Author Contributions
Conceptualization, Y.L. and J.J.; methodology, J.J.; software, Y.L.; validation, Y.L., J.J. and Y.W.; formal analysis, J.J.; investigation, R.L., W.H. and R.M.; resources, R.L.; data curation, W.H. and R.M; writing—original draft preparation, Y.L.; writing—review and editing, J.J.; visualization, Y.L.; supervision, Y.W.; project administration, J.J.; funding acquisition, J.J. All authors have read and agreed to the published version of the manuscript.
Figure 1.
Photoinduced energy/electron transfer pathways between solvent molecules and NBFRs. * Represents the excited triplet state of the molecule.
Figure 1.
Photoinduced energy/electron transfer pathways between solvent molecules and NBFRs. * Represents the excited triplet state of the molecule.
Figure 2.
The degradation of PBBA, PBEB, PBT and HBB under three different wavelengths (λ1: 180~400 nm, λ2: 334~465 nm, λ3: 400~700 nm). The initial concentration was 1 mg/L. The reaction temperature was 15 °C.
Figure 2.
The degradation of PBBA, PBEB, PBT and HBB under three different wavelengths (λ1: 180~400 nm, λ2: 334~465 nm, λ3: 400~700 nm). The initial concentration was 1 mg/L. The reaction temperature was 15 °C.
Figure 3.
The degradation of PBBA, PBEB, PBT and HBB with three different initial concentrations (0.25 mg/L, 0.5 mg/L, 1 mg/L) under light wavelength of 180~400 nm. The reaction temperature was 15 °C.
Figure 3.
The degradation of PBBA, PBEB, PBT and HBB with three different initial concentrations (0.25 mg/L, 0.5 mg/L, 1 mg/L) under light wavelength of 180~400 nm. The reaction temperature was 15 °C.
Figure 4.
The degradation of PBBA, PBEB, PBT and HBB with three different initial concentrations (0.25 mg/L, 0.5 mg/L, 1 mg/L) under light wavelength of 334~365 nm. The reaction temperature was 15 °C.
Figure 4.
The degradation of PBBA, PBEB, PBT and HBB with three different initial concentrations (0.25 mg/L, 0.5 mg/L, 1 mg/L) under light wavelength of 334~365 nm. The reaction temperature was 15 °C.
Figure 5.
The degradation of PBBA, PBEB, PBT and HBB in three different solvents (n-hexane, toluene, acetone) under light wavelengths of 180~400 nm. The initial concentration was 1 mg/L. The reaction temperature was 15 °C.
Figure 5.
The degradation of PBBA, PBEB, PBT and HBB in three different solvents (n-hexane, toluene, acetone) under light wavelengths of 180~400 nm. The initial concentration was 1 mg/L. The reaction temperature was 15 °C.
Figure 6.
Change curve of C–Br bond lengths of NBFRs with the dielectric constant.
Figure 6.
Change curve of C–Br bond lengths of NBFRs with the dielectric constant.
Figure 7.
The relationship between dipole moments of NBFRs (PBEB, PBT, PBBA) and dielectric constants.
Figure 7.
The relationship between dipole moments of NBFRs (PBEB, PBT, PBBA) and dielectric constants.
Figure 8.
Molecular structure diagram (marked with atomic position number) and the Fukui function mapped electron density isosurface (ρ = 0.01 a.u.): f0 (r), f+ (r), and f− (r) of HBB, PBT, PBEB, and PBBA (the dark blue on the isosurface denotes the larger positive value of the Fukui function).
Figure 8.
Molecular structure diagram (marked with atomic position number) and the Fukui function mapped electron density isosurface (ρ = 0.01 a.u.): f0 (r), f+ (r), and f− (r) of HBB, PBT, PBEB, and PBBA (the dark blue on the isosurface denotes the larger positive value of the Fukui function).
Figure 9.
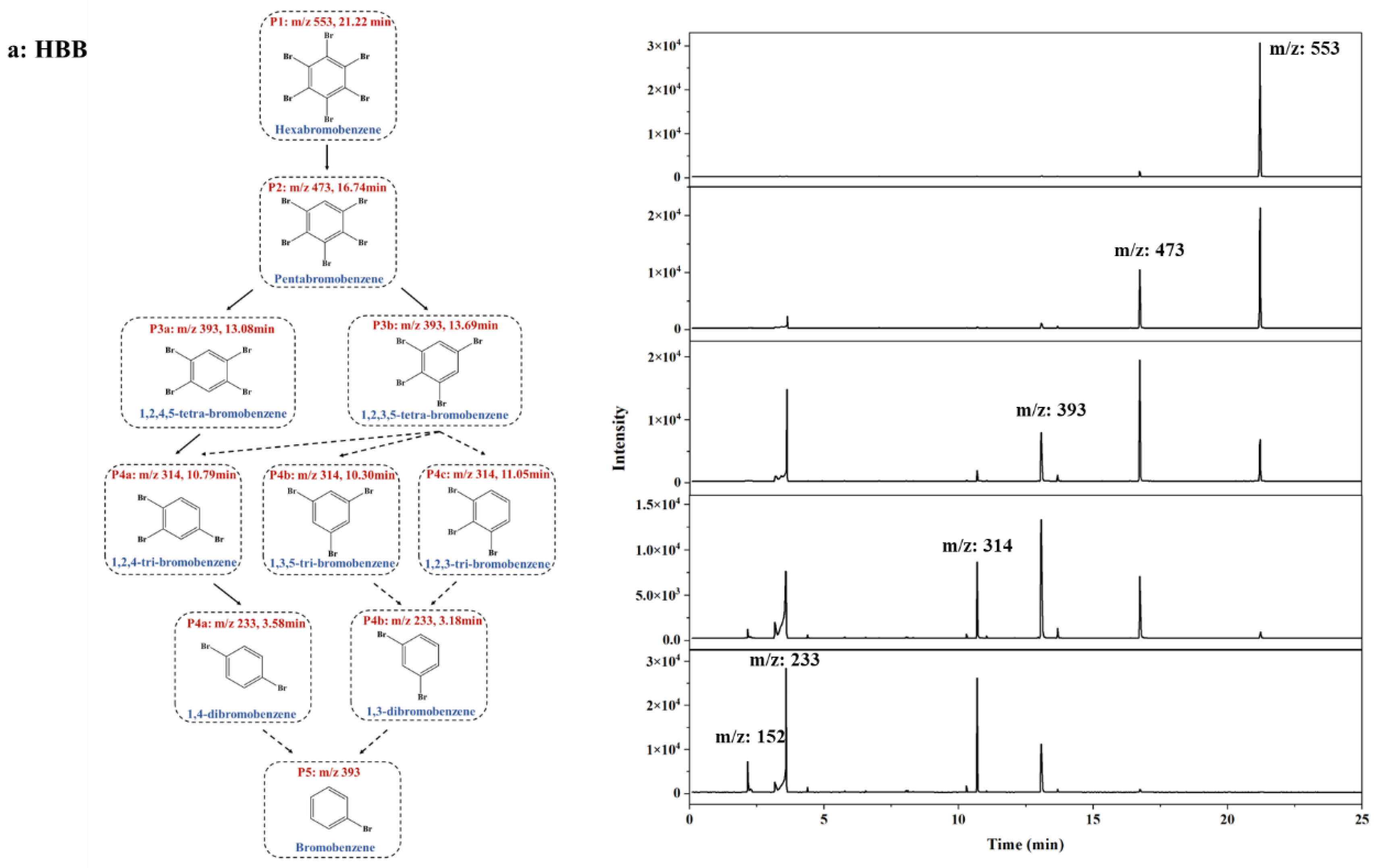
Proposed photolytic pathways and mass spectra of (a) HBB, (b) PBT, (c) PBEB, (d) PBBA. The solid arrow indicates the primary degradation pathway, and the dotted line indicates the minor pathway. (In the same group of peaks, the peaks with the highest proportion of peak area to the lowest were labeled a, b, c, and so on.) The mass spectrum of each substance from bottom to top is 0/2/10/30/60 min of photodegradation reaction.
Figure 9.
Proposed photolytic pathways and mass spectra of (a) HBB, (b) PBT, (c) PBEB, (d) PBBA. The solid arrow indicates the primary degradation pathway, and the dotted line indicates the minor pathway. (In the same group of peaks, the peaks with the highest proportion of peak area to the lowest were labeled a, b, c, and so on.) The mass spectrum of each substance from bottom to top is 0/2/10/30/60 min of photodegradation reaction.
Table 1.
Electron transfer reactions between NBFRs and solvent molecules.
Table 1.
Electron transfer reactions between NBFRs and solvent molecules.
| NO. | Reaction Equation | Equation |
|---|
| 1 | NBFRsT1* + SolventS0 → NBFRs·+ + Solvent·− | ΔG1 = VIET1 (NBFRs) − VEAS0 (Solvent) |
| 2 | NBFRsT1* + SolventS0 → NBFRs·− + Solvent·+ | ΔG2 = VIES0 (Solvent) − VEAT1 (NBFRs) |
| 3 | NBFRsT1* + SolventT1* → NBFRs·+ + Solvent·− | ΔG3 = VIET1 (NBFRs) − VEAT1 (Solvent) |
| 4 | NBFRsT1* + SolventT1* → NBFRs·− + Solvent·+ | ΔG4 = VIET1 (Solvent) − VEAT1 (NBFRs) |
| 5 | NBFRsS0 + SolventT1* → NBFRs·+ + Solvent·− | ΔG5 = VIES0 (NBFRs) − VEAT1 (Solvent) |
| 6 | NBFRsS0 + SolventT1* → NBFRs·− + Solvent·+ | ΔG6 = VIET1 (Solvent) − VEAS0 (NBFRs) |
Table 2.
Rate constant and half-life and correlation coefficient of PBBA, PBEB, PBT and HBB under three wavelength ranges in n-hexane and in an initial concentration of 1 mg/L. All data were fitted by the pseudo-first-order kinetics equation.
Table 2.
Rate constant and half-life and correlation coefficient of PBBA, PBEB, PBT and HBB under three wavelength ranges in n-hexane and in an initial concentration of 1 mg/L. All data were fitted by the pseudo-first-order kinetics equation.
| Optical Wavelength (nm) | Rate Coefficient (min−1) |
|---|
| PBBA | PBEB | PBT | HBB |
|---|
| 180~400 | 0.3008 ± 0.00478 | 0.1943 ± 0.00123 | 0.1800 ± 0.00040 | 0.1702 ± 0.00278 |
| 334~365 | 0.0433 ± 0.00131 | 0.0289 ± 0.00478 | 0.0280 ± 0.00461 | 0.0265 ± 0.00208 |
| 400~700 | 0.0099 ± 0.00118 | 0.0063 ± 0.00053 | 0.0058 ± 0.00041 | 0.0091 ± 0.00151 |
| | Half-life (min) |
| 180~400 | 2.31 | 3.57 | 3.85 | 4.07 |
| 334~365 | 16.03 | 25.06 | 25.86 | 26.45 |
| 400~700 | 71.93 | 112.12 | 120.4 | 79.93 |
| | R2 |
| 180~400 | 0.992 | 0.993 | 0.993 | 0.976 |
| 334~365 | 0.967 | 0.957 | 0.956 | 0.991 |
| 400~700 | 0.992 | 0.979 | 0.982 | 0.979 |
Table 3.
Rate constant, half-life and correlation coefficient of PBBA, PBEB, PBT and HBB in three initial concentrations in n-hexane under wavelength of 334~365 nm. All data were fitted by the pseudo-first-order kinetics equation.
Table 3.
Rate constant, half-life and correlation coefficient of PBBA, PBEB, PBT and HBB in three initial concentrations in n-hexane under wavelength of 334~365 nm. All data were fitted by the pseudo-first-order kinetics equation.
| Initial Concentration (mg/L) | Rate Coefficient (min−1) |
|---|
| PBBA | PBEB | PBT | HBB |
|---|
| 0.25 | 0.0736 ± 0.00000 | 0.0513 ± 0.00768 | 0.0379 ± 0.00004 | 0.0784 ± 0.00053 |
| 0.5 | 0.0683 ± 0.00363 | 0.0324 ± 0.00012 | 0.0307 ± 0.00004 | 0.0578 ± 0.00029 |
| 1 | 0.0433 ± 0.00131 | 0.0289 ± 0.00478 | 0.0280 ± 0.00461 | 0.0265 ± 0.00208 |
| | Half-life (min) |
| 0.25 | 9.42 | 13.98 | 18.31 | 8.85 |
| 0.5 | 10.2 | 21.43 | 22.61 | 12 |
| 1 | 16.03 | 25.06 | 25.86 | 26.45 |
| | R2 |
| 0.25 | 0.99 | 0.971 | 0.996 | 0.984 |
| 0.5 | 0.996 | 0.997 | 0.996 | 0.998 |
| 1 | 0.967 | 0.957 | 0.956 | 0.991 |
Table 4.
Rate constant, half-life and correlation coefficient of PBBA, PBEB, PBT and HBB in three initial concentrations in n-hexane under wavelength of 180~400 nm. All data were fitted by the pseudo first-order kinetics equation.
Table 4.
Rate constant, half-life and correlation coefficient of PBBA, PBEB, PBT and HBB in three initial concentrations in n-hexane under wavelength of 180~400 nm. All data were fitted by the pseudo first-order kinetics equation.
| Initial Concentration (mg/L) | Rate Coefficient (min−1) |
|---|
| PBBA | PBEB | PBT | HBB |
|---|
| 0.25 | 0.2804 ± 0.03584 | 0.1552 ± 0.00327 | 0.1477 ± 0.00527 | 0.1586 ± 0.00265 |
| 0.5 | 0.2885 ± 0.00380 | 0.1146 ± 0.00131 | 0.1141 ± 0.00780 | 0.1173 ± 0.00151 |
| 1 | 0.3008 ± 0.00478 | 0.1943 ± 0.00123 | 0.1800 ± 0.00040 | 0.1702 ± 0.00278 |
| | Half-life (min) |
| 0.25 | 2.53 | 4.47 | 4.70 | 4.37 |
| 0.5 | 2.47 | 6.05 | 6.12 | 5.91 |
| 1 | 2.31 | 3.57 | 3.85 | 4.07 |
| | R2 |
| 0.25 | 0.998 | 0.990 | 0.980 | 0.997 |
| 0.5 | 0.980 | 0.984 | 0.978 | 0.982 |
| 1 | 0.992 | 0.993 | 0.993 | 0.976 |
Table 5.
Rate constant, half-life and correlation coefficient of PBBA, PBEB, PBT and HBB in three organic solvents in an initial concentration of 1 mg/L under wavelengths of 180~400 nm. All data were fitted by the pseudo-first-order kinetics equation.
Table 5.
Rate constant, half-life and correlation coefficient of PBBA, PBEB, PBT and HBB in three organic solvents in an initial concentration of 1 mg/L under wavelengths of 180~400 nm. All data were fitted by the pseudo-first-order kinetics equation.
| Solvent | Rate Coefficient (min−1) |
|---|
| PBBA | PBEB | PBT | HBB |
|---|
| HEX | 0.3008 ± 0.00478 | 0.1943 ± 0.00123 | 0.1800 ± 0.00040 | 0.1702 ± 0.00278 |
| ACE | 0.0299 ± 0.00057 | 0.0170 ± 0.00045 | 0.0168 ± 0.00065 | 0.0124 ± 0.00020 |
| TOL | 0.0534 ± 0.00069 | 0.0512 ± 0.00229 | 0.0469 ± 0.00229 | 0.0408 ± 0.00131 |
| | Half-life (min) |
| HEX | 2.31 | 3.57 | 3.85 | 4.07 |
| ACE | 23.19 | 40.7 | 41.35 | 56.15 |
| TOL | 13 | 13.58 | 14.83 | 17.02 |
| | R2 |
| HEX | 0.992 | 0.993 | 0.993 | 0.976 |
| ACE | 0.965 | 0.942 | 0.934 | 0.933 |
| TOL | 0.994 | 0.991 | 0.985 | 0.986 |
Table 6.
Total energy (E), frontier orbital energy levels (EHOMO, ELUMO) and energy gap (ΔEgap) of NBFRs in different solvents.
Table 6.
Total energy (E), frontier orbital energy levels (EHOMO, ELUMO) and energy gap (ΔEgap) of NBFRs in different solvents.
| Compound | Solvents | ε | E (a.u.) | HOMO (eV) | LUMO (eV) | ΔEgap (eV) |
|---|
| HBB | GAS | 1.00 | −15658.84586 | −6.99061 | −2.26943 | 4.72118 |
| n-hexane | 1.58 | −15658.86152 | −6.98762 | −2.27596 | 4.71165 |
| toluene | 2.37 | −15658.86295 | −6.98462 | −2.27406 | 4.71057 |
| acetone | 20.70 | −15658.84914 | −6.95768 | −2.25338 | 4.70431 |
| PBBA | GAS | 1.00 | −13393.01210 | −6.93945 | −1.96303 | 4.97642 |
| n-hexane | 1.58 | −13393.03012 | −6.92612 | −1.96085 | 4.96526 |
| toluene | 2.37 | −13393.03222 | −6.92122 | −1.95813 | 4.96309 |
| acetone | 20.70 | −13393.03260 | −6.89210 | −1.93745 | 4.95465 |
| PBEB | GAS | 1.00 | −13166.38381 | −6.81618 | −1.84793 | 4.96826 |
| n-hexane | 1.58 | −13166.39881 | −6.79931 | −1.84575 | 4.95356 |
| toluene | 2.37 | −13166.40023 | −6.79632 | −1.84521 | 4.95111 |
| acetone | 20.70 | −13166.39808 | −6.77292 | −1.83296 | 4.93996 |
| PBT | GAS | 1.00 | −13127.06888 | −6.82326 | −1.85663 | 4.96662 |
| n-hexane | 1.58 | −13127.08355 | −6.80503 | −1.85201 | 4.95302 |
| toluene | 2.37 | −13127.08493 | −6.80149 | −1.85065 | 4.95084 |
| acetone | 20.70 | −13127.08302 | −6.77292 | −1.83459 | 4.93832 |
Table 7.
Vertical transition energies (ET1), vertical ionization energies (VIE) and vertical electron affinities (VIE) for the NBFRs and the solvent molecules (n-hexane; toluene; acetone) (eV).
Table 7.
Vertical transition energies (ET1), vertical ionization energies (VIE) and vertical electron affinities (VIE) for the NBFRs and the solvent molecules (n-hexane; toluene; acetone) (eV).
| Compound | Solvents | ET1 | VIES0 | VEAS0 | VIET1 | VEAT1 |
|---|
| n-hexane | n-hexane | 9.5261 | 8.8109 | −2.8572 | −0.7152 | 6.6689 |
| toluene | toluene | 3.7512 | 7.2657 | −0.6611 | 3.5145 | 3.0901 |
| acetone | acetone | 3.9244 | 7.4011 | 0.9553 | 3.4767 | 4.8797 |
| HBB | n-hexane | 3.2317 | 8.1835 | 1.2648 | 4.9483 | 4.5 |
| toluene | 3.2327 | 8.0939 | 1.355 | 4.8587 | 4.5902 |
| acetone | 3.2382 | 6.8619 | 2.3836 | 3.6267 | 5.6188 |
| PBBA | n-hexane | 3.3261 | 8.0651 | 1.1271 | 4.7378 | 4.4544 |
| toluene | 3.3264 | 7.9897 | 1.2075 | 4.6624 | 4.5348 |
| acetone | 3.3273 | 7.2988 | 1.9489 | 3.9715 | 5.2762 |
| PBEB | n-hexane | 3.3068 | 7.8546 | 0.8708 | 4.5393 | 4.1861 |
| toluene | 3.3078 | 7.7696 | 0.9626 | 4.4543 | 4.2779 |
| acetone | 3.3153 | 7.0433 | 1.7075 | 3.728 | 5.0228 |
| PBT | n-hexane | 3.3075 | 7.8543 | 0.8802 | 4.5376 | 4.1969 |
| toluene | 3.3086 | 7.7599 | 0.9766 | 4.4432 | 4.2933 |
| acetone | 3.3167 | 7.0326 | 1.7193 | 3.7159 | 5.036 |
Table 8.
Gibbs free energy values from ΔG1 to ΔG6 of the photoinduced electron transfer reactions between the solvent molecules and the NBFRs (eV).
Table 8.
Gibbs free energy values from ΔG1 to ΔG6 of the photoinduced electron transfer reactions between the solvent molecules and the NBFRs (eV).
| Compound | Solvents | ΔG1 | ΔG2 | ΔG3 | ΔG4 | ΔG5 | ΔG6 |
|---|
| HBB | n-hexane | 7.8055 | 4.3109 | −1.7206 | −5.2152 | 1.5146 | −1.9800 |
| toluene | 5.5199 | 2.6755 | 1.7687 | −1.0757 | 5.0039 | 2.1595 |
| acetone | 2.6714 | 1.7824 | −1.2530 | −2.1420 | 1.9822 | 1.0932 |
| PBBA | n-hexane | 7.5950 | 4.3565 | −1.9311 | −5.1696 | 1.3962 | −1.8423 |
| toluene | 5.3236 | 2.7310 | 1.5724 | −1.0202 | 4.8997 | 2.3071 |
| acetone | 3.0162 | 2.1250 | −0.9082 | −1.7994 | 2.4191 | 1.5279 |
| PBEB | n-hexane | 7.3965 | 4.6248 | −2.1296 | −4.9013 | 1.1857 | −1.5860 |
| toluene | 5.1155 | 2.9878 | 1.3643 | −0.7634 | 4.6796 | 2.5519 |
| acetone | 2.7727 | 2.3784 | −1.1517 | −1.5460 | 2.1636 | 1.7693 |
| PBT | n-hexane | 7.3948 | 4.6140 | −2.1313 | −4.9121 | 1.1854 | −1.5954 |
| toluene | 5.1043 | 2.9725 | 1.3531 | −0.7787 | 4.6698 | 2.5380 |
| acetone | 2.7606 | 2.3651 | −1.1638 | −1.5593 | 2.1529 | 1.7574 |