Coronavirus Vaccination: Spike Antibody Levels in Health Workers after Six Months—A Cross-Sectional Study
Abstract
:1. Introduction
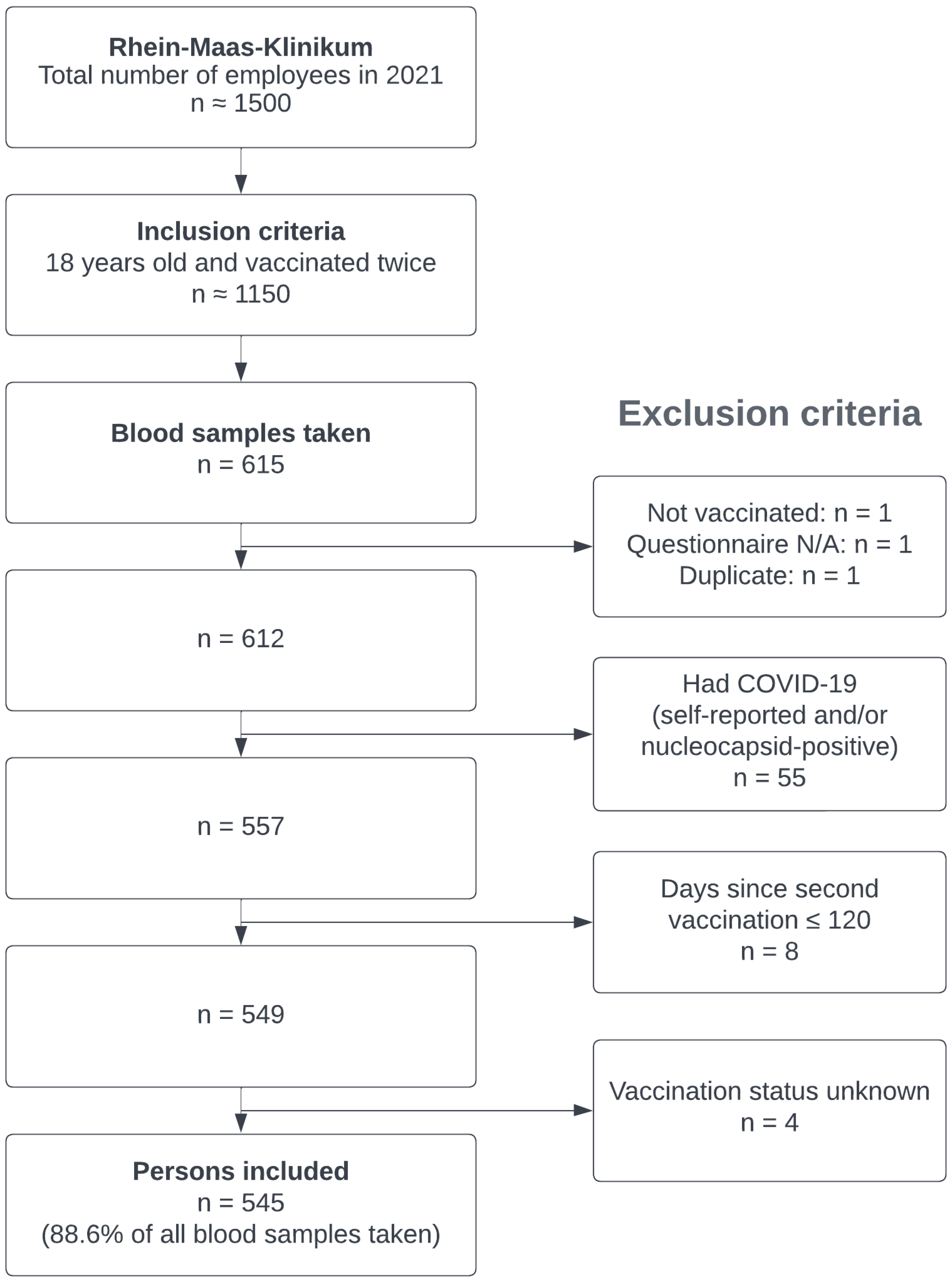
2. Materials and Methods
2.1. Recruiting
2.2. Sample Collection/Outcome
2.3. Explanatory Variables
2.4. Statistics
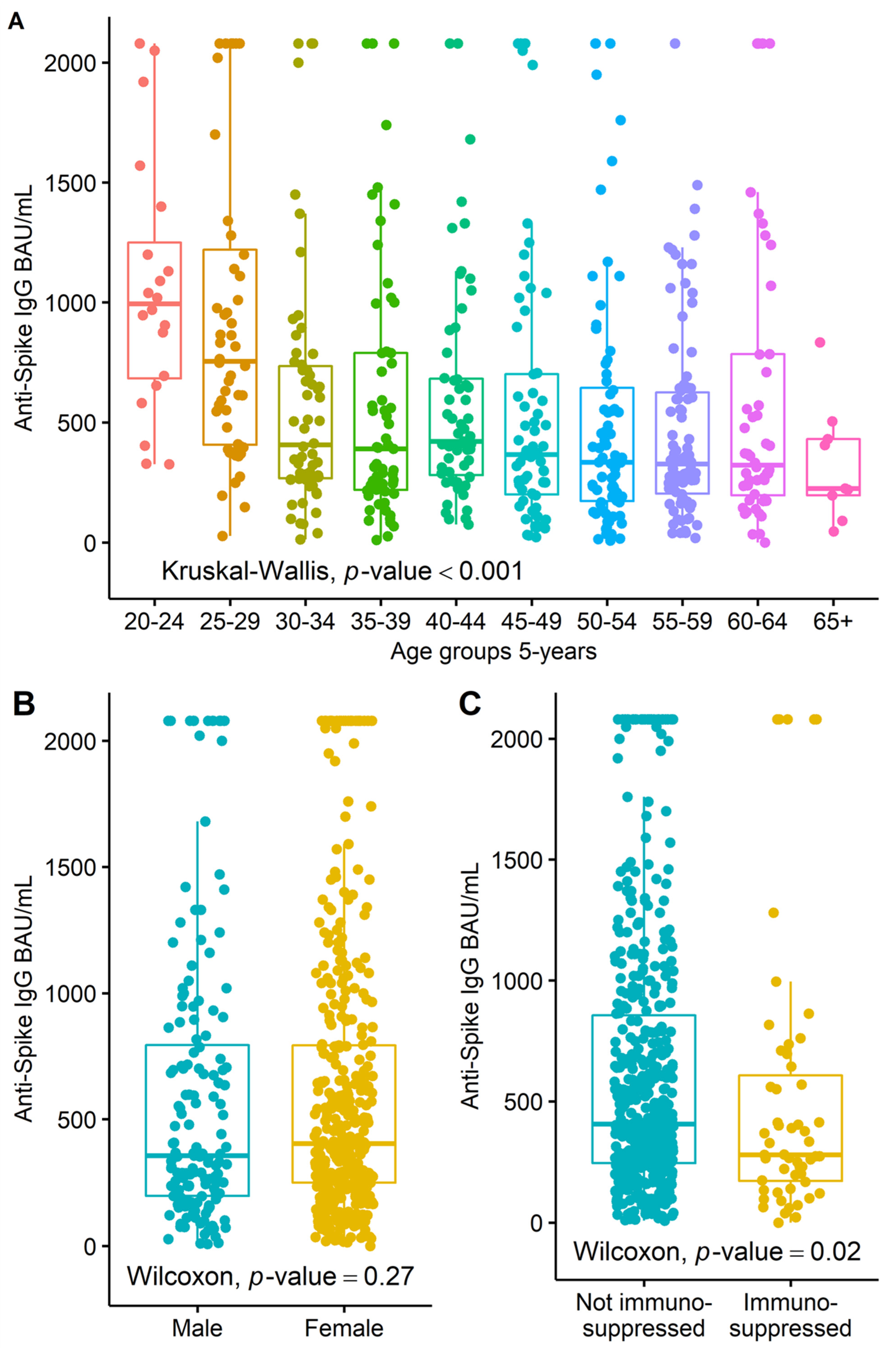
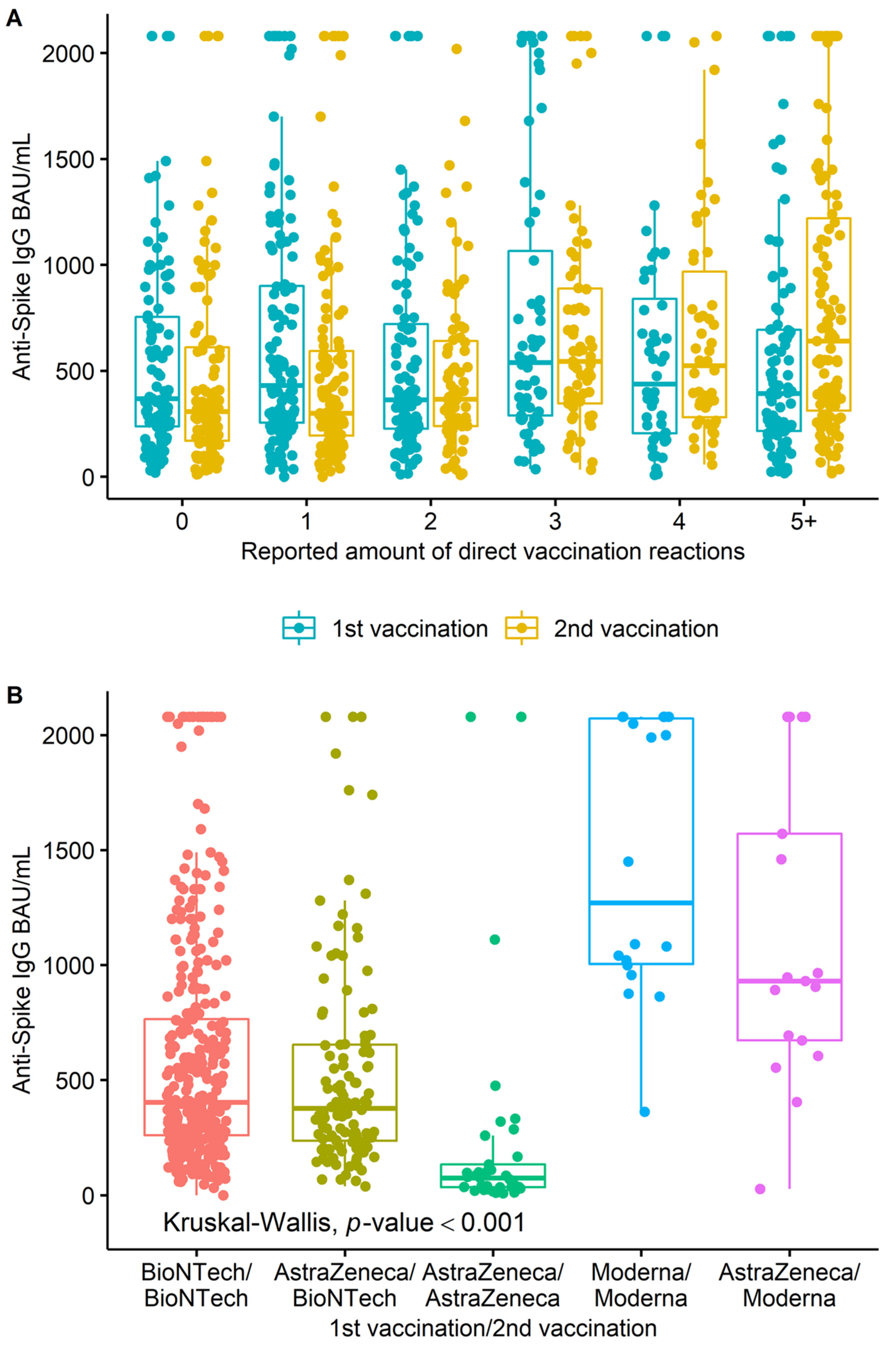
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, A.S.V.; Wood, R.; Gribben, C.; Caldwell, D.; Bishop, J.; Weir, A.; Kennedy, S.; Reid, M.; Smith-Palmer, A.; Goldberg, D.; et al. Risk of hospital admission with coronavirus disease 2019 in healthcare workers and their households: Nationwide linkage cohort study. BMJ 2020, 371, m3582. [Google Scholar] [CrossRef]
- Schadwinkel, A.; Schmid, R. Corona-Patienten: Ärzte und Pfleger auf dem Krankenbett. Spektrum.de. 2020. Available online: https://www.spektrum.de/news/aerzte-und-pfleger-auf-dem-krankenbett/1799309 (accessed on 27 November 2020).
- Krastinova, E.; Garrait, V.; Lecam, M.T.; Coste, A.; Varon, E.; Delacroix, I.; Si Ali, A.; Jung, C.; Smati, M.; Cherbit, M.; et al. Household transmission and incidence of positive SARS-CoV-2 RT-PCR in symptomatic healthcare workers, clinical course and outcome: A French hospital experience. Occup. Environ. Med. 2020, 78, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Nienhaus, A.; Altenburg, C.; Bokemeyer, B.; Schedlbauer, G.; Stranzinger, J. COVID-19 bei Beschäftigten im Gesundheitsdienst und in der Wohlfahrtspflege. ASU Arb. Soz. Umw. 2020, 55, 5. [Google Scholar]
- Nguyen, L.H.; Drew, D.A.; Graham, M.S.; Joshi, A.D.; Guo, C.G.; Ma, W.; Mehta, R.S.; Warner, E.T.; Sikavi, D.R.; Lo, C.H.; et al. Risk of COVID-19 among front-line health-care workers and the general community: A prospective cohort study. Lancet Public Health 2020, 5, e475–e483. [Google Scholar] [CrossRef]
- Prunas, O.; Warren, J.L.; Crawford, F.W.; Gazit, S.; Patalon, T.; Weinberger, D.M.; Pitzer, V.E. Vaccination with BNT162b2 reduces transmission of SARS-CoV-2 to household contacts in Israel. medRxiv 2021, 7, 21260393. [Google Scholar] [CrossRef] [PubMed]
- Salo, J.; Hägg, M.; Kortelainen, M.; Leino, T.; Saxell, T.; Siikanen, M.; Sääksvuori, L. The indirect effect of mRNA-based COVID-19 vaccination onunvaccinated household members. medRxiv 2021, 13, 1162. [Google Scholar] [CrossRef]
- Robert Koch-Institut “Aufklärungsmerkblatt zur COVID-19-Impfung mit mRNA-Impfstoff”. 2022. Available online: https://www.rki.de/DE/Content/Infekt/Impfen/Materialien/Downloads-COVID-19-Vektorimpfstoff/Aufklaerungsbogen-de.pdf?__blob=publicationFile (accessed on 15 February 2022).
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O′Brien, K.L.; Smith, P.G.; et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef]
- Lopez-Bernal, J.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of COVID-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef]
- Chemaitelly, H.; Ayoub, H.H.; AlMukdad, S.; Coyle, P.; Tang, P.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Hasan, M.R.; Al-Kanaani, Z.; et al. Duration of mRNA vaccine protection against SARS-CoV-2 Omicron BA.1 and BA.2 subvariants in Qatar. medRxiv 2022, 13, 3082. [Google Scholar] [CrossRef]
- BioNTech. Pfizer and BioNTech Provide Update on Booster Program in Light of the Delta-Variant; Press Release; BioNTech: Mainz, Germany, 2021. [Google Scholar]
- Fu, D.; Zhang, G.; Wang, Y.; Zhang, Z.; Hu, H.; Shen, S.; Wu, J.; Li, B.; Li, X.; Fang, Y.; et al. Structural basis for SARS-CoV-2 neutralizing antibodies with novel binding epitopes. PLoS Biol. 2021, 19, e3001209. [Google Scholar] [CrossRef]
- Feng, S.; Phillips, D.J.; White, T.; Sayal, H.; Aley, P.K.; Bibi, S.; Dold, C.; Fuskova, M.; Gilbert, S.C.; Hirsch, I.; et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 2032–2040. [Google Scholar] [CrossRef] [PubMed]
- Earle, K.A.; Ambrosino, D.M.; Fiore-Gartland, A.; Goldblatt, D.; Gilbert, P.B.; Siber, G.R.; Dull, P.; Plotkin, S.A. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 2021, 39, 4423–4428. [Google Scholar] [CrossRef]
- McCartney, P.R. Sex-Based Vaccine Response in theContext of COVID-19. J. Obstet. Gynecol. Neonatal. Nurs. 2020, 49, 4. [Google Scholar] [CrossRef] [PubMed]
- Collier, D.A.; Ferreira, I.A.T.M.; Kotagiri, P.; Datir, R.P.; Lim, E.Y.; Touizer, E.; Meng, B.; Abdullahi, A.; Collaboration, T.C.-N.B.C.; Elmer, A.; et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature 2021, 596, 417–422. [Google Scholar] [CrossRef]
- Stuven, P.; Muhlenbruch, G.; Evenschor-Ascheid, A.; Conzen, E.; Peters, C.; Schablon, A.; Nienhaus, A. COVID-19 infections in staff of an emergency care hospital after the first wave of the pandemic in Germany. GMS Hyg. Infect. Control 2022, 17, Doc04. [Google Scholar] [CrossRef]
- Tobin, J. Estimation of Relationships for Limited Dependent Variables. Econometrica 1958, 26, 24. [Google Scholar] [CrossRef]
- Perkmann, T.; Perkmann-Nagele, N.; Koller, T.; Mucher, P.; Radakovics, A.; Marculescu, R.; Wolzt, M.; Wagner, O.F.; Binder, C.J.; Haslacher, H. Anti-Spike Protein Assays to Determine SARS-CoV-2 Antibody Levels: A Head-to-Head Comparison of Five Quantitative Assays. Microbiol. Spectr. 2021, 9, e0024721. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 COVID-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef]
- Vassilaki, N.; Gargalionis, A.N.; Bletsa, A.; Papamichalopoulos, N.; Kontou, E.; Gkika, M.; Patas, K.; Theodoridis, D.; Manolis, I.; Ioannidis, A.; et al. Impact of Age and Sex on Antibody Response Following the Second Dose of COVID-19 BNT162b2 mRNA Vaccine in Greek Healthcare Workers. Microorganisms 2021, 9, 1725. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, S.E.; Shurin, G.V.; Yost, M.; Anderson, A.; Pinto, L.; Wells, A.; Shurin, M.R. Differential Antibody Response to mRNA COVID-19 Vaccines in Healthy Subjects. Microbiol. Spectr. 2021, 9, e0034121. [Google Scholar] [CrossRef]
- Lee, A.; Wong, S.Y.; Chai, L.Y.A.; Lee, S.C.; Lee, M.X.; Muthiah, M.D.; Tay, S.H.; Teo, C.B.; Tan, B.K.J.; Chan, Y.H.; et al. Efficacy of COVID-19 vaccines in immunocompromised patients: Systematic review and meta-analysis. BMJ 2022, 376, e068632. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.R.; Apostolidis, S.A.; Painter, M.M.; Mathew, D.; Pattekar, A.; Kuthuru, O.; Gouma, S.; Kuri-Cervantes, L.; Meng, W.; Adamski, S.; et al. Longitudinal Analysis Reveals Distinct Antibody and Memory B Cell Responses in SARS-CoV2 Naive and Recovered Individuals Following mRNA Vaccination. medRxiv 2021, 3, 21252872. [Google Scholar] [CrossRef]
- Steensels, D.; Pierlet, N.; Penders, J.; Mesotten, D.; Heylen, L. Comparison of SARS-CoV-2 Antibody Response Following Vaccination With BNT162b2 and mRNA-1273. JAMA 2021, 326, 1533–1535. [Google Scholar] [CrossRef]
- Stuart, A.S.V.; Shaw, R.H.; Liu, X.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.A.; Collins, A.M.; et al. Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-COV2): A single-blind, randomised, phase 2, non-inferiority trial. Lancet 2022, 399, 36–49. [Google Scholar] [CrossRef]
- Liu, X.; Shaw, R.H.; Stuart, A.S.V.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.A.; Collins, A.M.; et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): A single-blind, randomised, non-inferiority trial. Lancet 2021, 398, 856–869. [Google Scholar] [CrossRef]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef]
- Kedzierska, K.; Thomas, P.G. Count on us: T cells in SARS-CoV-2 infection and vaccination. Cell Rep. Med. 2022, 3, 100562. [Google Scholar] [CrossRef]
- Robert Koch Institut. Wöchentlicher Lagebericht des RKI zur Coronavirus-Krankheit-2019 (COVID-19)-23.06.2022–Aktualisierter Stand Für Deutschland; Robert Koch Institut: Berlin, Germany, 2022. [Google Scholar]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- World Health Organization. Who Director-General’s Opening Remarks at the Media Briefing on COVID-19. Available online: https://www.who.int/dg/speeches/detail/who-directorgeneral-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 15 June 2022).
- Report of the Who-China Joint Mission on Coronavirus Disease 2019 (COVID-19). Available online: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report (accessed on 15 June 2022).
- Coronavirus Disease 2019 (COVID-19) Situation Report-85, Data as Received by Who from National Authorities by 10:00 Cet, 14 April 2020. Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200414-sitrep-85-covid-19 (accessed on 15 June 2022).
- Zhao, J.Q.; Yuan, H.; Wang, W.; Liu, X.; Liao, Y.; Su, X.; Wang, J.; Yuan, T.; Li, J.; Li, S.; et al. Antibody Responses to SARS-CoV-2 in Patients with Novel Coronavirus Disease 2019. Clin. Infect. Dis. 2020, 71, 2027–2034. [Google Scholar] [CrossRef]
- Xiao, A.T.; Gao, C.; Zhang, S. Profile of Specific Antibodies to SARS-CoV-2: The First Report. J. Infect. 2020, 81, 147–178. [Google Scholar] [CrossRef]
- Shen, C.; Wang, Z.; Zhao, F.; Yang, Y.; Li, J.; Yuan, J.; Wang, F.; Li, D.; Yang, M.; Xing, L.; et al. Treatment of 5 Critically Ill Patients with Covid-19 with Convalescent Plasma. JAMA. 2020, 323, 1582–1589. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Laboratory Testing Strategy Recommendations for COVID-19: Interim Guidance. Available online: https://www.who.int/publications/i/item/laboratory-testing-strategy-recommendations-for-covid-19-interim-guidance (accessed on 15 June 2022).
- 29 Cfr Part 1910.1030; Bloodborne Pathogens. Occupational Safety and Health Administration US Departmen of Labor: Washington, DC, USA, 2006.
- US Department of Health and Human Services. Biosafety in Microbiological and Biomedical Laboratories, 5th ed.; US Government Printing Office: Washington, DC, USA, 2009. Available online: https://www.cdc.gov/labs/BMBL.html (accessed on 15 June 2022).
- World Health Organization. Laboratory Biosafety Manual, 3rd ed.; World Health Organization: Geneva, Switzerland, 2004; Available online: who.int/publications/i/item/9241546506 (accessed on 15 June 2022).
- Clinical and Laboratory Standards Institute (CLSI). Protection of Laboratory Workers from Occupationally Acquired Infections; Approved Guideline—Fourth Edition. Available online: https://ansi.cachefly.net/preview-pages/CLSI/preview_M29-A3.pdf (accessed on 15 June 2022).
- Clinical and Laboratory Standards Institute (CLSI). Procedures for the Handling and Processing of Blood Specimens for Common Laboratory Tests; Approved Guideline—Fourth Edition. Available online: https://clsi.org/media/1369/gp44a4_sample.pdf (accessed on 15 June 2022).
- Clinical and Laboratory Standards Institute (CLSI). Statistical Quality Control for Quantitative Measurement Procedures: Principles and Definitions. 4th Edition. Available online: https://clsi.org/standards/products/clinical-chemistry-and-toxicology/documents/c24/ (accessed on 15 June 2022).
- Wassell, J. Basic Qc Practices. 3rd Edition. Ann. Clin. Biochem. 2012, 49, 308–309. [Google Scholar] [CrossRef]
- Goldenberg, D.M.; Hansen, H.J.; Kelley, E.A.; Primus, F.J. “Sandwich”-Type Immunoassay of Carcinoembryonic Antigen in Patients Receiving Murine Monoclonal Antibodies for Diagnosis and Therapy. Clin. Chem. 1988, 34, 261–264. [Google Scholar]
- Schroff, R.W.; Foon, K.A.; Beatty, S.M.; Oldham, R.K.; Morgan, A.C., Jr. Human Anti-Murine Immunoglobulin Responses in Patients Receiving Monoclonal Antibody Therapy. Cancer Res. 1985, 45, 879–885. [Google Scholar]
- Stuart, M.C.; Boscato, L.M. Heterophilic Antibodies: A Problem for All Immunoassays. Clin. Chem. 1988, 34, 27–33. [Google Scholar]
| AstraZeneca/ AstraZeneca (n = 37) | AstraZeneca/ BioNTech (n = 116) | AstraZeneca/ Moderna (n = 17) | BioNTech/ BioNTech (n = 357) | Moderna/ Moderna (n = 18) | Total (n = 545) | |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 10 (27.0%) | 21 (18.1%) | 4 (23.5%) | 111 (31.1%) | 6 (33.3%) | 152 (27.9%) |
| Female | 27 (73.0%) | 95 (81.9%) | 13 (76.5%) | 246 (68.9%) | 12 (66.7%) | 393 (72.1%) |
| Age | ||||||
| Mean (SD) | 49.41 (10.24) | 46.48 (11.28) | 37.47 (14.97) | 44.57 (11.83) | 39.22 (13.06) | 44.91 (11.92) |
| Min–Max | 31.00–64.00 | 19.00–62.00 | 20.00–63.00 | 22.00–69.00 | 20.00–59.00 | 19.00–69.00 |
| Immunosuppression | ||||||
| Not immunosuppressed | 36 (97.3%) | 100 (89.3%) | 16 (94.1%) | 314 (89.0%) | 16 (88.9%) | 482 (89.8%) |
| Immunosuppressed | 1 (2.7%) | 12 (10.7%) | 1 (5.9%) | 39 (11.0%) | 2 (11.1%) | 55 (10.2%) |
| n-missing | 0 | 4 | 0 | 4 | 0 | 8 |
| 1st vaccination: number of directly reported reactions | ||||||
| 0 | 4 (11.1%) | 12 (10.4%) | 1 (5.9%) | 82 (23.1%) | 4 (22.2%) | 103 (19.0%) |
| 1 | 7 (19.4%) | 10 (8.7%) | 2 (11.8%) | 97 (27.3%) | 8 (44.4%) | 124 (22.9%) |
| 2 | 6 (16.7%) | 20 (17.4%) | 1 (5.9%) | 74 (20.8%) | 3 (16.7%) | 104 (19.2%) |
| 3 | 4 (11.1%) | 13 (11.3%) | 2 (11.8%) | 47 (13.2%) | 2 (11.1%) | 68 (12.6%) |
| 4 | 5 (13.9%) | 13 (11.3%) | 2 (11.8%) | 28 (7.9%) | 0 (0.0%) | 48 (8.9%) |
| 5+ | 10 (27.8%) | 47 (40.9%) | 9 (52.9%) | 27 (7.6%) | 1 (5.6%) | 94 (17.4%) |
| n-missing | 1 | 1 | 0 | 2 | 0 | 4 |
| 2nd vaccination: number of directly reported reactions | ||||||
| 0 | 18 (48.6%) | 21 (18.1%) | 1 (5.9%) | 65 (18.2%) | 2 (11.1%) | 107 (19.6%) |
| 1 | 7 (18.9%) | 28 (24.1%) | 2 (11.8%) | 79 (22.1%) | 3 (16.7%) | 119 (21.8%) |
| 2 | 7 (18.9%) | 14 (12.1%) | 4 (23.5%) | 51 (14.3%) | 3 (16.7%) | 79 (14.5%) |
| 3 | 2 (5.4%) | 16 (13.8%) | 2 (11.8%) | 44 (12.3%) | 3 (16.7%) | 67 (12.3%) |
| 4 | 1 (2.7%) | 15 (12.9%) | 1 (5.9%) | 32 (9.0%) | 1 (5.6%) | 50 (9.2%) |
| 5+ | 2 (5.4%) | 22 (19.0%) | 7 (41.2%) | 86 (24.1%) | 6 (33.3%) | 123 (22.6%) |
| Days between the second vaccination and blood sample | ||||||
| MW (SD) | 191.27 (4.91) | 190.78 (6.24) | 164.18 (8.14) | 180.94 (13.29) | 166.67 (10.31) | 182.74 (13.07) |
| Min–Max | 163.00–195.00 | 161.00–199.00 | 160.00–195.00 | 130.00–280.00 | 160.00–193.00 | 130.00–280.00 |
| Coronavirus SARS-CoV-2 spike IgG BAU/ml | ||||||
| MW (SD) | 233.21 (488.91) | 530.28 (453.27) | 1114.56 (655.04) | 605.76 (516.13) | 1454.22 (587.28) | 608.29 (547.30) |
| Min–Max | 8.10–2080.00 | 38.80–2080.00 | 27.60–2080.00 | 0.00–2080.00 | 362.00–2080.00 | 0.00–2080.00 |
| Reference range spike IgG | ||||||
| <33.8 | 9 (24.3%) | 0 (0.0%) | 1 (5.9%) | 2 (0.6%) | 0 (0.0%) | 12 (2.2%) |
| ≥33.8 | 28 (75.7%) | 116 (100.0%) | 16 (94.1%) | 355 (99.4%) | 18 (100.0%) | 533 (97.8%) |
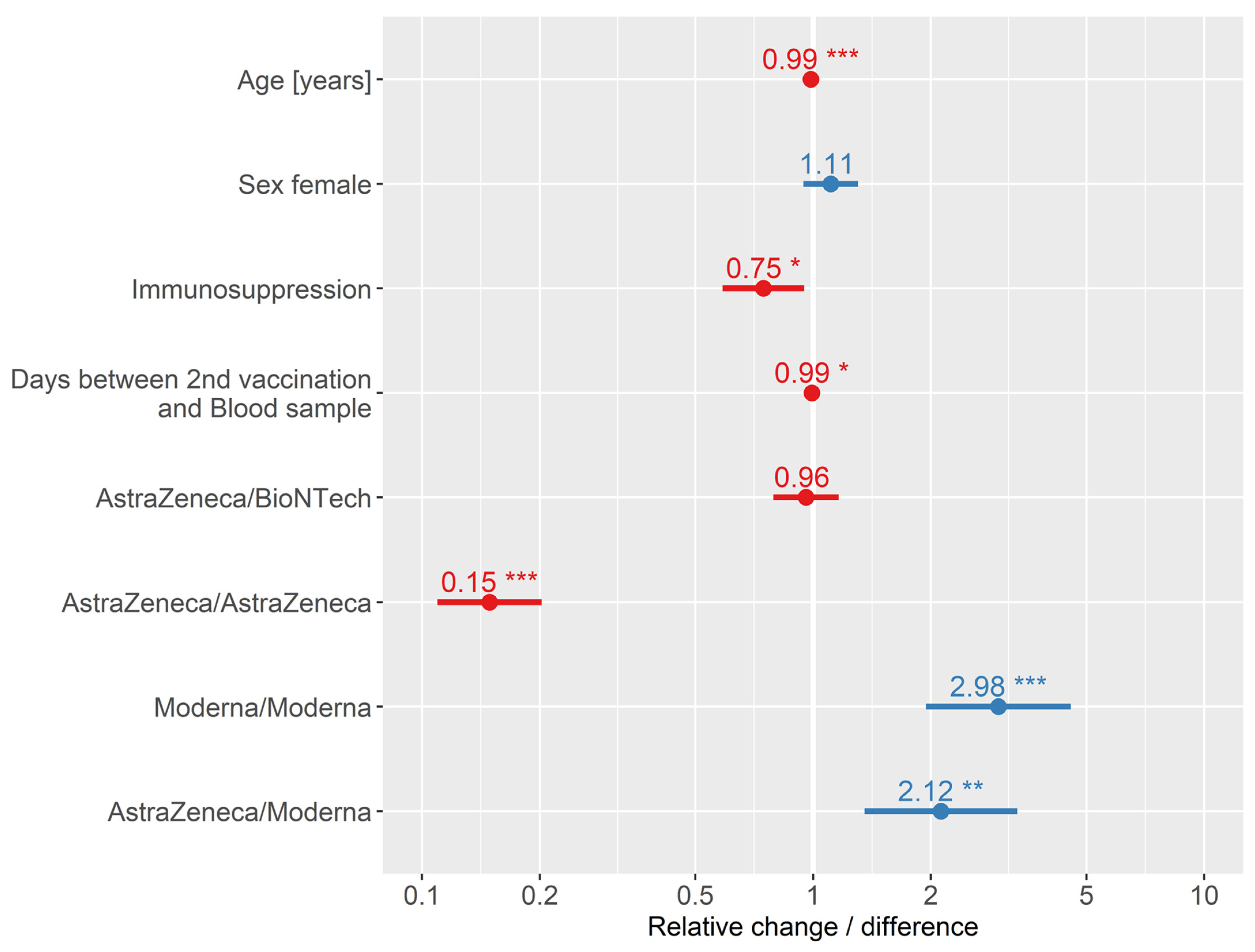
| Coefficients | Reference | Estimate Log | Std. Error | z Value | Relative Change/ Difference | 95% CI | p-Value | |
|---|---|---|---|---|---|---|---|---|
| LL | UL | |||||||
| Age | - | –0.014 | 0.003 | –4.462 | 0.99 | 0.98 | 0.99 | <0.001 |
| Sex | Male | 0.104 | 0.082 | 1.267 | 1.11 | 0.94 | 1.30 | 0.205 |
| Immunosuppressed | Not immunosuppressed | –0.292 | 0.122 | –2.391 | 0.75 | 0.59 | 0.95 | 0.017 |
| Days between blood samples and 2nd vaccination | - | –0.007 | 0.003 | –1.971 | 0.99 | 0.99 | 1.00 | 0.049 |
| 1st/2nd vaccination | ||||||||
| - AstraZeneca/BioNTech | BioNTech/BioNTech | –0.042 | 0.099 | –0.422 | 0.96 | 0.79 | 1.16 | 0.673 |
| - AstraZeneca/AstraZeneca | –1.904 | 0.157 | –12.159 | 0.15 | 0.11 | 0.20 | <0.001 | |
| - Moderna/Moderna | 1.091 | 0.217 | 5.020 | 2.98 | 1.94 | 4.56 | 0.001 | |
| - AstraZeneca/Moderna | 0.753 | 0.230 | 3.279 | 2.12 | 1.35 | 3.33 | <0.001 | |
| Intercept | 7.910 | 0.648 | 12.216 | - | - | - | <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damerau, L.; Mühlenbruch, G.; Evenschor-Ascheid, A.; Fussen, C.; Nienhaus, A.; Terschüren, C.; Herold, R.; Harth, V. Coronavirus Vaccination: Spike Antibody Levels in Health Workers after Six Months—A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2022, 19, 11422. https://doi.org/10.3390/ijerph191811422
Damerau L, Mühlenbruch G, Evenschor-Ascheid A, Fussen C, Nienhaus A, Terschüren C, Herold R, Harth V. Coronavirus Vaccination: Spike Antibody Levels in Health Workers after Six Months—A Cross-Sectional Study. International Journal of Environmental Research and Public Health. 2022; 19(18):11422. https://doi.org/10.3390/ijerph191811422
Chicago/Turabian StyleDamerau, Lukas, Georg Mühlenbruch, Agnes Evenschor-Ascheid, Christine Fussen, Albert Nienhaus, Claudia Terschüren, Robert Herold, and Volker Harth. 2022. "Coronavirus Vaccination: Spike Antibody Levels in Health Workers after Six Months—A Cross-Sectional Study" International Journal of Environmental Research and Public Health 19, no. 18: 11422. https://doi.org/10.3390/ijerph191811422
APA StyleDamerau, L., Mühlenbruch, G., Evenschor-Ascheid, A., Fussen, C., Nienhaus, A., Terschüren, C., Herold, R., & Harth, V. (2022). Coronavirus Vaccination: Spike Antibody Levels in Health Workers after Six Months—A Cross-Sectional Study. International Journal of Environmental Research and Public Health, 19(18), 11422. https://doi.org/10.3390/ijerph191811422







