; Abdullah Alqadami, A.; Alshehri, S.M. Nickel ferrite bearing nitrogen-doped mesoporous carbon as efficient adsorbent for the removal of highly toxic metal ion from aqueous medium. Chem. Eng. J. 2017, 330, 1351–1360. [Google Scholar] [CrossRef]
- Martin, J.E.; Herzing, A.A.; Yan, W.; Li, X.Q.; Koel, B.E.; Kiely, C.J.; Zhang, W. Determination of the oxide layer thickness in core-shell zerovalent iron nanoparticles. Langmuir 2008, 24, 4329–4334. [Google Scholar] [CrossRef]
- Leupin, O.X.; Hug, S.J. Oxidation and removal of arsenic (III) from aerated groundwater by filtration through sand and zero-valent iron. Water Res. 2005, 39, 1729–1740. [Google Scholar] [CrossRef]
- Yan, W.; Ramos, M.A.V.; Koel, B.E.; Zhang, W.X. As(III) sequestration by iron nanoparticles: Study of solid-phase redox transformations with X-ray photoelectron spectroscopy. J. Phys. Chem. C 2012, 116, 5303–5311. [Google Scholar] [CrossRef]
- Bakshi, S.; Banik, C.; Rathke, S.J.; Laird, D.A. Arsenic sorption on zero-valent iron-biochar complexes. Water Res. 2018, 137, 153–163. [Google Scholar] [CrossRef]
- Liu, X.; Xu, H.; Wang, L.; Qu, Z.; Yan, N. Surface nano-traps of Fe0/COFs for arsenic(III) depth removal from wastewater in non-ferrous smelting industry. Chem. Eng. J. 2020, 381, 122559. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, M.; Dong, H.; Li, H.; Pan, B. Simultaneous Oxidation and Sequestration of As(III) from Water by Using Redox Polymer-Based Fe(III) Oxide Nanocomposite. Environ. Sci. Technol. 2017, 51, 6326–6334. [Google Scholar] [CrossRef]
- Bhowmick, S.; Chakraborty, S.; Mondal, P.; Van Renterghem, W.; Van den Berghe, S.; Roman-Ross, G.; Chatterjee, D.; Iglesias, M. Montmorillonite-supported nanoscale zero-valent iron for removal of arsenic from aqueous solution: Kinetics and mechanism. Chem. Eng. J. 2014, 243, 14–23. [Google Scholar] [CrossRef]
- Cui, J.; Jin, Q.; Li, Y.; Li, F. Oxidation and removal of As(iii) from soil using novel magnetic nanocomposite derived from biomass waste. Environ. Sci. Nano 2019, 6, 478–488. [Google Scholar] [CrossRef]


| Kinetic Models | Parameters | As(III) | As(V) |
|---|---|---|---|
| Qexp (mg/g) | 40.71 | 39.52 | |
| Pseudo-first-order kinetics | k1 (g /mg/ h) | 19.00 | 6.35 |
| qe (mg/g) | 40.13 | 37.69 | |
| R2 | 0.998 | 0.979 | |
| Pseudo-second-order kinetics | k2 (g /mg/ h) | 1.119 | 0.2841 |
| qe (mg/g) | 40.75 | 39.01 | |
| R2 | 0.998 | 0.993 |
| Langmuir Model | Freundlich Model | ||||||
|---|---|---|---|---|---|---|---|
| qm (mg/g) | KL | R2 | RL | Kf | 1/n | R2 | |
| As(III) | |||||||
| nZVI | 93.62 | 0.0037 | 0.999 | 0.57–0.98 | 0.686 | 0.777 | 0.995 |
| S-nZVI | 165.86 | 0.090 | 0.967 | 0.05–0.69 | 20.693 | 0.523 | 0.867 |
| As(V) | |||||||
| nZVI | 50.29 | 0.0085 | 0.997 | 0.37–0.96 | 0.302 | 0.624 | 0.974 |
| S-nZVI | 95.76 | 0.044 | 0.863 | 0.10–0.82 | 1.891 | 0.0346 | 0.974 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ainiwaer, M.; Zeng, X.; Yin, X.; Wen, J.; Su, S.; Wang, Y.; Zhang, Y.; Zhang, T.; Zhang, N. Thermodynamics, Kinetics, and Mechanisms of the Co-Removal of Arsenate and Arsenite by Sepiolite-Supported Nanoscale Zero-Valent Iron in Aqueous Solution. Int. J. Environ. Res. Public Health 2022, 19, 11401. https://doi.org/10.3390/ijerph191811401
Ainiwaer M, Zeng X, Yin X, Wen J, Su S, Wang Y, Zhang Y, Zhang T, Zhang N. Thermodynamics, Kinetics, and Mechanisms of the Co-Removal of Arsenate and Arsenite by Sepiolite-Supported Nanoscale Zero-Valent Iron in Aqueous Solution. International Journal of Environmental Research and Public Health. 2022; 19(18):11401. https://doi.org/10.3390/ijerph191811401
Chicago/Turabian StyleAiniwaer, Meihaguli, Xibai Zeng, Xianqiang Yin, Jiong Wen, Shiming Su, Yanan Wang, Yang Zhang, Tuo Zhang, and Nan Zhang. 2022. "Thermodynamics, Kinetics, and Mechanisms of the Co-Removal of Arsenate and Arsenite by Sepiolite-Supported Nanoscale Zero-Valent Iron in Aqueous Solution" International Journal of Environmental Research and Public Health 19, no. 18: 11401. https://doi.org/10.3390/ijerph191811401
APA StyleAiniwaer, M., Zeng, X., Yin, X., Wen, J., Su, S., Wang, Y., Zhang, Y., Zhang, T., & Zhang, N. (2022). Thermodynamics, Kinetics, and Mechanisms of the Co-Removal of Arsenate and Arsenite by Sepiolite-Supported Nanoscale Zero-Valent Iron in Aqueous Solution. International Journal of Environmental Research and Public Health, 19(18), 11401. https://doi.org/10.3390/ijerph191811401









