Availability of Financial and Medical Resources for Screening Providers and Its Impact on Cancer Screening Uptake and Intervention Programs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Cancer Screening
2.3. Indicators of Financial Resources
2.4. Indicators of Medical Resources
2.5. Indicators of Screening Interventions
2.6. Other Indicators
2.7. Statistical Analysis
3. Results
3.1. Characteristics of Collected Data
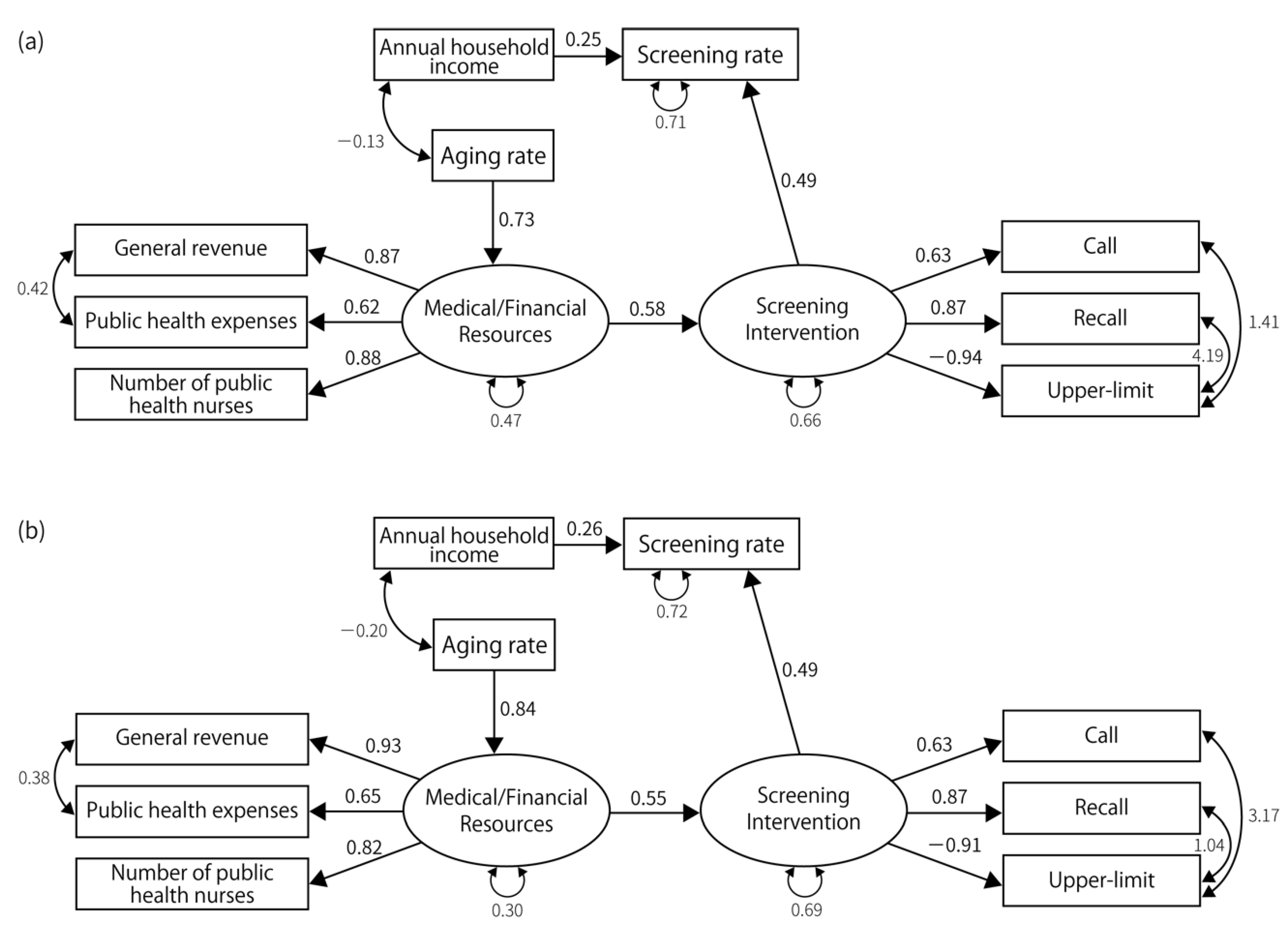
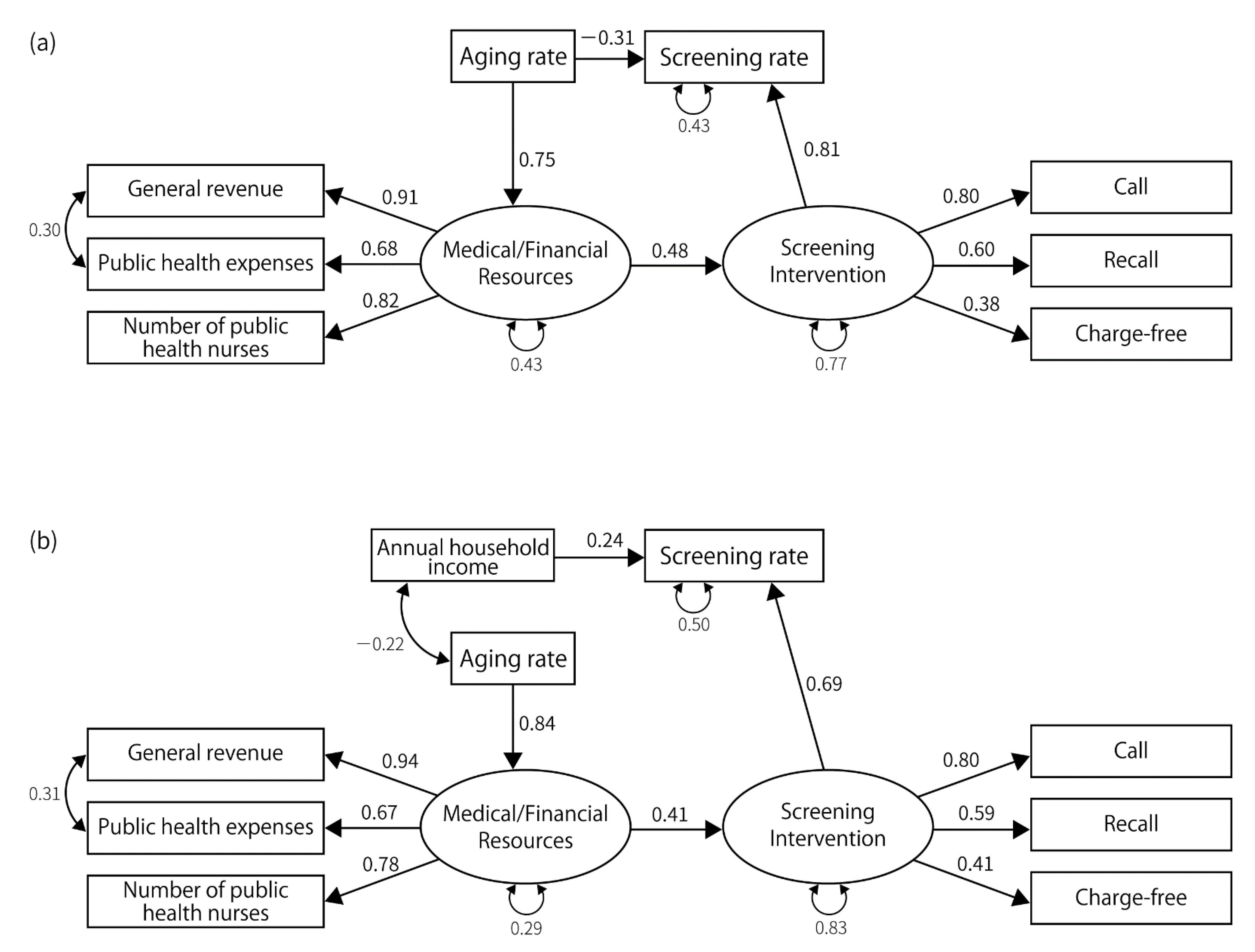
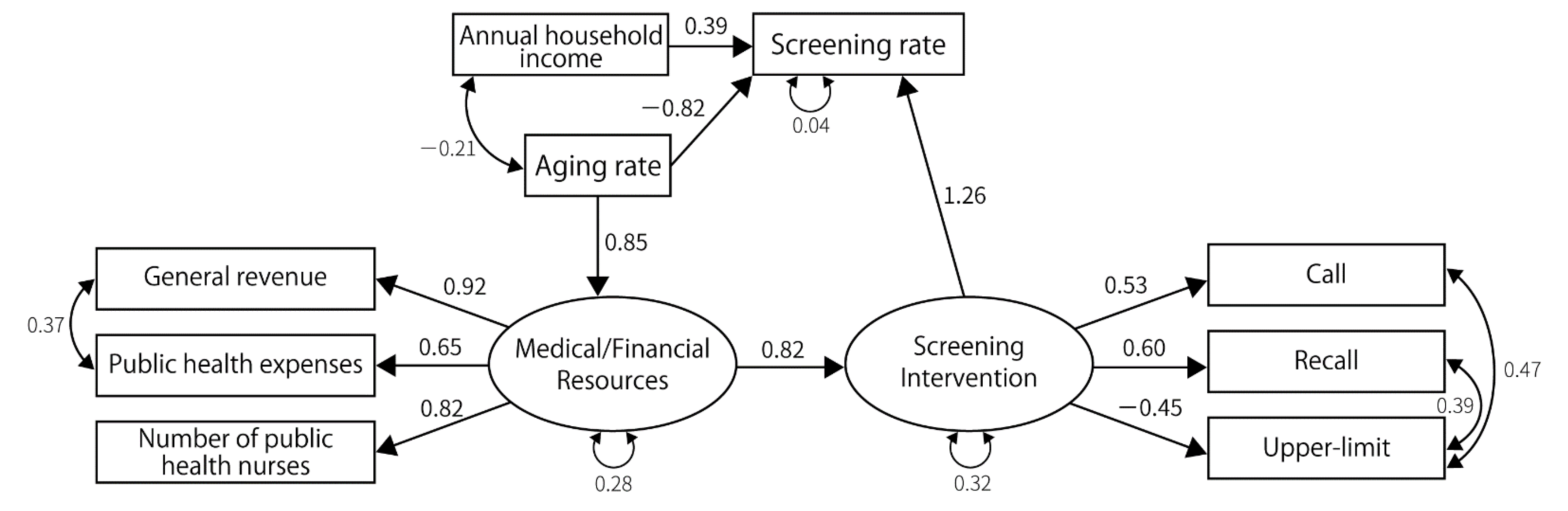
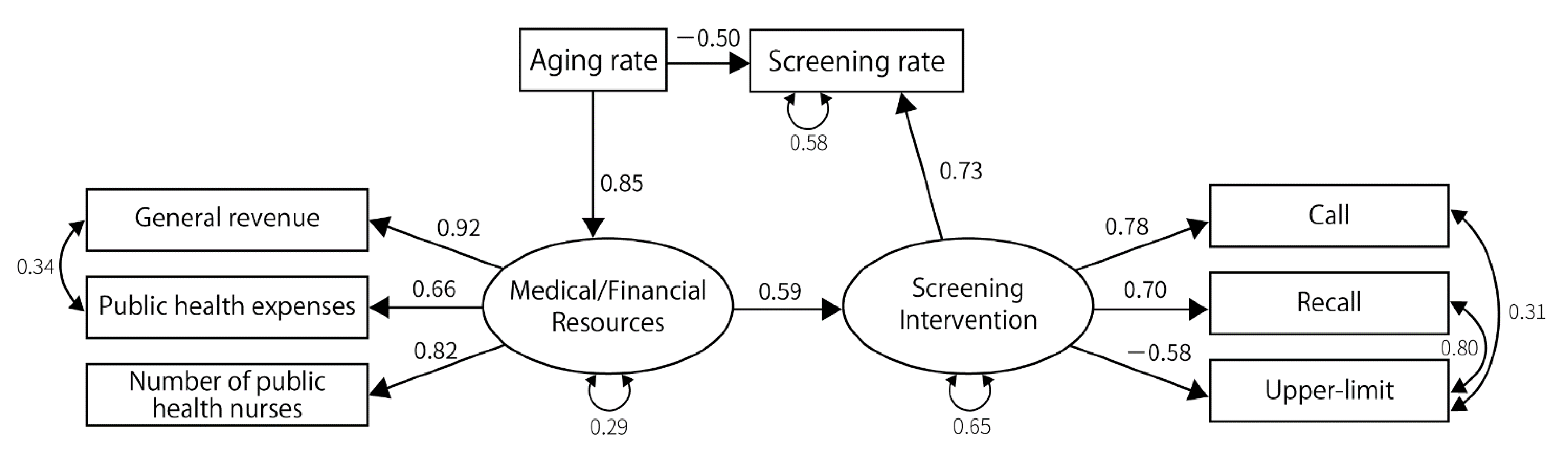
3.2. Results of SEM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Regarding Gastric Cancer Screening Participants
Appendix B. The Impact of the Number of Medical Nurses
References
- Smith, R.A.; Andrews, K.S.; Brooks, D.; Fedewa, S.A.; Manassaram-Baptiste, D.; Saslow, D.; Wender, R.C. Cancer screening in the United States, 2019: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J. Clin. 2019, 69, 184–210. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Lee, Y.Y.; Suh, M.; Lee, E.Y.; Mai, T.T.X.; Ki, M.; Oh, J.K.; Cho, H.; Park, B.; Jun, J.K.; et al. Socioeconomic inequalities in cervical and breast cancer screening among women in Korea, 2005–2015. Yonsei Med. J. 2018, 59, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Meissner, H.I.; Breen, N.; Klabunde, C.N.; Vernon, S.W. Patterns of colorectal cancer screening uptake among men and women in the United States. Cancer Epidemiol. Biomark. Prev. 2006, 15, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.L.; Kinney, A.Y.; Levin, T.R. Factors associated with colorectal cancer screening in a population-based study: The impact of gender, health care source, and time. Prev. Med. 2004, 38, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Blanks, R.G.; Benson, V.S.; Alison, R.; Brown, A.; Reeves, G.K.; Beral, V.; Patnick, J.; Green, J. Nationwide bowel cancer screening programme in England: Cohort study of lifestyle factors affecting participation and outcomes in women. Br. J. Cancer. 2015, 112, 1562–1567. [Google Scholar] [CrossRef]
- Fukuda, Y.; Nakamura, K.; Takano, T.; Nakao, H.; Imai, H. Socioeconomic status and cancer screening in Japanese males: Large inequlaity in middle-aged and urban residents. Environ. Health Prev. Med. 2007, 12, 90–96. [Google Scholar] [CrossRef]
- Musa, J.; Achenbach, C.J.; O’Dwyer, L.C.; Evans, C.T.; McHugh, M.; Hou, L.; Simon, M.A.; Murphy, R.L.; Jordan, N. Effect of cervical cancer education and provider recommendation for screening on screening rates: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0183924. [Google Scholar] [CrossRef]
- Hoffmeister, M.; Holleczek, B.; Zwink, N.; Stock, C.; Stegmaier, C.; Brenner, H. Screening for bowel cancer: Increasing participation via personal invitation. Dtsch. Ärzteblatt Int. 2017, 114, 87–93. [Google Scholar] [CrossRef]
- Ornstein, S.M.; Garr, D.R.; Jenkins, R.G.; Rust, P.F.; Arnon, A. Computer-generated physician and patient reminders. Tools to improve population adherence to selected preventive services. J. Fam. Pract. 1991, 32, 82–90. [Google Scholar]
- Barr, J.K.; Franks, A.L.; Lee, N.C.; Antonucci, D.M.; Rifkind, S.; Schachter, M. A randomized intervention to improve ongoing participation in mammography. Am. J. Manag. Care 2001, 7, 887–894. [Google Scholar] [PubMed]
- Sabik, L.M.; Vichare, A.M.; Dahman, B.; Bradley, C.J. Co-payment policies and breast and cervical cancer screening in Medicaid. Am. J. Manag. Care 2020, 26, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Noman, S.; Shahar, H.K.; Abdul Rahman, H.; Ismail, S.; Abdulwahid Al-Jaberi, M.; Azzani, M. The effectiveness of educational interventions on breast cancer screening uptake, knowledge, and beliefs among women: A systematic review. Int. J. Environ. Res. Public Health 2020, 18, 263. [Google Scholar] [CrossRef] [PubMed]
- Saei Ghare Naz, M.S.G.; Kariman, N.; Ebadi, A.; Ozgoli, G.; Ghasemi, V.; Rashidi Fakari, F. Educational interventions for cervical cancer screening behavior of women: A systematic review. Asian Pac. J. Cancer Prev. 2018, 19, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Kaku, T.; Hirata, N.; Shinkoda, H.; Noguchi, Y. Influences on women health care after change and reduction of financial resources for cytological screening of cervical cancer in Fukuoka Prefecture, Japan. Mem. Kyushu Univ. Sch. Health Sci. 2003, 1, 23–28. [Google Scholar] [CrossRef]
- Takaku, R. Do municipalities want to increase checkup rates of cancer screening tests? Iryo Shakai 2011, 21, 249–264. (In Japanese) [Google Scholar] [CrossRef]
- OECD Reviews of Public Health. A Healthier Tomorrow: Japan. Available online: https://www.oecd-ilibrary.org/sites/9789264311602-7-en/index.html?itemId=/content/component/9789264311602-7-en (accessed on 30 June 2022).
- e-Stat. Portal Site of Official Statistics of Japan. Available online: https://www.e-stat.go.jp/en (accessed on 30 June 2022).
- Ministry of Health, Labour and Welfare. Guidelines for Implementation of Cancer Prevention Focused Health Education and Cancer Screening. Available online: https://www.mhlw.go.jp/content/10900000/000838645.pdf (accessed on 30 June 2022).
- Suh, M.; Song, S.; Cho, H.N.; Park, B.; Jun, J.K.; Choi, E.; Kim, Y.; Choi, K.S. Trends in participation rates for the national cancer screening program in Korea, 2002–2012. Cancer Res. Treat. 2017, 49, 798–806. [Google Scholar] [CrossRef]
- Ritvo, P.; Myers, R.E.; Paszat, L.; Serenity, M.; Perez, D.F.; Rabeneck, L. Gender differences in attitudes impeding colorectal cancer screening. BMC Public Health 2013, 13, 500. [Google Scholar] [CrossRef]
- Clarke, N.; Gallagher, P.; Kearney, P.M.; McNamara, D.; Sharp, L. Impact of gender on decisions to participate in faecal immunochemical test-based colorectal cancer screening: A qualitative study. Psycho-Oncology 2016, 25, 1456–1462. [Google Scholar] [CrossRef]
- Hamashima, C. Cancer screening guidelines and policy making: 15 years of experience in cancer screening guideline development in Japan. Jpn. J. Clin. Oncol. 2018, 48, 278–286. [Google Scholar] [CrossRef]
- Cancer Registry and Statistics. Cancer Information Service: National Cancer Center, Japan. Available online: https://ganjoho.jp/reg_stat/statistics/stat/screening/dl_screening.html (accessed on 30 June 2022).
- MacCallum, R.C.; Browne, M.W.; Sugawara, H.M. Power analysis and determination of sample size for covariance structure modeling. Psychol. Methods 1996, 1, 130–149. [Google Scholar] [CrossRef]
- Hooper, D.; Coughlan, J.P. Structural equation modelling: Guidelines for determining model fit. Electron. J. Bus. Res. Methods 2008, 6, 53–60. [Google Scholar] [CrossRef]
- Hu, L.; Bentler, P.M. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ. Model. Multidiscip. J. 1999, 6, 1–55. [Google Scholar] [CrossRef]
- Ihaka, R.; Gentleman, R. R: A Language for Data Analysis and Graphics. J. Comput. Graph. Stat. 1996, 5, 299–314. [Google Scholar] [CrossRef]
- Rosseel, Y. lavaan: An R package for structural equation modeling. J. Stat. Softw. 2012, 48, 1–36. [Google Scholar] [CrossRef]
- Epskamp, S. semPlot: Path Diagrams and Visual Analysis of Various SEM Packages’ Output. R Package Version 1.1.2. Available online: https://CRAN.R-project.org/package=semPlot (accessed on 30 June 2022).
- Kanno, M. Use of cost-effectiveness evaluation in local government administration in cancer screening: From the perspective of someone who competes to secure budget. J. Natl. Inst. Public Health 2013, 62, 617–624. (In Japanese) [Google Scholar]
- Tsounis, A.; Sarafis, P.; Alexopoulos, E.C. Austerity and its consequences on cancer screening in Greece. Lancet 2014, 384, 2110. [Google Scholar] [CrossRef]
- Kosaka, K.; Kawahara, T.; Tsubono, Y.; Aida, J. Research on the Development of Effective Means to Improve Screening Rates for Cancer. Available online: https://www.mhlw.go.jp/shingi/2007/06/dl/s0626-13m_0001.pdf (accessed on 30 June 2022).
- Bentler, P.M.; Chou, C.P. Practical issues in structural modeling. Sociol. Methods Res. 1987, 16, 78–117. [Google Scholar] [CrossRef]


| Gastric Cancer | Lung Cancer | Colorectal Cancer | Breast Cancer | Cervical Cancer | ||||
|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | |||
| Number of Prefectures | n = 47 | n = 47 | n = 47 | n = 47 | n = 47 | n = 47 | n = 47 | n = 47 |
| Cancer screening rate (%) | 9.3 (6.8, 11.5) | 7.4 (5.6, 9.4) | 23.2 (13.5, 28.9) | 21.2 (12.8, 27.0) | 20.3 (17.6, 27.8) | 21.2 (17.0, 26.6) | 24.2 (20.6, 28.9) | 26.3 (23.1, 30.3) |
| Variables Related to Screening Intervention * | ||||||||
| Call (%) | 80.0 (71.6, 90.0) | 79.7 (69.1, 90.0) | 81.5 (74.2, 92.2) | 81.5 (69.5, 88.4) | 81.0 (72.7, 90.0) | |||
| Recall (%) | 31.4 (22.1, 52.1) | 28.0 (18.7, 44.9) | 42.1 (28.2, 52.8) | 44.0 (33.3, 55.1) | 44.8 (34.5, 55.6) | |||
| Charge-free (%) | 7.0 (3.0, 20.5) | 28.0 (19.3, 45.9) | 11.1 (5.1, 24.5) | 6.3 (0.9, 16.5) | 8.0 (3.2, 20.0) | |||
| >0% (n, %) | 38 (80.9) | - | 45 (95.7) | 35 (74.5) | 38 (80.9) | |||
| After-hours (%) | 87.0 (80.0, 94.0) | 86.4 (73.7, 92.1) | 87.0 (77.6, 93.1) | 81.8 (62.9, 90.5) | 76.0 (57.4, 89.5) | |||
| >median (n, %) | 24 (51) | 24 (51) | 24 (51) | 24 (51) | 24 (51) | |||
| Extra-region (%) | 1.7 (0.0, 5.0) | 1.7 (0.0, 5.0) | 0.00 (0.0, 5.2) | 3.1 (0.0, 7.3) | 1.7 (0.0, 5.8) | |||
| >0% (n, %) | 24 (51.1) | 24 (51.1) | 22 (46.8) | 31 (66.0) | 24 (51.1) | |||
| Modality extension (%) | 15.1 (7.0, 27.8) | 12.5 (4.5, 20.6) | 1.7 (0.0, 7.4) | 80.0 (50.1, 94.7) | 11.1 (7.5, 23.8) | |||
| >0% (n, %) | 42 (89.4) | 40 (85.1) | 24 (51.1) | 44 (93.6) | 39 (83.0) | |||
| Out of evidence (%) | 90.5 (83.7, 100.0) | 90.5 (83.7, 100.0) | 90.5 (83.7, 100.0) | 90.5 (83.7, 100.0) | 90.5 (83.7, 100.0) | |||
| <100% (n, %) | 13 (27.7) | 13 (27.7) | 13 (27.7) | 13 (27.7) | 13 (27.7) | |||
| Upper limit (%) | 28.6 (13.5, 52.9) | 13.3 (3.5, 28.8) | 7.3 (2.0, 18.0) | 52.0 (30.2, 67.7) | 29.6 (12.2, 52.0) | |||
| >0% (number, %) | - | 36 (76.6) | 36 (76.6) | - | - | |||
| Data Type | Values |
|---|---|
| Basic Statistics | |
| Mean annual household income (103 yen) | 5.2 (4.9, 5.7) |
| Population aged ≥ 65 (%) | 25.5 (24.3, 26.6) |
| Medical/financial resources | |
| Public health expenses (103 yen) | 20.6 (17.5, 22.9) |
| General revenue per capita (103 yen) | 246.9 (218.3, 266.7) |
| Number of nurses (per 103 people) | 10.3 (8.6, 11.6) |
| Number of public health nurses (per 103 people) | 0.5 (0.4, 0.6) |
| Number of hospitals and clinics (per 103 people) | 0.9 (0.8, 1.0) |
| Number of medical doctors (per 103 people) | 2.4 (2.2, 2.8) |
| Latent Factors | Measured Variables | Gastric Cancer | Lung Cancer | Colorectal Cancer | Breast Cancer | Cervical Cancer | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | ||||||||||||
| Estimate | p-Value | Estimate | p-Value | Estimate | p-Value | Estimate | p-Value | Estimate | p-Value | Estimate | p-Value | Estimate | p-Value | Estimate | p-Value | ||
| Latent variable measurement model | |||||||||||||||||
| Resource | Number of public health nurses | 0.88 | - | 0.79 | - | 0.82 | - | 0.78 | - | 0.80 | - | 0.79 | - | 0.80 | - | 0.83 | - |
| Public health expenses | 0.62 | <0.001 | 0.72 | <0.001 | 0.68 | <0.001 | 0.67 | <0.001 | 0.73 | <0.001 | 0.72 | <0.001 | 0.72 | <0.001 | 0.67 | <0.001 | |
| General revenue per capita | 0.87 | <0.001 | 0.96 | <0.001 | 0.91 | <0.001 | 0.94 | <0.001 | 0.95 | <0.001 | 0.97 | <0.001 | 0.95 | <0.001 | 0.91 | <0.001 | |
| Policy | Recall | 0.80 | - | 0.66 | - | 0.60 | - | 0.59 | - | 0.74 | - | 0.74 | - | 0.60 | - | 0.53 | - |
| Call | 0.55 | 0.002 | 0.35 | 0.02 | 0.80 | 0.001 | 0.80 | 0.001 | 0.73 | <0.001 | 0.74 | 0.005 | 0.54 | <0.001 | 0.61 | <0.001 | |
| Set upper limit | −0.90 | 0.002 | −0.77 | 0.005 | −0.47 | 0.014 | −0.43 | 0.025 | |||||||||
| No-charge | 0.38 | 0.03 | 0.41 | 0.023 | |||||||||||||
| Regressions | |||||||||||||||||
| Response variables | Explanatory variables | ||||||||||||||||
| Screening rate | Medical/financial resources | 0.58 | 0.003 | ||||||||||||||
| Screening interventions | 0.53 | 0.015 | 0.60 | 0.004 | 0.81 | 0.001 | 0.69 | 0.001 | 1.14 | 0.001 | 1.23 | 0.005 | |||||
| Annual household income | 0.27 | 0.017 | 0.34 | 0.003 | 0.24 | 0.038 | 0.51 | <0.001 | 0.41 | 0.002 | 0.38 | <0.001 | |||||
| Aging rate | −0.31 | 0.021 | −0.40 | 0.029 | −0.70 | <0.001 | −0.93 | <0.001 | |||||||||
| Screening interventions | Medical/financial resources | 0.62 | <0.001 | 0.67 | 0.001 | 0.48 | 0.016 | 0.41 | 0.032 | 0.48 | 0.009 | 0.434 | 0.014 | 0.78 | <0.001 | 0.77 | 0.001 |
| Resource | Aging rate | 0.73 | <0.001 | 0.82 | <0.001 | 0.75 | <0.001 | 0.84 | <0.001 | 0.74 | <0.001 | 0.821 | <0.001 | 0.83 | <0.001 | 0.85 | <0.001 |
| Model fit indices | |||||||||||||||||
| GFI | 0.872 | 0.892 | 0.943 | 0.887 | 0.874 | 0.876 | 0.93 | 0.909 | |||||||||
| AGFI | 0.738 | 0.78 | 0.87 | 0.789 | 0.717 | 0.753 | 0.85 | 0.782 | |||||||||
| SRMR | 0.09 | 0.091 | 0.07 | 0.093 | 0.111 | 0.115 | 0.081 | 0.084 | |||||||||
| CFI | 0.955 | 0.946 | 1.000 | 0.937 | 0.904 | 0.890 | 0.945 | 0.917 | |||||||||
| RMSEA | 0.087 | 0.095 | <0.001 | 0.096 | 0.134 | 0.144 | 0.102 | 0.149 | |||||||||
| χ2 test (p value) | 0.128 | 0.085 | 0.683 | 0.077 | 0.018 | 0.007 | 0.060 | 0.010 | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, K.; Nakamura, S.; Watanabe, K.; Sakaguchi, M.; Narimatsu, H. Availability of Financial and Medical Resources for Screening Providers and Its Impact on Cancer Screening Uptake and Intervention Programs. Int. J. Environ. Res. Public Health 2022, 19, 11477. https://doi.org/10.3390/ijerph191811477
Takahashi K, Nakamura S, Watanabe K, Sakaguchi M, Narimatsu H. Availability of Financial and Medical Resources for Screening Providers and Its Impact on Cancer Screening Uptake and Intervention Programs. International Journal of Environmental Research and Public Health. 2022; 19(18):11477. https://doi.org/10.3390/ijerph191811477
Chicago/Turabian StyleTakahashi, Koshi, Sho Nakamura, Kaname Watanabe, Masahiko Sakaguchi, and Hiroto Narimatsu. 2022. "Availability of Financial and Medical Resources for Screening Providers and Its Impact on Cancer Screening Uptake and Intervention Programs" International Journal of Environmental Research and Public Health 19, no. 18: 11477. https://doi.org/10.3390/ijerph191811477





