Factors Affecting Glycemic Control among Saudi Children with Type 1 Diabetes Mellitus in Aseer Region, Southwestern Saudi Arabia
Abstract
:1. Introduction
2. Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ngwiri, T.; Were, F.; Predieri, B.; Ngugi, P.; Iughetti, L. Glycemic Control in Kenyan Children and Adolescents with Type 1 Diabetes Mellitus. Int. J. Endocrinol. 2015, 2015, 761759. [Google Scholar] [CrossRef] [PubMed]
- Aljabri, K.S.; Bokhari, S.A. Glycemic Control of Patients with Type 1 Diabetes Mellitus in Saudi Community. J. Diabetes Metab. 2013, 4, 256. [Google Scholar] [CrossRef]
- Al-Herbish, A.S.; El-Mouzan, M.I.; Al-Salloum, A.A.; Al-Qurachi, M.M.; Al-Omar, A.A. Prevalence of type 1 diabetes mellitus in Saudi Arabian children and adolescents. Saudi Med. J. 2008, 29, 1285–1288. [Google Scholar] [PubMed]
- International Diabetes Federation’s Diabetes Atlas. List of Countries by Incidence of Type 1 Diabetes Ages 0 to 14. Diabetes-UK. 2016, pp. 8–10. Available online: https://www.diabetes.org.uk/About_us/News_Landing_Page/UK-has-worlds-5th-highest-rate-of-Type-1-diabetes-in-children/List-of-countries-by-incidence-of-Type-1-diabetes-ages-0-to-14/ (accessed on 2 January 2013).
- Melin, E.O.; Thunander, M.; Landin-Olsson, M.; Thulesius, H.O. Depression, obesity, and smoking were independently associated with inadequate glycemic control in patients with type 1 diabetes. Eur. J. Endocrinol. 2013, 168, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Egede, L.E.; Nietert, P.J.; Zheng, D. Depression and all-cause and coronary heart disease mortality among adults with and without diabetes. Diabetes Care 2005, 28, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Seher, Ç.; Halil, S.; Özgür, T.; Eren, E.; Ömer, T. Factors Influencing Glycemic Control in Children with Type 1 Diabetes. GüncelPediatri 2010, 8, 7–19. [Google Scholar]
- Elgerbi, A.E.M. Factors associated with poor glycemic control among children with type 1 diabetes at Zawia province. Univ. Bull. 2014, 16, 151–164. [Google Scholar]
- Al-Agha, A.E.; Alafif, M.M.; Abd-Elhameed, I.A. Glycemic control, complications, and associated autoimmune diseases in children and adolescents with type 1 diabetes in Jeddah, Saudi Arabia. Saudi Med. J. 2015, 36, 26–31. [Google Scholar] [CrossRef]
- Korczak, D.J.; Pereira, S.; Koulajian, K.; Matejcek, A.; Giacca, A. Type 1 diabetes mellitus and major depressive disorder: Evidence for a biological link. Diabetologia 2011, 54, 2483–2493. [Google Scholar] [CrossRef]
- Lustman, P.; Clouse, R. Depression in diabetic patients: The relationship between mood and glycemic control. J. Diabetes Its Complicat. 2005, 19, 113–122. [Google Scholar] [CrossRef]
- Bloomgarden, Z.T. Cardiovascular disease in diabetes. Diabetes Care 2010, 33, e49–e54. [Google Scholar] [CrossRef] [PubMed]
- Selvin, E.; Coresh, J.; Golden, S.H.; Boland, L.L. Glycemic control, atherosclerosis, and risk factors for cardiovascular disease in individuals with diabetes: The atherosclerosis risk in communities study. Diabetes Care 2005, 28, 1965–1973. [Google Scholar] [CrossRef] [Green Version]
- Hamburg, N.M.; McMackin, C.J.; Huang, A.L.; Shenouda, S.M.; Widlansky, M.E.; Schulz, E.; Gokce, N.; Ruderman, N.B.; Keaney, J.F., Jr.; Vita, J.A. Physical inactivity rapidly induces insulin resistance and microvascular dysfunction in healthy volunteers. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2650–2656. [Google Scholar] [CrossRef] [PubMed]
- Ekelund, U.; Brage, S.; Griffin, S.J.; Wareham, N.J. Objectively measured moderate- and vigorous-intensity physical activity but not sedentary time predicts insulin resistance in high-risk individuals. Diabetes Care 2009, 32, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44 (Suppl. 1), S15–S33. [Google Scholar] [CrossRef]
- Clements, M.A.; Lind, M.; Raman, S.; Patton, S.R.; Lipska, K.J.; Fridlington, A.G.; Tang, F.; Jones, P.G.; Wu, Y.; A Spertus, J.; et al. Age at diagnosis predicts deterioration in glycaemic control among children and adolescents with type 1 diabetes. BMJ Open Diabetes Res. Care 2014, 2, e000039. [Google Scholar] [CrossRef]
- Yazidi, M.; Chihaoui, M.; Chaker, F.; Rjeb, O.; Slimane, H. Factors predicting glycemic control in type 1 diabetic patient. Open Med. J. 2016, 3, 153–158. [Google Scholar] [CrossRef]
- Andrade, C.S.; Ribeiro, G.I.S.; Teles Santos, C.A.S.; Silva Neves, R.C.; Moreira, E.D., Jr. Factors associated with high levels of glycated haemoglobin in patients with type 1 diabetes: A multicentre study in Brazil. BMJ Open 2017, 7, e018094. [Google Scholar] [CrossRef]
- Sastre, J.; Pines, P.J.; Moreno, J.; Aguirre, M.; Blanco, B.; Calderón, D.; Herranz, S.; Roa, C.; Lopez, J.; El Grupo de Estudio DIACAM. Situacion de control metabolico ypautas de tratamiento en pacientes con diabetes tipo 1 en Castilla-La Mancha: Estudio de diabetes tipo 1 en Castilla-La Mancha. Endocrinol. Nutr. 2012, 59, 539–546. [Google Scholar] [CrossRef]
- Angamo, M.T.; Melese, B.H.; Ayen, W.Y. Determinants of glycemic control among insulin-treated diabetic patients in Southwest Ethiopia: Hospital-based cross-sectional study. PLoS ONE 2013, 8, e61759. [Google Scholar]
- Colom, C.; Chico, A.; Carreras, G.; Aulinas, A.; Pujol, I.; Perez, A. Control glucémico y complicaciones crónicas a 20 años del comienzo de la diabetes tipo 1. Resultados de una unidad especializada. Av. Diabetol. 2015, 31, 113–119. [Google Scholar] [CrossRef]
- Dahl-Jorgensen, K. Modern insulin therapy in children and adolescents. Acta Paediatr. 1999, 88, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.N.; Quinn, N.; Volkening, L.K.; Laffel, L.M.B. Impact of carbohydrate counting on glycemic control in children with type 1 diabetes. Diabetes Care 2009, 32, 1014–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, B.S.; Anderson, B.J.; Butler, D.A.; Antisdel, J.E.; Brackett, J.; Laffel, L.M. Predictors of glycemic control and short-term adverse outcomes in youth with type 1 diabetes. J. Pediatr. 2001, 139, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Haller, M.J.; Stalvey, M.S.; Silverstein, J.H. Predictors of control of diabetes: Monitoring may be the key. J. Pediatr. 2004, 144, 660–666. [Google Scholar] [CrossRef]
- Laurenzi, A.; Bolla, A.M.; Panigoni, G.; Doria, V.; Uccellatore, A.; Peretti, E.; Saibene, A.; Galimberti, G.; Bosi, E.; Scavini, M. Effects of carbohydrate counting on glucose control and quality of life over 24 weeks in adult patients with type 1 diabetes on continuous subcutaneous insulin infusion. Diabetes Care 2011, 34, 823–827. [Google Scholar] [CrossRef]
- Tascini, G.; Berioli, M.G.; Cerquiglini, L.; Santi, E.; Mancini, G.; Rogari, F.; Toni, G.; Esposito, S. Carbohydrate counting in children and adolescents with type 1 diabetes. Nutrients 2018, 10, 109. [Google Scholar] [CrossRef]
- Gupta, L.; Khandelwal, D.; Kalra, S. Applied carbohydrate counting. J. Pak. Med. Assoc. 2017, 67, 1456–1457. [Google Scholar]
- Morris, D.; Boyle, D.; Mcmahon, A.; Greene, A.; Macdonald, M.; Newton, R.; DARTS/MEMO Collaboration. Adherence to insulin treatment, glycemic control, and ketoacidosis in insulin-dependent diabetes mellitus. Lancet 1997, 350, 1505–1510. [Google Scholar] [CrossRef]
- Meltzer, L.J.; Johnson, S.B.; Prine, J.M.; Banks, R.A.; Desrosiers, P.M.; Silverstein, J.H. Disordered eating, body mass, and glycemic control in adolescents with type 1 diabetes. Diabetes Care 2001, 24, 678–682. [Google Scholar] [CrossRef]
- Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Kitzler, T.M.; Bachar, M.; Skrabal, F.; Kotanko, P. Evaluation of treatment adherence in type 1 diabetes: A novel approach. Eur. J. Clin. Investig. 2007, 37, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Parga, M.; Llorente, R. Discriminant analysis of treatment adherence in insulin-dependent diabetes mellitus. Psychol. Spain 2005, 9, 41–48. [Google Scholar]
- Kaufman, F.R.; Halvorson, M.; Carpenter, S. Association between diabetes control and visits to a multidisciplinary pediatric diabetes clinic. Pediatrics 1999, 103, 948–951. [Google Scholar] [CrossRef] [PubMed]
- Svoren, B.M.; Volkening, L.K.; Butler, D.A.; Moreland, E.C.; Anderson, B.J.; Laffel, L.M. Temporal trends in the treatment of pediatric type 1 diabetes and impact on acute outcomes. J. Pediatr. 2007, 150, 279–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speight, J.; Holmes-Truscott, E.; Harvey, D.M.; Hendrieckx, C.; Hagger, V.; Harris, S.E.; Knight, B.; Mcintyre, H.D. Structured type 1 diabetes education delivered in routine care in Australia reduces diabetes-related emergencies and severe diabetes-related distress: The OzDAFNE program. Diabetes Res. Clin. Pract 2016, 112, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Ba-Essa, E.M.; Mobarak, E.I.; Alghamdi, A.; Al-Daghri, N.M. Intensified glucose self-monitoring with education in Saudi DM patients. Int. J. Clin. Exp. Med. 2015, 8, 19374. [Google Scholar]
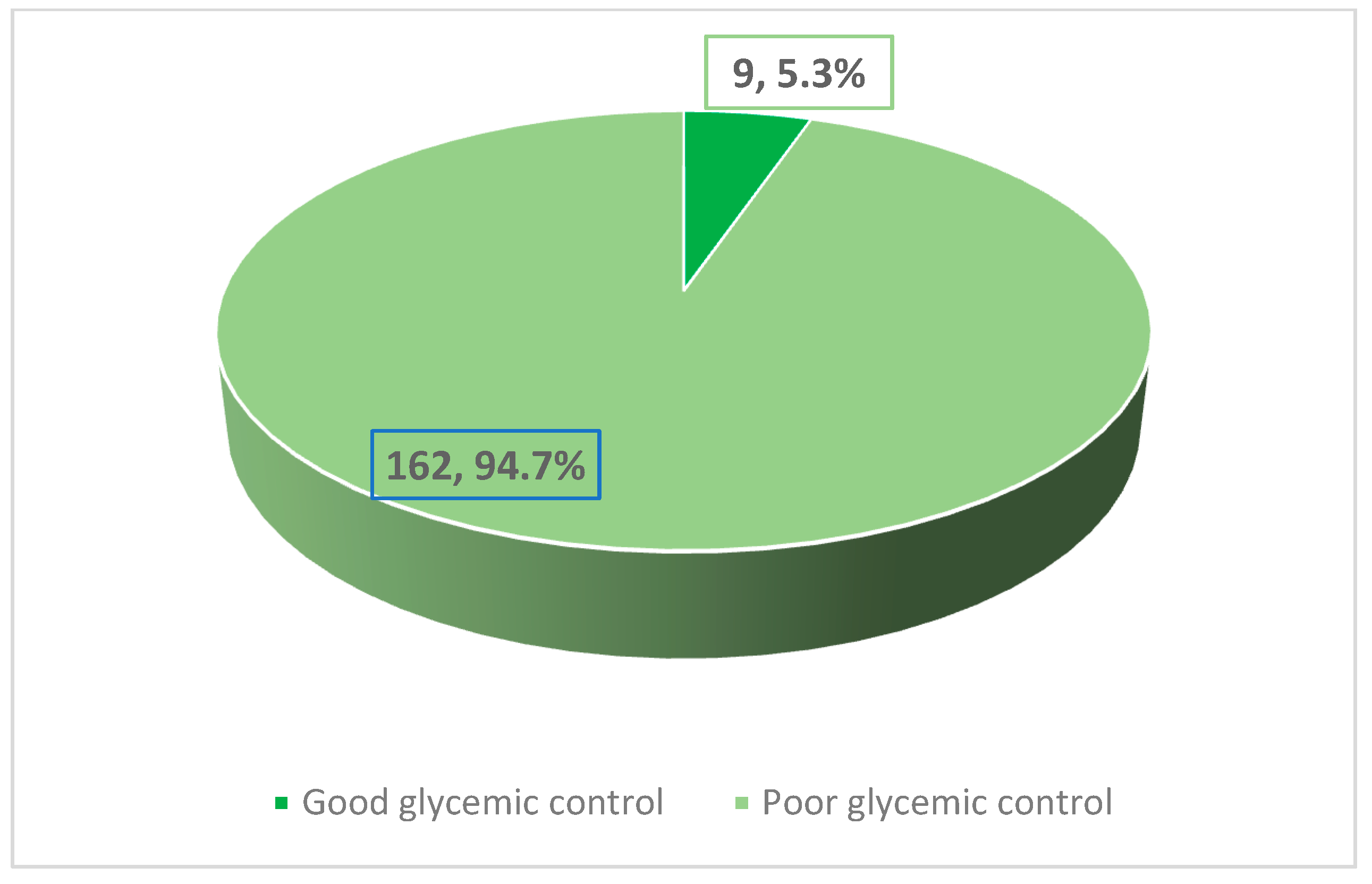
| HbA1C | p-Value | ||
|---|---|---|---|
| Good Glycemic Control n = 9 n (%) | Poor Glycemic Control n = 162 n (%) | ||
| Gender Male (n 92) Female (n 79) | 7 (7.6) 2 (2.5) | 85 (92.4) 77 (97.5) | 0.127 * |
| Current parental marital status Married (n = 166) Unmarried (n = 5) | 8 (4.8) 1 (20.0) | 156 (95.2) 4 (80.0) | 0.239 * |
| Caregiver Mothers only (n = 128) Both parents (n = 24) Patient self-care (n = 14) Others (n = 5) | 7 (5.5) 0 (0.0) 2 (14.3) 0 (0.0) | 121 (94.5) 24 (100) 12 (85.7) 5 (100) | 0.272 ** |
| Educational level of the caregiver Illiterate (n = 6) Elementary school (n = 42) Intermediate school (n = 20) High school (n = 38) University (n = 65) | 0 (0.0) 4 (9.5) 0 (0.0) 2 (5.3) 3 (4.6) | 6 (100) 38 (90.5) 20 (100) 36 (94.7) 62 (95.4) | 0.553 ** |
| Family income (SR/month) <5000 (n = 15) 5000–10,000 (n = 76) 10,001 < 15,000 (n = 57) ≥15,000 (n = 23) | 1 (6.7) 5 (6.6) 3 (5.3 0 (0.0) | 14 (93.3) 71 (93.4) 54 (94.7) 23 (100) | 0.6599 ** |
| Housing type Private (n = 120) Rented (n = 51) | 6 (5.0) 3 (5.9) | 114 (95.0) 48 (94.1) | 0.536 ** |
| Age at diagnosis (years) <3 (n = 32) 3–6 (n = 65) >6 (n = 74) | 2 (6.3) 2 (3.1) 5 (6.8) | 30 (93.8) 63 (96.9) 69 (93.2) | 0.601 ** |
| HbA1C | p-Value | ||
|---|---|---|---|
| Good Glycemic Control n = 9 n (%) | Poor Glycemic Control n = 162 n (%) | ||
| Duration of DM (years) ≤3 (n = 94) >3 (n = 77) | 8 (8.5) 1 (1.3) | 86 (91.5) 76 (98.7) | 0.035 * |
| History of hospitalization No (n = 59) Once (n = 61) Twice (n = 35) >Twice (n = 16) ICU admission Yes (n = 53) No (n = 118) | 1 (1.7) 5 (8.2) 2 (5.7) 1 (6.3) 4 (7.5) 5 (4.2) | 58 (98.3) 56 (91.8) 33 (94.3) 15 (93.7) 49 (92.5) 113 (95.8) | 0.457 ** 0.290 * |
| Carbohydrate count usage (n = 170) Yes (n = 22) No (n = 148) | 4 (18.2) 5 (3.4) | 18 (81.8) 143 (96.6) | 0.017 * |
| Diet usage Yes (n = 35) No (n = 136) | 2 (5.7) 7 (5.1) | 33 (94.3) 129 (94.9) | 0.583 * |
| Physician revisit interval Once/month (n = 8) Once/3 months (n = 150) Once/6 months (13) | 0 (0.0) 9 (6.0) 0 (0.0) | 8 (100) 141 (94.0) 13 (100) | 0.514 ** |
| Adherence to insulin therapy Excellent (n = 117) Good (n = 52) Bad (n = 2) | 7 (6.0) 2 (3.8) 0 (0.0) | 110 (94.0) 50 (96.2) 2 (100) | 0.802 ** |
| Type of insulin Multi-dosage/day (n = 105) Two doses/day (n = 62) Insulin pump (n = 4) | 6 (5.7) 3 (4.8) 0 (0.0) | 99 (94.3) 59 (95.2) 4 (100) | 0.866 ** |
| Knowledge of the family about glucagon Yes (n = 153) No (n = 18) | 7 (4.6) 2 (11.1) | 146 (95.4) 16 (88.9) | 0.242 * |
| Glucagon injection Yes (n = 151) No (n = 20) | 8 (5.3) 1 (5.0) | 143 (94.7) 19 (95.0) | 0.716 ** |
| Availability of diabetes educator Yes (n = 141) No (n = 13) Do not know (n = 17) | 7 (5.0) 1 (7.7) 1 (5.9) | 134 (95.0) 12 (92.3) 16 (94.1) | 0.908 ** |
| Sufficiency of information from a diabetes educator Yes (n = 133) No (n = 37) | 7 (5.3) 2 (5.4) | 126 (94.7) 35 (94.6) | 0.622 * |
| Availability of glucose test device No (n = 2) Yes, from the government (n = 140) Yes, on patient’s exposure (n = 29) | 0 (0.0) 8 (5.7) 1 (3.4) | 2 (100) 132 (94.3) 28 (96.6) | 0.835 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Qahtani, S.M.; Shati, A.A.; Alqahtani, Y.A.; AlAsmari, A.A.; Almahdi, M.A.; Al Hassan, A.A.; Alhassany, A.M.; Shathan, R.A.; Aldosari, R.M.; AlQahtani, A.S.; et al. Factors Affecting Glycemic Control among Saudi Children with Type 1 Diabetes Mellitus in Aseer Region, Southwestern Saudi Arabia. Int. J. Environ. Res. Public Health 2022, 19, 11558. https://doi.org/10.3390/ijerph191811558
Al-Qahtani SM, Shati AA, Alqahtani YA, AlAsmari AA, Almahdi MA, Al Hassan AA, Alhassany AM, Shathan RA, Aldosari RM, AlQahtani AS, et al. Factors Affecting Glycemic Control among Saudi Children with Type 1 Diabetes Mellitus in Aseer Region, Southwestern Saudi Arabia. International Journal of Environmental Research and Public Health. 2022; 19(18):11558. https://doi.org/10.3390/ijerph191811558
Chicago/Turabian StyleAl-Qahtani, Saleh M., Ayed A. Shati, Youssef A. Alqahtani, Ali A. AlAsmari, Mohammed A. Almahdi, Amjad A. Al Hassan, Ali M. Alhassany, Rana A. Shathan, Rawa M. Aldosari, Abdullah S. AlQahtani, and et al. 2022. "Factors Affecting Glycemic Control among Saudi Children with Type 1 Diabetes Mellitus in Aseer Region, Southwestern Saudi Arabia" International Journal of Environmental Research and Public Health 19, no. 18: 11558. https://doi.org/10.3390/ijerph191811558






