Economic Evaluation of Pharmacist-Led Digital Health Interventions: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
- P: Adult patients
- I: Digital intervention(s) by pharmacists
- C: Usual care (i.e., comparator treatment in published studies)
- O: Health-related clinical outcomes from patients
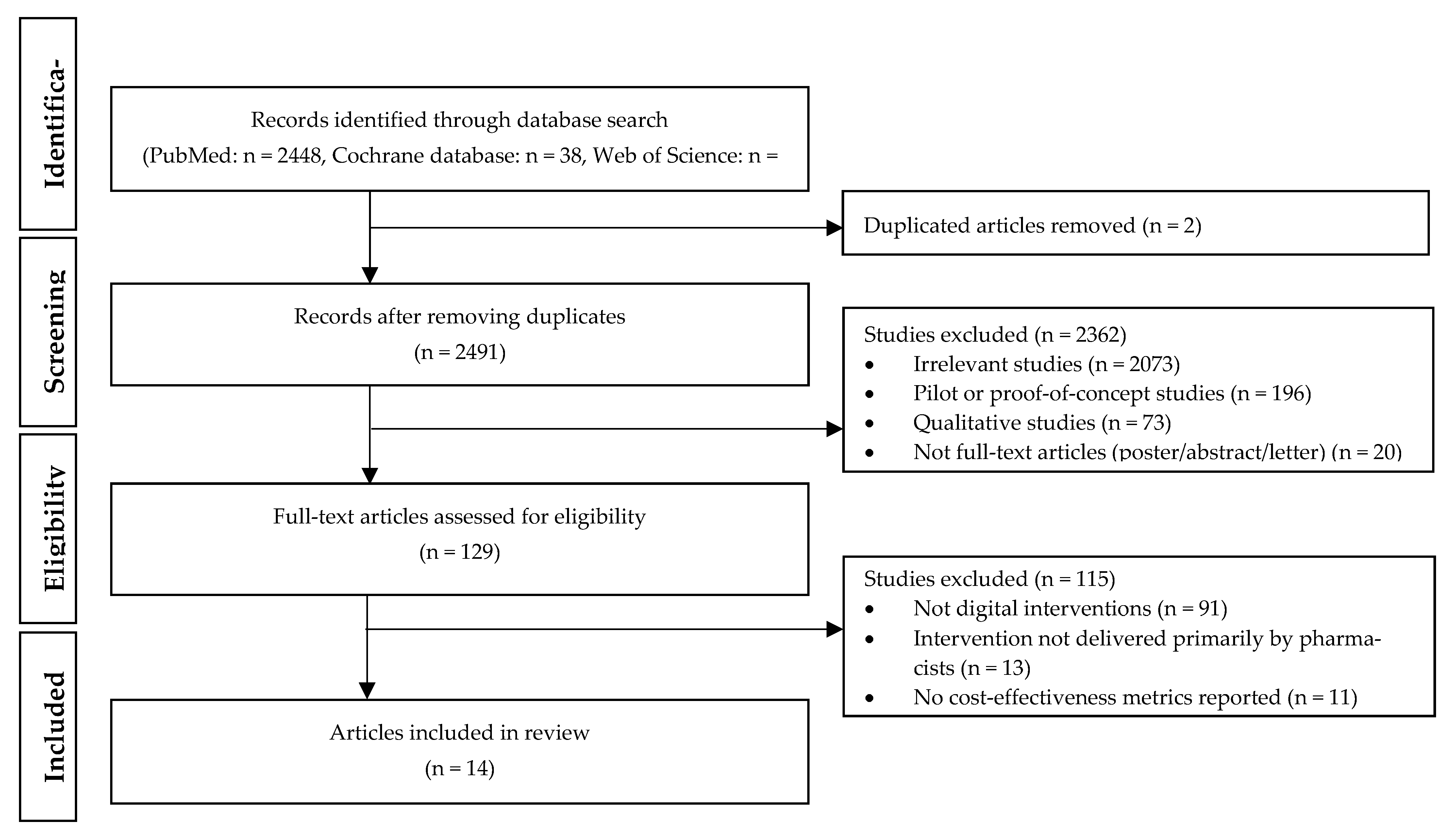
2.2. Inclusion/Exclusion Criteria and Selection Process
2.3. Quality Assessment of the Studies
3. Results
3.1. Overview of the Included Studies
3.2. Telephone-Based Intervention
3.3. Computer- or Web-Based Intervention
3.4. Videotape-Based Intervention
3.5. Smartphone-Based Intervention
3.6. Multiple Techologies-Based Intervention
3.7. Study Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, C.E.; Harrington, R.A.; Desai, S.A.; Mahaffey, K.W.; Turakhia, M.P. Characteristics of digital health studies registered in ClinicalTrials. gov. JAMA Intern. Med. 2019, 179, 838–840. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, A.D.; Neinstein, A.; Khanna, R. Balancing innovation and safety when integrating digital tools into health care. Ann Intern Med. 2018, 169, 592. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration. What Is Digital Health? Available online: https://www.fda.gov/medical-devices/digital-health-center-excellence/what-digital-health (accessed on 7 September 2022).
- Manejwala, O. Yes, personalized digital interventions actually work. Med. Econom. Available online: https://www.medicaleconomics.com/view/yes-personalized-digital-interventions-actually-work (accessed on 7 September 2022).
- Dameff, C.; Clay, B.; Longhurst, C.A. Personal health records: More promising in the smartphone era? JAMA 2019, 321, 339–340. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, W.; Zhou, K.; Waddell, E.; Myers, T.; Dorsey, E.R. Improving access to care: Telemedicine across medical domains. Annu. Rev. Public Health 2021, 42, 463–481. [Google Scholar] [CrossRef]
- Ji, X.; Chow, E.; Abdelhamid, K.; Naumova, D.; Mate, K.K.V.; Bergeron, A.; Lebouché, B. Utility of mobile technology in medical interpretation: A literature review of current practices. Patient Educ. Couns. 2021, 104, 2137–2145. [Google Scholar] [CrossRef]
- Willis, V.C.; Thomas Craig, K.J.; Jabbarpour, Y.; Scheufele, E.L.; Arriaga, Y.E.; Ajinkya, M.; Rhee, K.B.; Bazemore, A. Digital health interventions to enhance prevention in primary care: Scoping review. JMIR Med. Inform. 2022, 10, e33518. [Google Scholar] [CrossRef]
- Crilly, P.; Kayyali, R. A systematic review of randomized controlled trials of telehealth and digital technology use by community pharmacists to improve public health. Pharmacy 2020, 8, 137. [Google Scholar] [CrossRef]
- Park, T.; Muzumdar, J.; Kim, H. Digital health interventions by clinical pharmacists: A systematic review. Int. J. Environ. Res. Public Health 2022, 19, 532. [Google Scholar] [CrossRef]
- Spiro, S. Digital transformation of pharmacists’ clinical services. J. Am. Pharm. Assoc. 2019, 59, S8–S12. [Google Scholar] [CrossRef]
- Fishman, P.A.; Cook, A.J.; Anderson, M.L.; Ralston, J.D.; Catz, S.L.; Carrell, D.; Carlson, J.; Green, B.B. Improving BP control through electronic communications: An economic evaluation. Am. J. Manag. Care 2013, 19, 709–716. [Google Scholar]
- Avery, A.J.; Rodgers, S.; Cantrill, J.A.; Armstrong, S.; Cresswell, K.; Eden, M.; Elliott, R.A.; Howard, R.; Kendrick, D.; Morris, C.J.; et al. A pharmacist-led information technology intervention for medication errors (PINCER): A multicentre, cluster randomised, controlled trial and cost-effectiveness analysis. Lancet 2012, 379, 1310–1319. [Google Scholar] [CrossRef]
- Hope, C.; Overhage, J.M.; Seger, A.; Teal, E.; Mills, V.; Fiskio, J.; Gandhi, T.K.; Bates, D.W.; Murray, M.D. A tiered approach is more cost effective than traditional pharmacist-based review for classifying computer-detected signals as adverse drug events. J. Biomed. Inform. 2003, 36, 92–98. [Google Scholar] [CrossRef]
- Amador-Fernández, N.; Benrimoj, S.I.; García-Mochón, L.; García-Cárdenas, V.; Dineen-Griffin, S.; Gastelurrutia, M.Á.; Gómez-Martínez, J.C.; Colomer-Molina, V.; Martínez-Martínez, F. A cost utility analysis alongside a cluster-randomised trial evaluating a minor ailment service compared to usual care in community pharmacy. BMC Health Serv. Res. 2021, 21, 1253. [Google Scholar] [CrossRef]
- Dineen-Griffin, S.; Vargas, C.; Williams, K.A.; Benrimoj, S.I.; Garcia-Cardenas, V. Cost utility of a pharmacist-led minor ailment service compared with usual pharmacist care. Cost Eff. Resour. Alloc. 2020, 18, 24. [Google Scholar] [CrossRef]
- Iribarren, S.J.; Cato, K.; Falzon, L.; Stone, P.W. What is the economic evidence for mHealth? A systematic review of economic evaluations of mHealth solutions. PLoS ONE 2017, 12, e0170581. [Google Scholar] [CrossRef]
- Steinhubl, S.R.; Muse, E.D.; Topol, E.J. Can mobile health technologies transform health care? JAMA 2013, 310, 2395–2396. [Google Scholar] [CrossRef]
- Painter, J.T.; Fortney, J.C.; Austen, M.A.; Pyne, J.M. Cost-effectiveness of telemedicine-based collaborative care for posttraumatic stress disorder. Psychiatr. Serv. 2017, 68, 1157–1163. [Google Scholar] [CrossRef]
- Pyne, J.M.; Fortney, J.C.; Tripathi, S.P.; Maciejewski, M.L.; Edlund, M.J.; Williams, D.K. Cost-effectiveness analysis of a rural telemedicine collaborative care intervention for depression. Arch. Gen. Psychiatry 2010, 67, 812–821. [Google Scholar] [CrossRef]
- de la Torre-Díez, I.; López-Coronado, M.; Vaca, C.; Aguado, J.S.; de Castro, C. Cost-utility and cost-effectiveness studies of telemedicine, electronic, and mobile health systems in the literature: A systematic review. Telemed. J. E. Health 2015, 21, 81–85. [Google Scholar] [CrossRef]
- Whitten, P.S.; Mair, F.S.; Haycox, A.; May, C.R.; Williams, T.L.; Hellmich, S. Systematic review of cost effectiveness studies of telemedicine interventions. BMJ 2002, 324, 1434–1437. [Google Scholar] [CrossRef]
- Zhai, Y.K.; Zhu, W.J.; Cai, Y.L.; Sun, D.X.; Zhao, J. Clinical- and cost-effectiveness of telemedicine in type 2 diabetes mellitus: A systematic review and meta-analysis. Medicine 2014, 93, e312. [Google Scholar] [CrossRef] [PubMed]
- Pease, A.; Zomer, E.; Liew, D.; Lo, C.; Earnest, A.; Zoungas, S. Cost-effectiveness of health technologies in adults with type 1 diabetes: A systematic review and narrative synthesis. Syst. Rev. 2020, 9, 171. [Google Scholar] [CrossRef] [PubMed]
- Elbert, N.J.; van Os-Medendorp, H.; van Renselaar, W.; Ekeland, A.G.; Hakkaart-van Roijen, L.; Raat, H.; Nijsten, T.E.; Pasmans, S.G. Effectiveness and cost-effectiveness of ehealth interventions in somatic diseases: A systematic review of systematic reviews and meta-analyses. J. Med. Intern. Res. 2014, 16, e110. [Google Scholar] [CrossRef] [PubMed]
- Naslund, J.A.; Marsch, L.A.; McHugo, G.J.; Bartels, S.J. Emerging mHealth and eHealth interventions for serious mental illness: A review of the literature. J. Ment. Health 2015, 24, 321–332. [Google Scholar] [CrossRef]
- Badawy, S.M.; Kuhns, L.M. Economic evaluation of text-messaging and smartphone-based interventions to improve medication adherence in adolescents with chronic health conditions: A systematic review. JMU 2016, 4, e121. [Google Scholar] [CrossRef]
- Ofman, J.J.; Sullivan, S.D.; Neumann, P.J.; Chiou, C.F.; Henning, J.M.; Wade, S.W.; Hay, J.W. Examining the value and quality of health economic analyses: Implications of utilizing the QHES. J. Manag. Care Pharm. 2003, 9, 53–61. [Google Scholar] [CrossRef]
- Szucs, T.D.; Pfeil, A.M. A systematic review of the cost effectiveness of herpes zoster vaccination. Pharmacoeconomics 2013, 31, 125–136. [Google Scholar] [CrossRef]
- Spiegel, B.M.; Targownik, L.E.; Kanwal, F.; Derosa, V.; Dulai, G.S.; Gralnek, I.M.; Chiou, C.F. The quality of published health economic analyses in digestive diseases: A systematic review and quantitative appraisal. Gastroenterology 2004, 127, 403–411. [Google Scholar] [CrossRef]
- Park, T.; Griggs, S.K.; Suh, D.C. Cost effectiveness of monoclonal antibody therapy for rare diseases: A systematic review. BioDrugs 2015, 29, 259–274. [Google Scholar] [CrossRef]
- Faleh AlMutairi, M.; Tourkmani, A.M.; Alrasheedy, A.A.; ALHarbi, T.J.; Bin Rsheed, A.M.; ALjehani, M.; AlRuthia, Y. Cost-effectiveness of telemedicine care for patients with uncontrolled type 2 diabetes mellitus during the COVID-19 pandemic in Saudi Arabia. Ther. Adv. Chronic Dis. 2021, 12, 131–135. [Google Scholar] [CrossRef]
- Margusino-Framiñán, L.; Cid-Silva, P.; Castro-Iglesias, Á.; Mena-de-Cea, Á.; Rodríguez-Osorio, I.; Pernas-Souto, B.; Vázquez-Rodríguez, P.; López-Calvo, S.; Martín-Herranz, I. Teleconsultation for the Pharmaceutical Care of HIV Outpatients in Receipt of Home Antiretrovirals Delivery: Clinical, Economic, and Patient-Perceived Quality Analysis. Telemed. E-health 2019, 25, 399–406. [Google Scholar] [CrossRef]
- Bosmans, J.E.; Brook, O.H.; van Hout, H.P.; de Bruijne, M.C.; Nieuwenhuyse, H.; Bouter, L.M.; Stalman, W.A.; Van Tulder, M.W. Cost effectiveness of a pharmacy-based coaching programme to improve adherence to antidepressants. Pharmacoeconomics 2007, 25, 25–37. [Google Scholar] [CrossRef][Green Version]
- Dehmer, S.P.; Maciosek, M.V.; Trower, N.K.; Asche, S.E.; Bergdall, A.R.; Nyboer, R.A.; O’Connor, P.J.; Pawloski, P.A.; Sperl-Hillen, J.M.; Green, B.B.; et al. Economic evaluation of the home blood pressure telemonitoring and pharmacist case management to control hypertension (Hyperlink) Trial. J. Am. Coll. Clin. Pharm. 2018, 1, 21–30. [Google Scholar] [CrossRef]
- Lázaro Cebas, A.; Caro Teller, J.M.; García Muñoz, C.; González Gómez, C.; Ferrari Piquero, J.M.; Lumbreras Bermejo, C.; Romero Garrido, J.A.; Benedí González, J. Intervention by a clinical pharmacist carried out at discharge of elderly patients admitted to the internal medicine department: Influence on readmissions and costs. BMC Health Serv. Res. 2022, 22, 167. [Google Scholar] [CrossRef]
- Lowres, N.; Neubeck, L.; Salkeld, G.; Krass, I.; McLachlan, A.J.; Redfern, J.; Bennett, A.A.; Briffa, T.; Bauman, A.; Martinez, C.; et al. Feasibility and cost-effectiveness of stroke prevention through community screening for atrial fibrillation using iPhone ECG in pharmacies. Thromb. Haemost. 2014, 111, 1167–1176. [Google Scholar] [CrossRef]
- Padwal, R.S.; So, H.; Wood, P.W.; Mcalister, F.A.; Siddiqui, M.; Norris, C.M.; Jeerakathil, T.; Stone, J.; Valaire, S.; Mann, B.; et al. Cost-effectiveness of home blood pressure telemonitoring and case management in the secondary prevention of cerebrovascular disease in Canada. J. Clin. Hypertens. 2019, 21, 159–168. [Google Scholar] [CrossRef]
- Hirth, R.A.; Chernew, M.E.; Miller, E.; Fendrick, A.M.; Weissert, W.G. Willingness to pay for a quality-adjusted life year: In search of a standard. Med. Decis. Making 2000, 20, 332–342. [Google Scholar] [CrossRef]
- Braithwaite, R.S.; Meltzer, D.O.; King, J.T., Jr.; Leslie, D.; Roberts, M.S. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med. Care. 2008, 46, 349–356. [Google Scholar] [CrossRef]
- Finley, P.R.; Rens, H.R.; Pont, J.T.; Gess, S.L.; Louie, C.; Bull, S.A.; Bero, R.A. Impact of a collaborative pharmacy practice model on the treatment of depression in primary care. Am. J. Health Syst. Pharm. 2002, 59, 1518–1526. [Google Scholar] [CrossRef]
- Adler, D.A.; Bungay, K.M.; Wilson, I.B.; Pei, Y.; Supran, S.; Peckham, E.; Cynn, D.J.; Rogers, W.H. The impact of a pharmacist intervention on 6-month outcomes in depressed primary care patients. Gen. Hosp. Psychiatry 2004, 26, 199–209. [Google Scholar] [CrossRef]
- BinDhim, N.F.; McGeechan, K.; Trevena, L. Smartphone smoking cessation application (SSC App) trial: A multicountry double-blind automated randomised controlled trial of a smoking cessation decision-aid “app”. BMJ Open 2018, 8, e017105. [Google Scholar]
- Bricker, J.B.; Mull, K.E.; Kientz, J.A.; Vilardaga, R.; Mercer, L.D.; Akioka, K.J.; Heffner, J.L. Randomized, controlled pilot trial of a smartphone app for smoking cessation using acceptance and commitment therapy. Drug. Alcohol Depend. 2014, 143, 87–94. [Google Scholar] [CrossRef]
- Siriwoen, R.; Chongsuwat, R.; Tansakul, S.; Siri, S. Effectiveness of a weight management program applying mobile health technology as a supporting tool for overweight and obese working women. Asia Pac. J. Public Health 2018, 30, 572–581. [Google Scholar] [CrossRef]
- Tregarthen, J.; Kim, J.P.; Sadeh-Sharvit, S.; Neri, E.; Welch, H.; Lock, J. Comparing a tailored self-help mobile app with a standard self-monitoring app for the treatment of eating disorder symptoms: Randomized controlled trial. JMIR Ment. Health 2019, 6, e14972. [Google Scholar] [CrossRef]
- Pavic, M.; Klaas, V.; Theile, G.; Kraft, J.; Tröster, G.; Blum, D.; Guckenberger, M. Mobile health technologies for continuous monitoring of cancer patients in palliative care aiming to predict health status deterioration: A feasibility study. J. Palliat. Med. 2020, 23, 678–685. [Google Scholar] [CrossRef]
- Lin, W.Y.; Ke, H.L.; Chou, W.C.; Chang, P.C.; Tsai, T.H.; Lee, M.Y. Realization and technology acceptance test of a wearable cardiac health monitoring and early warning system with multi-channel MCGs and ECG. Sensors 2018, 18, 3538. [Google Scholar] [CrossRef]
- Avellar, S.A.; Thomas, J.; Kleinman, R.; Sama-Miller, E.; Woodruff, S.E.; Coughlin, R.; Westbrook, T.P.R. External validity: The next step for systematic reviews? Eval. Rev. 2017, 41, 283–325. [Google Scholar] [CrossRef]
| Questions | Points |
|---|---|
| 1. Was the study objective presented in a clear, specific, and measurable manner? | 7 |
| 2. Was the perspective of the analysis (societal, third-party payer, etc.) stated and were reasons for its selection either stated or implied? a,b | 4 |
| 3. Were variable estimates used in the analysis from the best available source (i.e., randomized control trial—best, expert opinion—worst)? c | 8 |
| 4. If estimates came from a subgroup analysis, were the groups prespecified at the beginning of the study? | 1 |
| 5. Was uncertainty handled by statistical analysis to address random events or sensitivity analysis to cover a range of assumption? | 9 |
| 6. Was incremental analysis performed between alternatives for resources and costs? | 6 |
| 7. Was the methodology for data abstraction (including the value of health states for cost-utility analysis and other benefits) stated? b | 5 |
| 8. Did the analytic horizon allow time for all relevant and important outcomes? Were benefits and costs that went beyond 1 year discounted (3% to 5%) and justification given for the discount rate if the discount rate was arbitrarily determined? b | 7 |
| 9. Was the measurement of costs appropriate and the methodology for the estimation of quantities, unit costs, and price years clearly described? a,b | 8 |
| 10. Were the primary outcome measure(s) for the economic evaluation clearly stated and did they include the major short-term, long-term, and negative outcomes? a | 6 |
| 11. Were the health outcomes measures/scales valid and reliable? If previously tested valid and reliable measures were not available, was justification given for the measures/scales used? | 7 |
| 12. Were the traditional economic model (including structure), study methods and analysis, and the components of the numerator and denominator displayed in a clear, transparent manner? a,b | 8 |
| 13. Were the choice of the traditional economic model, main assumptions of the traditional economic model, and limitations of the study stated and justified? a,b | 7 |
| 14. Did the author(s) explicitly discuss direction and magnitude of potential biases? a | 6 |
| 15. Were the conclusions/recommendations of the study justified and based on the study results? | 8 |
| 16. Was there a statement disclosing the source of funding for the study? | 3 |
| Total points | 100 |
| Author (Year) | Type of Analysis | Country | Perspective | Currency | Economic Model | Time Horizon | Discount Rate | Funding Source |
|---|---|---|---|---|---|---|---|---|
| Amador-Fernandez et al. (2021) [15] | CUA | Spain | Patient and health system | EUR | No model (data directly from a clinical trial) | 6 months | Not needed | Yes (Public) |
| Avery et al. (2012) [13] | CEA | United Kingdom | UK National Health Service | GBP | Simple probabilistic decision-analytic model | 6 months | Not needed | Yes (Public) |
| Bosmans et al. (2007) [34] | CEA | Netherland | Societal | EUR | No model (data directly from a clinical trial) | 6 months | Not needed | Yes (Public) |
| Dehmar et al. (2018) [35] | CEA | United States | Health service | USD | No model (data directly from a clinical trial) | 12 months | Not needed | Yes (Public) |
| Dineen-Griffin et al. (2020) [16] | CUA | Australia | Societal | AUD | Decision tree | 14 days | Not needed | Yes (Public) |
| Faleh AlMutairi et al. (2021) [32] | CEA | Saudi Arabia | Health service provider | SAR | No model (data from retrospective chart review) | 4 months | Not needed | No |
| Fishman et al. (2013) [12] | CEA | United States | Health plan | USD | No model (data directly from a clinical trial) | Lifetime | 3%, 5%, 7% | Yes (Public) |
| Hope et al. (2003) [14] | CEA | United States | Not reported | USD | Not reported | 4 months | Not needed | Yes (Public) |
| Lazaro Cebas et al. (2022) [36] | CBA | Spain | Not reported | EUR | No model (data directly from a clinical trial) | 9 months | Not needed | Yes (Public) |
| Lowres et al. (2014) [37] | CUA | Australia | Australian health funder | AUD | Not reported | 10 years | 5% | Yes (Private and Public) |
| Margusino-Framinan et al. (2022) [33] | CMA | Spain | Patient and societal | EUR | No model (data directly from a clinical trial) | 12 months | Not needed | No |
| Padwal et al. (2018) [38] | CUA | Canada | Health care payer | CAD | Markov model | Lifetime | 1.5% | Yes (Public) |
| Painter et al. (2017) [19] | CUA | United States | Payer | USD | No model (data directly from a clinical trial) | 12 months | Not needed | Yes (Public) |
| Pyne et al. (2010) [20] | CUA | United States | Veterans health administration | USD | No model (data directly from a clinical trial) | 6 and 12 months | Not needed | Yes (Public) |
| Author | Study Population | Intervention | Comparator | Types of Costs Included | Cost: Intervention vs. Comparator | Effectiveness: Intervention vs. Comparator | Incremental Cost-Effectiveness Ratio (ICER) a |
|---|---|---|---|---|---|---|---|
| Telephone-based intervention | |||||||
| Dehmer et al. [35] | Patients with hypertension | Telemonitoring of blood pressure | Usual care | Clinic-based (office visit, laboratory, radiology), pharmacy, and hospital costs | Change from baseline: USD −186 vs. USD 96 | Incremental % of achieving blood pressure control for the intervention: 18.4% b | USD 7337 per person achieving blood pressure control |
| Faleh AlMutairi et al. [32] | Patients with diabetes | Telemedicine care | Traditional care | Medications, laboratory tests, medical supplies, shipping, phone calls, and clinical visits | Saudi Riyal (SAR) 4820 vs. SAR 4151 | Difference in HbA1c: 1.82 vs. 1.54 | SAR 2373 per 1% reduction in the level of HbA1c |
| Lazaro Cebas et al. [36] | Polymedicated elderly patients aged ≥65 years | Phone call follow-up after discharge | No follow-up | Clinical pharmacist salary, cost per admission in elderly patients | Incremental cost for the intervention to prevent one readmission: EUR 3091 b | 30-day hospital readmission: 16.43% vs. 20.13% | Total cost saving: EUR 1301 |
| Margusino-Framinan et al. [33] | Patients with HIV | Pre-post design (Intervention: teleconsultation with home drug delivery/mail-order pharmacy) | Direct (transportation, hospital pharmacy consultation service, home drug delivery) cost and indirect (productivity) cost | Cost saving of EUR 137 per patient per year for the intervention | No significant difference in HIV viral load and CD4+ level after the intervention | EUR 137 patient/year costs-saved and 18.5 h/patient/year working time gained | |
| Padwal et al. [38] | Patients with cerebrovascular disease | Home blood pressure telemonitoring with pharmacist case management | Usual care | Pharmacist and physician cost, blood pressure device cost, drug cost, etc. | CAD 21,640 vs. CAD 23,020 | Quality-adjusted life year (QALY): 8.83 vs. 8.00 | The intervention was dominant, achieving improved health at a reduced cost |
| Painter et al. [19] | Veterans with posttraumatic stress disorder (PTSD) | Telemedicine-based collaborative care | Usual care | Outpatient and pharmacy costs | Incremental cost for the intervention: USD 2495 | Incremental QALY: 0.008 | USD 185,565/QALY |
| Pyne et al. [20] | Patients with depression | Telemedicine-based collaborative care | Usual care | (Base-case analysis) Outpatient and drug costs (Secondary analysis) Base-case costs and inpatient costs | Incremental cost for the intervention was significant (β = USD 1528, p < 0.001) | Incremental QALY for the intervention was significant (β = 0.018, p = 0.04) | (Base-case analysis) USD 85,634/QALY (Secondary analysis) USD 111,999–USD 132,175/QALY |
| Computer- or web-based intervention | |||||||
| Avery et al. [13] | Patients electronically prescribed from a general practitioner | Computer-generated feedback for medication errors, educational outreach, and dedicated support | Computer-generated feedback for medication errors | Costs for generating error reports, training pharmacists, meetings, and time spent in each practice outside meetings following up errors | GBP 1050 vs. GBP 93 | Primary outcomes: (1) NSAID-related error: 3% vs. 4% (2) Beta blocker-related error: 2% vs. 3% (3) ACE inhibitor- or loop diuretic-related error: 5% vs. 8% | GBP 66 per error avoided |
| Fishman et al. [12] | Adults with hypertension alone (no diagnosis of diabetes, cardiovascular, or other serious conditions) | Home blood pressure monitoring + pharmacist’s web-based management of patients’ blood pressures | Home blood pressure monitoring | Physical and human resources used to provide an intervention or usual care | USD 400 vs. USD 67 | Discounted change in life expectancy: (1) Women: 0.44 vs. 0.29 (2) Men: 0.53 vs. 0.35 | (1) Women: USD 2220/year (2) Men: USD 1850/year |
| Hope et al. [14] | Adults with outpatient appointment at ambulatory care clinics | Tiered review method (computer-based review before a clinician’s review) | Traditional pharmacist review method | Training cost, data analyst, nurse, and pharmacist | USD 22,606 vs. USD 44,580 | 777 adverse drug events (ADEs) and 666 medication errors (MEs) b | Cost per ADE identified: USD 42.40 with the tiered method vs. USD 68.70 with the traditional method |
| Videotape-based intervention | |||||||
| Bosmans et al. [34] | Patients with a new prescription for a non-tricyclic antidepressant | Coaching program (three contacts with pharmacist and a take-home video) | Usual care | Direct medical costs and indirect costs | EUR 3275 vs. EUR 2961 | (1) Adherence: No significant difference between the two groups (mean difference: 2.1%, 95% CI: −5.6 to 9.8) (2) Improvements in depressive symptoms measured by the Hopkins Symptom Checklist (SCL): No significant difference between the two groups (mean difference: −0.15, 95% CI: −0.54 to 0.23) | (1) EUR 149 per 1% improvement in adherence (2) EUR 2550 per point improvement on the SCL |
| Smartphone-based intervention | |||||||
| Lowres et al. [37] | Patients aged 65 or older with no severe medical condition | Screening of atrial fibrillation (AF) using iPhone electrocardiogram (iECG) | No screening of AF | Diagnostic assessment of AF costs, anticoagulation, and monitoring costs | Not reported separately for the intervention and comparator | Not reported separately for the intervention and comparator | AUD 5988/QALY and AUD 30,481 for preventing one stroke |
| Multiple technologies system-based intervention | |||||||
| Amador-Fernandez et al. [15] | Patients presenting minor ailments or requesting a non-prescription medication for minor ailments | Face-to-face consultation on a web-based program plus telephone follow-up | Usual care | Health professionals’ consultation time, medication costs, pharmacists’ training costs, and investment of the pharmacy and consultation costs | EUR 20 vs. EUR 13 | QALY: 0.0248 vs. 0.0245 | EUR 24,733/QALY |
| Dineen-Griffin et al. [16] | Patients with minor ailments | Face-to-face consultation using the technology-integrated platforms plus telephone follow-up | Usual care | Direct costs | (Base-case analysis) AUD 27 vs. AUD 20 (Multi-way sensitivity analysis)AUD 34 vs. AUD 23 | (Base-case analysis) QALY: 0.0296 vs. 0.0264 (Multi-way sensitivity analysis) QALY: 0.0296 vs. 0.0264 | (Base-case analysis) AUD 2277/QALY (Multi-way sensitivity analysis) AUD 3502/QALY |
| QHES Item | Amador-Fernandez et al. [15] | Avery et al. [13] | Bosmans et al. [34] | Dehmer et al. [35] | Dineen-Griffin et al. [16] | Faleh AlMutairi et al. [32] | Fishman et al. [12] | Hope et al. [14] | Lazaro Cebas et al. [36] | Lowres et al. [37] | Margusino-Framinan et al. [33] | Padwal et al. [38] | Painter et al. [19] | Pyne et al. [20] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | FP | FP | FP | FP | FP | FP | FP | FP | FP | FP | FP | FP | FP | FP |
| 2 | PP | PP | PP | PP | FP | PP | PP | NP | NP | PP | FP | PP | PP | PP |
| 3 | FP | FP | FP | FP | FP | FP | FP | FP | FP | FP | FP | FP | FP | FP |
| 4 | FP | FP | NA | NA | NA | NA | NA | NA | FP | NA | NA | NA | FP | FP |
| 5 | FP | FP | FP | FP | FP | FP | FP | NP | FP | FP | FP | FP | FP | FP |
| 6 | FP | FP | FP | FP | FP | FP | FP | FP | FP | FP | NP | FP | FP | FP |
| 7 | FP | FP | FP | FP | FP | FP | FP | FP | FP | FP | FP | FP | FP | FP |
| 8 | NA | NA | NA | NA | NA | NA | FP | NA | NA | FP | NA | FP | NA | NA |
| 9 | FP | PP | FP | PP | FP | PP | FP | PP | PP | FP | NP | FP | PP | FP |
| 10 | PP | PP | PP | PP | PP | PP | PP | PP | PP | FP | PP | FP | PP | PP |
| 11 | FP | FP | FP | FP | FP | FP | FP | FP | FP | FP | FP | FP | FP | FP |
| 12 | PP | PP | PP | PP | FP | PP | PP | PP | PP | PP | PP | FP | PP | PP |
| 13 | PP | PP | PP | PP | FP | PP | PP | PP | PP | PP | PP | FP | PP | PP |
| 14 | PP | PP | FP | NP | NP | NP | PP | NP | NP | PP | PP | NP | NP | NP |
| 15 | FP | FP | FP | FP | FP | FP | FP | FP | FP | FP | FP | FP | FP | FP |
| 16 | FP | FP | FP | FP | FP | FP | FP | FP | FP | FP | NP | FP | FP | FP |
| Quality | Good | Good | Good | Fair | Good | Fair | Good | Fair | Fair | Good | Fair | Good | Fair | Good |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, T.; Kim, H.; Song, S.; Griggs, S.K. Economic Evaluation of Pharmacist-Led Digital Health Interventions: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 11996. https://doi.org/10.3390/ijerph191911996
Park T, Kim H, Song S, Griggs SK. Economic Evaluation of Pharmacist-Led Digital Health Interventions: A Systematic Review. International Journal of Environmental Research and Public Health. 2022; 19(19):11996. https://doi.org/10.3390/ijerph191911996
Chicago/Turabian StylePark, Taehwan, Hyemin Kim, Seunghyun Song, and Scott K. Griggs. 2022. "Economic Evaluation of Pharmacist-Led Digital Health Interventions: A Systematic Review" International Journal of Environmental Research and Public Health 19, no. 19: 11996. https://doi.org/10.3390/ijerph191911996
APA StylePark, T., Kim, H., Song, S., & Griggs, S. K. (2022). Economic Evaluation of Pharmacist-Led Digital Health Interventions: A Systematic Review. International Journal of Environmental Research and Public Health, 19(19), 11996. https://doi.org/10.3390/ijerph191911996






