Hereditary Ovarian Cancer: Towards a Cost-Effective Prevention Strategy
Abstract
:1. Introduction
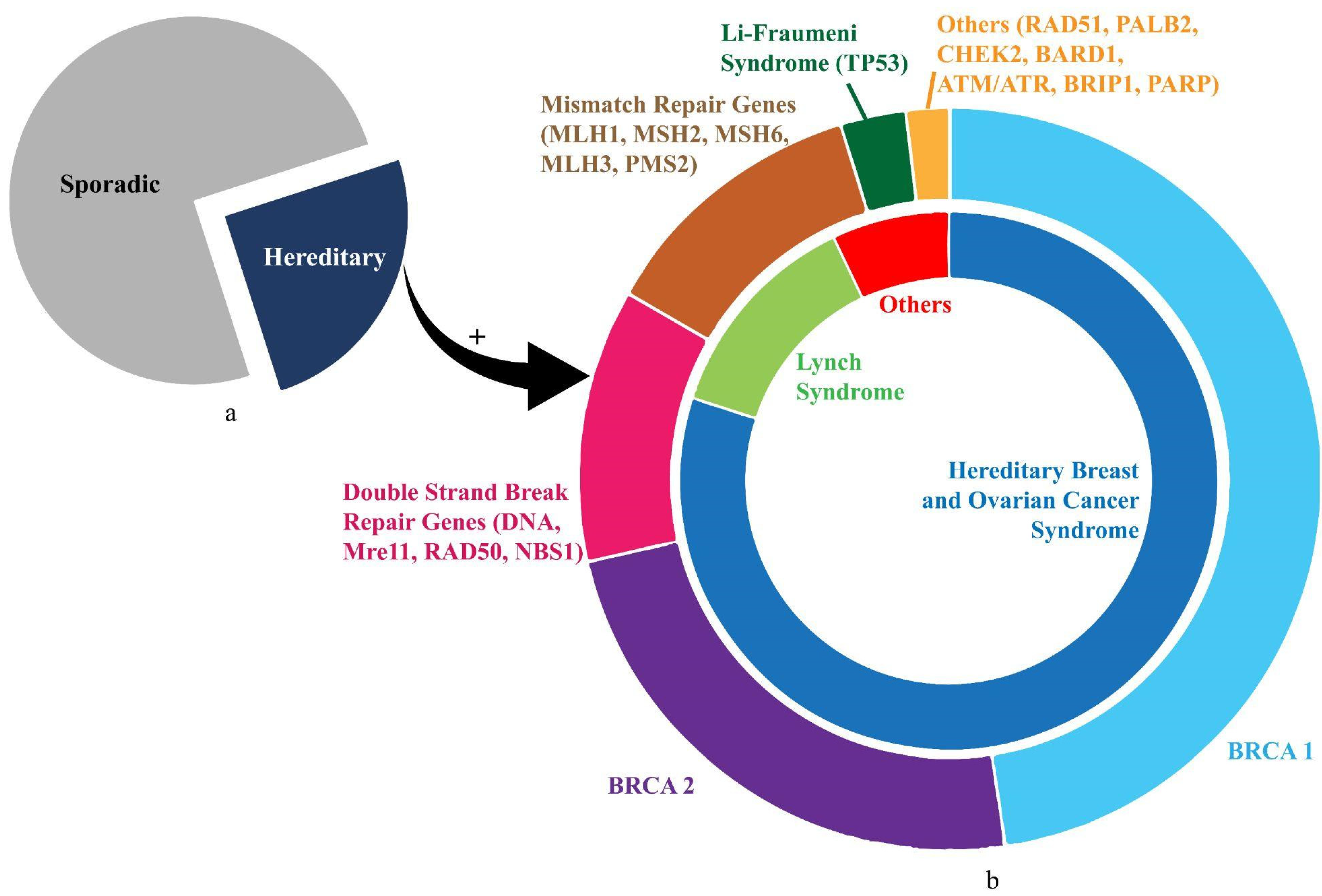
2. Hereditary Ovarian Cancer (HOC)
3. Prevention Strategies for Hereditary Ovarian Cancer
3.1. Genetic Testing and Counselling
3.2. Screening
3.3. Hormonal Chemoprevention
3.3.1. Combined Oral Contraceptive Pills (COCP)
3.3.2. Other Hormonal Agents
3.4. Surgical Prevention
3.4.1. Bilateral Tubal Ligation (BTL)
3.4.2. Risk-Reduction Bilateral Salpingo-Oophorectomy (RRBSO)
3.4.3. Risk-Reducing Bilateral Salpingectomy (RRBS)
3.4.4. Hysterectomy
4. International Guidelines
4.1. Society of Gynaecologic Oncology (SGO)
4.2. European Society of Medical Oncology (ESMO)
4.3. American College of Obstetrics and Gynaecologists (ACOG)
4.4. Manchester International Consensus Group (MICG)
4.5. E. United States Preventive Services Task Force (USPSTF)
4.6. American Society of Clinical Oncology (ASCO)
4.7. National Comprehensive Cancer Network (NCCN)
5. Comparative Analysis of Cost-Effective Prevention Strategies
5.1. General Population
| Population | Year | Reference | Risk-Reducing Strategy | Cost Effectiveness of Intervention (ICER) | Comments | ||
|---|---|---|---|---|---|---|---|
| General | 2012 | [80] | Annual screening CA125 to predict candidates for TVS | USD 89,000/YLS, 13% mortality reduction | |||
| Semi-annual screening CA125 to predict candidates for TVS | USD 117,000/YLS, 20% reduction in mortality | ||||||
| 2016 | [81] | MMS—Sequential ROCA | GBP 9000/QALY | ||||
| 2017 | [82] | MMS with per unit cost of GBP 20 per patient | GBP 90,000/LYG | ||||
| MMS with per unit cost of GBP 15 per patient | GBP 78,000/LYG | ||||||
| 2018 | [83] | MMS vs. no screening | 15% mortality reduction, ICER: USD 105,000–155,000 | ||||
| 2018 | [84] | ROCA-based MMS commenced at age 50 for 20 years | 6% decrease in mortality with USD 590,000/LYG | ||||
| ROCA-based MMS commenced at age 50 for 30 years | 9% decrease in mortality with USD 760,000/LYG | ||||||
| 2015 | [86] | RRBM | USD 27,000/LYG | ||||
| Hereditary Ovarian Cancer | BRCA 1/2 | 1998 | [87] | RRBO at 30 years of age | 2.6 years survival improvement and QALY of 0.5 in favour of PO | ||
| 2006 | [88] | RRBSO at age 35 | Most cost-effective with quality adjustment | ||||
| 2008 | [89] | RRBSO | 85% decrease in BRCA1 OC, no statistically significant effect on BRCA2 OC | ||||
| 2008 | [90] | RRBSO + RRBM | EUR 496/LYG | ||||
| RRBSO alone | EUR 1284/LYG | ||||||
| 2011 | [91] | RRBM vs. RRBSO vs. RRBM+RRBSO vs. chemoprevention vs. surveillance | BRCA1 | PBSO—USD 1741/QALY | |||
| BRCA2 | PBSO—USD 4587/QALY | ||||||
| 2013 | [92] | RRBS vs. RRSDO vs. RRBSO | BRCA1 | PSDO—USD 37,800/QALY | RRBSO yielded highest risk reduction, life expectancy and lowest cost, RRSDO had highest ICER | ||
| BRCA2 | PSDO—USD 89,700/QALY | ||||||
| 2018 | [93] | RRBM vs. RRBSO vs. RRBM+RRBSO at age 30 vs. RRBM+RRBSO at age 40 | PBM + PBSO at age 30—cost of EUR 29,000 and 17.7 QALY gained or 19.9 LYG | ||||
| Lynch Syndrome | 2008 | [94] | Annual screening from age 30 followed by RRH + RRBSO at age 40 vs. Only screening from age 30 vs. Only RRH +RRBSO at age 40 or 30 vs. No intervention | Annual screening from age 30 followed by PH + PBSO at age 40—USD 195,000/QALY | |||
| 2011 | [95] | RRBSO+RRH at age 30 | USD 23,400 per patient and QALY-26 | ||||
| Strong Family History | 2019 | [96] | No mutation testing vs. Cascade testing followed by RRS | Cascade testing followed by RRS—USD 9000–10,000 per QALY | |||
| 2019 | [97] | Intensified surveillance followed RRS (RRBM/ RRBSO/ RRBM+RRBSO) | EUR 17,000/QALY and EUR 22,000/LYG | Prevented one-third of malignancies, RRBM + RRBSO was the most cost-effective RRS | |||
| Ashkenazi Jewish Women | 1999 | [98] | Surveillance followed RRS (RRBM + RRBSO) | USD 21,000/LYG | |||
| 2009 | [99] | Screening + RRBSO vs. no screening | USD 8300/QALY | ||||
| 2014 | [100] | Population-based screening vs. Family-based screening, both followed by RRS | Population-based screening—GBP 2079/QALY | Done in population with index cases of 4 AJ Grandparents | |||
| 2017 | [101] | Population-based screening followed by RRBSO | 1 AJ Grandparent | GBP 2793/USD 7110/QALY (UK/US) | Highly cost-effective even in varying AJ ancestry | ||
| 2 AJ Grandparents | GBP 301/USD 7366/QALY (UK/US) | ||||||
| 3 AJ Grandparents | GBP 1759/USD 14,032/QALY (UK/US) | ||||||
| 4 AJ Grandparents | GBP 2589/USD 17,786/QALY (UK/US) | ||||||
| Sephardi Jewish Women | 2018 | [102] | Population based screening vs. Family based screening | £67/$308/QALY (UK/US) | |||
5.2. Women with HOC Risk
5.3. BRCA Mutation Carriers
5.4. LS Carriers
5.5. Women with Strong Family History
5.6. Ashkenazi Jewish (AJ) Women
5.7. Sephardi Jewish (SJ) Women
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- International Agency for Research on Cancer. Global Cancer Observatory: Cancer Today. Available online: https://gco.iarc.fr/today/online-analysis-table (accessed on 5 June 2022).
- Cancer Research UK. Ovarian Cancer Statistics. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/ovarian-cancer (accessed on 5 June 2022).
- American Cancer Society. Key Statistics for Ovarian Cancer. Available online: https://www.cancer.org/cancer/ovarian-cancer/about/key-statistics.html (accessed on 5 June 2022).
- Cheung, A.; Shah, S.; Parker, J.; Soor, P.; Limbu, A.; Sheriff, M.; Boussios, S. Non-Epithelial Ovarian Cancers: How Much Do We Really Know? Int. J. Environ. Res. Public. Health 2022, 19, 1106. [Google Scholar] [CrossRef] [PubMed]
- Boussios, S.; Moschetta, M.; Zarkavelis, G.; Papadaki, A.; Kefas, A.; Tatsi, K. Ovarian sex-cord stromal tumours and small cell tumours: Pathological, genetic and management aspects. Crit. Rev. Oncol. Hematol. 2017, 120, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Boussios, S.; Attygalle, A.; Hazell, S.; Moschetta, M.; McLachlan, J.; Okines, A.; Banerjee, S. Malignant Ovarian Germ Cell Tumors in Postmenopausal Patients: The Royal Marsden Experience and Literature Review. Anticancer Res. 2015, 35, 6713–6722. [Google Scholar] [PubMed]
- Boussios, S.; Karathanasi, A.; Zakynthinakis-Kyriakou, N.; Tsiouris, A.K.; Chatziantoniou, A.A.; Kanellos, F.S.; Tatsi, K. Ovarian carcinosarcoma: Current developments and future perspectives. Crit. Rev. Oncol. Hematol. 2019, 134, 46–55. [Google Scholar] [CrossRef] [PubMed]
- McCluggage, W.G. Morphological subtypes of ovarian carcinoma: A review with emphasis on new developments and pathogenesis. Pathology 2011, 43, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Kurman, R.J.; Shih, I.M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer--shifting the paradigm. Hum. Pathol. 2011, 42, 918–931. [Google Scholar] [CrossRef] [PubMed]
- Koshiyama, M.; Matsumura, N.; Konishi, I. Recent concepts of ovarian carcinogenesis: Type I and type II. Biomed. Res. Int. 2014, 2014, 934261. [Google Scholar] [CrossRef]
- Pavlidis, N.; Rassy, E.; Vermorken, J.B.; Assi, T.; Kattan, J.; Boussios, S.; Smith-Gagen, J. The outcome of patients with serous papillary peritoneal cancer, fallopian tube cancer, and epithelial ovarian cancer by treatment eras: 27 years data from the SEER registry. Cancer Epidemiol. 2021, 75, 102045. [Google Scholar] [CrossRef]
- Boussios, S.; Moschetta, M.; Tatsi, K.; Tsiouris, A.K.; Pavlidis, N. A review on pregnancy complicated by ovarian epithelial and non-epithelial malignant tumors: Diagnostic and therapeutic perspectives. J. Adv. Res. 2018, 12, 1–9. [Google Scholar] [CrossRef]
- Ghose, A.; Gullapalli, S.V.N.; Chohan, N.; Bolina, A.; Moschetta, M.; Rassy, E.; Boussios, S. Applications of Proteomics in Ovarian Cancer: Dawn of a New Era. Proteomes. 2022, 10, 16. [Google Scholar] [CrossRef]
- Toss, A.; Tomasello, C.; Razzaboni, E.; Contu, G.; Grandi, G.; Cagnacci, A.; Schilder, R.J.; Cortesi, L. Hereditary ovarian cancer: Not only BRCA 1 and 2 genes. Biomed. Res. Int. 2015, 2015, 341723. [Google Scholar] [CrossRef]
- Biglia, N.; Sgandurra, P.; Bounous, V.E.; Maggiorotto, F.; Piva, E.; Pivetta, E.; Ponzone, R.; Pasini, B. Ovarian cancer in BRCA1 and BRCA2 gene mutation carriers: Analysis of prognostic factors and survival. Ecancermedicalscience 2016, 10, 639. [Google Scholar] [CrossRef]
- Shah, S.; Cheung, A.; Kutka, M.; Sheriff, M.; Boussios, S. Epithelial Ovarian Cancer: Providing Evidence of Predisposition Genes. Int. J. Environ. Res. Public. Health 2022, 19, 8113. [Google Scholar] [CrossRef]
- Revythis, A.; Limbu, A.; Mikropoulos, C.; Ghose, A.; Sanchez, E.; Sheriff, M.; Boussios, S. Recent Insights into PARP and Immuno-Checkpoint Inhibitors in Epithelial Ovarian Cancer. Int. J. Environ. Res. Public. Health 2022, 19, 8577. [Google Scholar] [CrossRef]
- Boussios, S.; Karathanasi, A.; Cooke, D.; Neille, C.; Sadauskaite, A.; Moschetta, M.; Zakynthinakis-Kyriakou, N.; Pavlidis, N. PARP Inhibitors in Ovarian Cancer: The Route to “Ithaca”. Diagnostics 2019, 9, 55. [Google Scholar] [CrossRef]
- Tung, N.; Garber, J.E. PARP inhibition in breast cancer: Progress made and future hopes. NPJ Breast Cancer 2022, 8, 47. [Google Scholar] [CrossRef]
- Boussios, S.; Rassy, E.; Shah, S.; Ioannidou, E.; Sheriff, M.; Pavlidis, N. Aberrations of DNA repair pathways in prostate cancer: A cornerstone of precision oncology. Expert. Opin. Ther. Targets 2021, 25, 329–333. [Google Scholar] [CrossRef]
- Lancaster, J.M.; Powell, C.B.; Chen, L.M.; Richardson, D.L.; SGO Clinical Practice Committee. Society of Gynecologic Oncology statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol. Oncol. 2015, 136, 3–7. [Google Scholar] [CrossRef]
- Walker, J.L.; Powell, C.B.; Chen, L.M.; Carter, J.; Bae Jump, V.L.; Parker, L.P.; Borowsky, M.E.; Gibb, R.K. Society of Gynecologic Oncology recommendations for the prevention of ovarian cancer. Cancer 2015, 121, 2108–2120. [Google Scholar] [CrossRef]
- Paluch-Shimon, S.; Cardoso, F.; Sessa, C.; Balmana, J.; Cardoso, M.J.; Gilbert, F.; Senkus, E.; ESMO Guidelines Committee. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann. Oncol. 2016, 27, v103–v110. [Google Scholar] [CrossRef]
- Practice Bulletin No. 182 Summary: Hereditary Breast and Ovarian Cancer Syndrome. Obstet. Gynecol. 2017, 130, 657–659.
- ACOG Practice Bulletin No. 147: Lynch syndrome. Obstet. Gynecol. 2014, 124, 1042–1054.
- Crosbie, E.J.; Ryan, N.A.J.; Arends, M.J.; Bosse, T.; Burn, J.; Cornes, J.M.; Crawford, R.; Eccles, D.; Frayling, I.M.; Ghaem-Maghami, S.; et al. The Manchester International Consensus Group recommendations for the management of gynecological cancers in Lynch syndrome. Genet. Med. 2019, 21, 2390–2400. [Google Scholar] [CrossRef]
- US Preventive Services Task Force; Owens, D.K.; Davidson, K.W.; Krist, A.H.; Barry, M.J.; Cabana, M.; Caughey, A.B.; Doubeni, C.A.; Epling, J.W., Jr.; Kubik, M.; et al. Risk Assessment, Genetic Counseling, and Genetic Testing for BRCA-Related Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2019, 322, 652–665. [Google Scholar]
- Konstantinopoulos, P.A.; Norquist, B.; Lacchetti, C.; Armstrong, D.; Grisham, R.N.; Goodfellow, P.J.; Kohn, E.C.; Levine, D.A.; Liu, J.F.; Lu, K.H.; et al. Germline and Somatic Tumor Testing in Epithelial Ovarian Cancer: ASCO Guideline. J. Clin. Oncol. 2020, 38, 1222–1245. [Google Scholar] [CrossRef]
- Daly, M.B.; Pal, T.; Berry, M.P.; Buys, S.S.; Dickson, P.; Domchek, S.M.; Elkhanany, A.; Friedman, S.; Goggins, M.; Hutton, M.L.; et al. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2021, 19, 77–102. [Google Scholar] [CrossRef]
- Rebbeck, T.R.; Kauff, N.D.; Domchek, S.M. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J. Natl. Cancer Inst. 2009, 101, 80–87. [Google Scholar] [CrossRef]
- Iodice, S.; Barile, M.; Rotmensz, N.; Feroce, I.; Bonanni, B.; Radice, P.; Bernard, L.; Maisonneuve, P.; Gandini, S. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: A meta-analysis. Eur. J. Cancer 2010, 46, 2275–2284. [Google Scholar] [CrossRef]
- Temkin, S.M.; Bergstrom, J.; Samimi, G.; Minasian, L. Ovarian Cancer Prevention in High-risk Women. Clin. Obstet. Gynecol. 2017, 60, 738–757. [Google Scholar] [CrossRef]
- Ali, A.T. Towards Prevention of Ovarian Cancer. Curr. Cancer Drug Targets. 2018, 18, 522–537. [Google Scholar] [CrossRef]
- Weissman, S.M.; Weiss, S.M.; Newlin, A.C. Genetic testing by cancer site: Ovary. Cancer J. 2012, 18, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Hunn, J.; Rodriguez, G.C. Ovarian cancer: Etiology, risk factors, and epidemiology. Clin. Obstet. Gynecol. 2012, 55, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Pal, T.; Permuth-Wey, J.; Betts, J.A.; Krischer, J.P.; Fiorica, J.; Arango, H.; LaPolla, J.; Hoffman, M.; Martino, M.A.; Wakeley, K.; et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer 2005, 104, 2807–2816. [Google Scholar] [CrossRef]
- Pal, T.; Akbari, M.R.; Sun, P.; Lee, J.H.; Fulp, J.; Thompson, Z.; Coppola, D.; Nicosia, S.; Sellers, T.A.; McLaughlin, J.; et al. Frequency of mutations in mismatch repair genes in a population-based study of women with ovarian cancer. Br. J. Cancer 2012, 107, 1783–1790. [Google Scholar] [CrossRef]
- Wooster, R.; Weber, B.L. Breast and ovarian cancer. N. Engl. J. Med. 2003, 348, 2339–2347. [Google Scholar] [CrossRef] [PubMed]
- Anglian Breast Cancer Study Group. Prevalence and penetrance of BRCA1 and BRCA2 mutations in a population-based series of breast cancer cases. Br. J. Cancer 2000, 83, 1301–1308. [Google Scholar] [CrossRef]
- Chen, S.; Parmigiani, G. Meta-analysis of BRCA1 and BRCA2 penetrance. J. Clin. Oncol. 2007, 25, 1329–1333. [Google Scholar] [CrossRef]
- Walsh, T.; Casadei, S.; Lee, M.K.; Pennil, C.C.; Nord, A.S.; Thornton, A.M.; Roeb, W.; Agnew, K.J.; Stray, S.M.; Wickramanayake, A.; et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc. Natl. Acad. Sci. USA 2011, 108, 18032–18037. [Google Scholar] [CrossRef]
- Ratajska, M.; Antoszewska, E.; Piskorz, A.; Brozek, I.; Borg, Å.; Kusmierek, H.; Biernat, W.; Limon, J. Cancer predisposing BARD1 mutations in breast-ovarian cancer families. Breast Cancer Res. Treat. 2012, 131, 89–97. [Google Scholar] [CrossRef]
- Rafnar, T.; Gudbjartsson, D.F.; Sulem, P.; Jonasdottir, A.; Sigurdsson, A.; Jonasdottir, A.; Besenbacher, S.; Lundin, P.; Stacey, S.N.; Gudmundsson, J.; et al. Mutations in BRIP1 confer high risk of ovarian cancer. Nat. Genet. 2011, 43, 1104–1107. [Google Scholar] [CrossRef]
- Yang, X.; Leslie, G.; Doroszuk, A.; Schneider, S.; Allen, J.; Decker, B.; Dunning, A.M.; Redman, J.; Scarth, J.; Plaskocinska, I.; et al. Cancer Risks Associated With Germline PALB2 Pathogenic Variants: An International Study of 524 Families. J. Clin. Oncol. 2020, 38, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Meindl, A.; Hellebrand, H.; Wiek, C.; Erven, V.; Wappenschmidt, B.; Niederacher, D.; Freund, M.; Lichtner, P.; Hartmann, L.; Schaal, H.; et al. Germline mutations in breast and ovarian cancer pedigrees establish RAD51C as a human cancer susceptibility gene. Nat. Genet. 2010, 42, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Heikkinen, K.; Karppinen, S.M.; Soini, Y.; Mäkinen, M.; Winqvist, R. Mutation screening of Mre11 complex genes: Indication of RAD50 involvement in breast and ovarian cancer susceptibility. J. Med. Genet. 2003, 40, e131. [Google Scholar] [CrossRef] [Green Version]
- Ramus, S.J.; Song, H.; Dicks, E.; Tyrer, J.P.; Rosenthal, A.N.; Intermaggio, M.P.; Fraser, L.; Gentry-Maharaj, A.; Hayward, J.; Philpott, S.; et al. Germline Mutations in the BRIP1, BARD1, PALB2, and NBN Genes in Women With Ovarian Cancer. J. Natl. Cancer Inst. 2015, 107, djv214. [Google Scholar] [CrossRef]
- Boussios, S.; Rassy, E.; Moschetta, M.; Ghose, A.; Adeleke, S.; Sanchez, E.; Sheriff, M.; Chargari, C.; Pavlidis, N. BRCA Mutations in Ovarian and Prostate Cancer: Bench to Bedside. Cancers. 2022, 14, 3888. [Google Scholar] [CrossRef] [PubMed]
- Backes, F.J.; Cohn, D.E. Lynch syndrome. Clin. Obstet. Gynecol. 2011, 54, 199–214. [Google Scholar] [CrossRef]
- Grindedal, E.M.; Renkonen-Sinisalo, L.; Vasen, H.; Evans, G.; Sala, P.; Blanco, I.; Gronwald, J.; Apold, J.; Eccles, D.M.; Sánchez, A.A.; et al. Survival in women with MMR mutations and ovarian cancer: A multicentre study in Lynch syndrome kindreds. J. Med. Genet. 2010, 47, 99–102. [Google Scholar] [CrossRef]
- Piombino, C.; Cortesi, L.; Lambertini, M.; Punie, K.; Grandi, G.; Toss, A. Secondary Prevention in Hereditary Breast and/or Ovarian Cancer Syndromes Other Than BRCA. J. Oncol. 2020, 2020, 6384190. [Google Scholar] [CrossRef]
- Kurian, A.W.; Ward, K.C.; Howlader, N.; Deapen, D.; Hamilton, A.S.; Mariotto, A.; Miller, D.; Penberthy, L.S.; Katz, S.J. Genetic Testing and Results in a Population-Based Cohort of Breast Cancer Patients and Ovarian Cancer Patients. J. Clin. Oncol. 2019, 37, 1305–1315. [Google Scholar] [CrossRef]
- Buys, S.S.; Partridge, E.; Greene, M.H.; Prorok, P.C.; Reding, D.; Riley, T.L.; Hartge, P.; Fagerstrom, R.M.; Ragard, L.R.; Chia, D.; et al. Ovarian cancer screening in the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial: Findings from the initial screen of a randomized trial. Am. J. Obstet. Gynecol. 2005, 193, 1630–1639. [Google Scholar] [CrossRef]
- Buys, S.S.; Partridge, E.; Black, A.; Johnson, C.C.; Lamerato, L.; Isaacs, C.; Reding, D.J.; Greenlee, R.T.; Yokochi, L.A.; Kessel, B.; et al. Effect of screening on ovarian cancer mortality: The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA 2011, 305, 2295–2303. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, I.J.; Menon, U.; Ryan, A.; Gentry-Maharaj, A.; Burnell, M.; Kalsi, J.K.; Amso, N.N.; Apostolidou, S.; Benjamin, E.; Cruickshank, D.; et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): A randomised controlled trial. Lancet 2016, 387, 945–956. [Google Scholar] [CrossRef]
- Menon, U.; Gentry-Maharaj, A.; Burnell, M.; Singh, N.; Ryan, A.; Karpinskyj, C.; Carlino, G.; Taylor, J.; Massingham, S.K.; Raikou, M.; et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): A randomised controlled trial. Lancet 2021, 397, 2182–2193. [Google Scholar] [CrossRef]
- Walker, M.; Jacobson, M.; Sobel, M. Management of ovarian cancer risk in women with BRCA1/2 pathogenic variants. CMAJ 2019, 191, E886–E893. [Google Scholar] [CrossRef] [PubMed]
- Elezaby, M.; Lees, B.; Maturen, K.E.; Barroilhet, L.; Wisinski, K.B.; Schrager, S.; Wilke, L.G.; Sadowski, E. BRCA Mutation Carriers: Breast and Ovarian Cancer Screening Guidelines and Imaging Considerations. Radiology 2019, 291, 554–569. [Google Scholar] [CrossRef]
- Neff, R.T.; Senter, L.; Salani, R. BRCA mutation in ovarian cancer: Testing, implications and treatment considerations. Ther. Adv. Med. Oncol. 2017, 9, 519–531. [Google Scholar] [CrossRef]
- Narod, S.A.; Risch, H.; Moslehi, R.; Dørum, A.; Neuhausen, S.; Olsson, H.; Provencher, D.; Radice, P.; Evans, G.; Bishop, S.; et al. Oral contraceptives and the risk of hereditary ovarian cancer. Hereditary Ovarian Cancer Clinical Study Group. N. Engl. J. Med. 1998, 339, 424–428. [Google Scholar] [CrossRef]
- Moorman, P.G.; Havrilesky, L.J.; Gierisch, J.M.; Coeytaux, R.R.; Lowery, W.J.; Peragallo Urrutia, R.; Dinan, M.; McBroom, A.J.; Hasselblad, V.; Sanders, G.D.; et al. Oral contraceptives and risk of ovarian cancer and breast cancer among high-risk women: A systematic review and meta-analysis. J. Clin. Oncol. 2013, 31, 4188–4198. [Google Scholar] [CrossRef]
- Havrilesky, L.J.; Moorman, P.G.; Lowery, W.J.; Gierisch, J.M.; Coeytaux, R.R.; Urrutia, R.P.; Dinan, M.; McBroom, A.J.; Hasselblad, V.; Sanders, G.D.; et al. Oral contraceptive pills as primary prevention for ovarian cancer: A systematic review and meta-analysis. Obstet. Gynecol. 2013, 122, 139–147. [Google Scholar] [CrossRef]
- Narod, S.A.; Dubé, M.P.; Klijn, J.; Lubinski, J.; Lynch, H.T.; Ghadirian, P.; Provencher, D.; Heimdal, K.; Moller, P.; Robson, M.; et al. Oral contraceptives and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. J. Natl. Cancer Inst. 2002, 94, 1773–1779. [Google Scholar] [CrossRef]
- Haile, R.W.; Thomas, D.C.; McGuire, V.; Felberg, A.; John, E.M.; Milne, R.L.; Hopper, J.L.; Jenkins, M.A.; Levine, A.J.; Daly, M.M.; et al. Ontario Cancer Genetics Network Investigators, Whittemore AS. BRCA1 and BRCA2 mutation carriers, oral contraceptive use, and breast cancer before age 50. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1863–1870. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.; Park, E.Y.; Kwon, S.Y.; Shin, S.; Emerson, R.E.; Shin, Y.H.; DeMayo, F.J.; Lydon, J.P.; Coffey, D.M.; Hawkins, S.M.; et al. Targeting progesterone signaling prevents metastatic ovarian cancer. Proc. Natl. Acad. Sci. USA 2020, 117, 31993–32004. [Google Scholar] [CrossRef] [PubMed]
- Narod, S.A.; Sun, P.; Ghadirian, P.; Lynch, H.; Isaacs, C.; Garber, J.; Weber, B.; Karlan, B.; Fishman, D.; Rosen, B.; et al. Tubal ligation and risk of ovarian cancer in carriers of BRCA1 or BRCA2 mutations: A case-control study. Lancet. 2001, 357, 1467–1470. [Google Scholar] [CrossRef]
- Cibula, D.; Widschwendter, M.; Májek, O.; Dusek, L. Tubal ligation and the risk of ovarian cancer: Review and meta-analysis. Hum. Reprod. Update 2011, 17, 55–67. [Google Scholar] [CrossRef]
- Rice, M.S.; Murphy, M.A.; Tworoger, S.S. Tubal ligation, hysterectomy and ovarian cancer: A meta-analysis. J. Ovarian. Res. 2012, 5, 13. [Google Scholar] [CrossRef]
- Kauff, N.D.; Satagopan, J.M.; Robson, M.E.; Scheuer, L.; Hensley, M.; Hudis, C.A.; Ellis, N.A.; Boyd, J.; Borgen, P.I.; Barakat, R.R.; et al. Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N. Engl. J. Med. 2002, 346, 1609–1615. [Google Scholar] [CrossRef]
- Rebbeck, T.R.; Lynch, H.T.; Neuhausen, S.L.; Narod, S.A.; Van’t Veer, L.; Garber, J.E.; Evans, G.; Isaacs, C.; Daly, M.B.; Matloff, E.; et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N. Engl. J. Med. 2002, 346, 1616–1622. [Google Scholar] [CrossRef]
- Sekine, M.; Nishino, K.; Enomoto, T. BRCA Genetic Test and Risk-Reducing Salpingo-Oophorectomy for Hereditary Breast and Ovarian Cancer: State-of-the-Art. Cancers 2021, 13, 2562. [Google Scholar] [CrossRef]
- Rocca, W.A.; Grossardt, B.R.; de Andrade, M.; Malkasian, G.D.; Melton, L.J., 3rd. Survival patterns after oophorectomy in premenopausal women: A population-based cohort study. Lancet Oncol. 2006, 7, 821–828. [Google Scholar] [CrossRef]
- Stan, D.L.; Shuster, L.T.; Wick, M.J.; Swanson, C.L.; Pruthi, S.; Bakkum-Gamez, J.N. Challenging and complex decisions in the management of the BRCA mutation carrier. J. Women’s Health 2013, 22, 825–834. [Google Scholar] [CrossRef]
- Labidi-Galy, S.I.; Papp, E.; Hallberg, D.; Niknafs, N.; Adleff, V.; Noe, M.; Bhattacharya, R.; Novak, M.; Jones, S.; Phallen, J.; et al. High grade serous ovarian carcinomas originate in the fallopian tube. Nat. Commun. 2017, 8, 1093. [Google Scholar] [CrossRef] [PubMed]
- Falconer, H.; Yin, L.; Grönberg, H.; Altman, D. Ovarian cancer risk after salpingectomy: A nationwide population-based study. J. Natl. Cancer Inst. 2015, 107, dju410. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, L.C.; Lindor, N.M. The Role of Risk-Reducing Surgery in Hereditary Breast and Ovarian Cancer. N. Engl. J. Med. 2016, 374, 454–468. [Google Scholar] [CrossRef] [PubMed]
- McAlpine, J.N.; Hanley, G.E.; Woo, M.M.; Tone, A.A.; Rozenberg, N.; Swenerton, K.D.; Gilks, C.B.; Finlayson, S.J.; Huntsman, D.G.; Miller, D.M.; et al. Opportunistic salpingectomy: Uptake, risks, and complications of a regional initiative for ovarian cancer prevention. Am. J. Obstet. Gynecol. 2014, 210, 471.e1-11. [Google Scholar] [CrossRef]
- Adeleke, S.; Haslam, A.; Choy, A.; Diaz-Cano, S.; Galante, J.R.; Mikropoulos, C.; Boussios, S. Microsatellite instability testing in colorectal patients with Lynch syndrome: Lessons learned from a case report and how to avoid such pitfalls. Per. Med. 2022, 19, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Mariotto, A.B.; Yabroff, K.R.; Shao, Y.; Feuer, E.J.; Brown, M.L. Projections of the cost of cancer care in the United States: 2010–2020. J. Natl. Cancer Inst. 2011, 103, 117–128. [Google Scholar] [CrossRef]
- Drescher, C.W.; Hawley, S.; Thorpe, J.D.; Marticke, S.; McIntosh, M.; Gambhir, S.S.; Urban, N. Impact of screening test performance and cost on mortality reduction and cost-effectiveness of multimodal ovarian cancer screening. Cancer Prev. Res. Phila. 2012, 5, 1015–1024. [Google Scholar] [CrossRef]
- Kearns, B.; Chilcott, J.; Whyte, S.; Preston, L.; Sadler, S. Cost-effectiveness of screening for ovarian cancer amongst postmenopausal women: A model-based economic evaluation. BMC Med. 2016, 14, 200. [Google Scholar] [CrossRef]
- Menon, U.; McGuire, A.J.; Raikou, M.; Ryan, A.; Davies, S.K.; Burnell, M.; Gentry-Maharaj, A.; Kalsi, J.K.; Singh, N.; Amso, N.N.; et al. The cost-effectiveness of screening for ovarian cancer: Results from the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Br. J. Cancer 2017, 117, 619–627. [Google Scholar] [CrossRef]
- Moss, H.A.; Berchuck, A.; Neely, M.L.; Myers, E.R.; Havrilesky, L.J. Estimating Cost-effectiveness of a Multimodal Ovarian Cancer Screening Program in the United States: Secondary Analysis of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). JAMA. Oncol. 2018, 4, 190–195. [Google Scholar] [CrossRef]
- Naumann, R.W.; Brown, J. Ovarian cancer screening with the Risk of Ovarian Cancer Algorithm (ROCA): Good, bad, or just expensive? Gynecol. Oncol. 2018, 149, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Przybycin, C.G.; Kurman, R.J.; Ronnett, B.M.; Shih, I.M.; Vang, R. Are all pelvic (nonuterine) serous carcinomas of tubal origin? Am. J. Surg. Pathol. 2010, 34, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.S.; McAlpine, J.N.; Hanley, G.E.; Finlayson, S.J.; Cohen, T.; Miller, D.M.; Gilks, C.B.; Huntsman, D.G. Costs and benefits of opportunistic salpingectomy as an ovarian cancer prevention strategy. Obstet. Gynecol. 2015, 125, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Grann, V.R.; Panageas, K.S.; Whang, W.; Antman, K.H.; Neugut, A.I. Decision analysis of prophylactic mastectomy and oophorectomy in BRCA1-positive or BRCA2-positive patients. J. Clin. Oncol. 1998, 16, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.; Jacobson, J.S.; Heitjan, D.F.; Zivin, J.G.; Hershman, D.; Neugut, A.I.; Grann, V.R. Cost-effectiveness of preventive strategies for women with a BRCA1 or a BRCA2 mutation. Ann. Intern. Med. 2006, 144, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Kauff, N.D.; Domchek, S.M.; Friebel, T.M.; Robson, M.E.; Lee, J.; Garber, J.E.; Isaacs, C.; Evans, D.G.; Lynch, H.; Eeles, R.A.; et al. Risk-reducing salpingo-oophorectomy for the prevention of BRCA1- and BRCA2-associated breast and gynecologic cancer: A multicenter, prospective study. J. Clin. Oncol. 2008, 26, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Norum, J.; Hagen, A.I.; Maehle, L.; Apold, J.; Burn, J.; Møller, P. Prophylactic bilateral salpingo-oophorectomy (PBSO) with or without prophylactic bilateral mastectomy (PBM) or no intervention in BRCA1 mutation carriers: A cost-effectiveness analysis. Eur. J. Cancer 2008, 44, 963–971. [Google Scholar] [CrossRef]
- Grann, V.R.; Patel, P.R.; Jacobson, J.S.; Warner, E.; Heitjan, D.F.; Ashby-Thompson, M.; Hershman, D.L.; Neugut, A.I. Comparative effectiveness of screening and prevention strategies among BRCA1/2-affected mutation carriers. Breast Cancer Res. Treat. 2011, 125, 837–847. [Google Scholar] [CrossRef]
- Kwon, J.S.; Tinker, A.; Pansegrau, G.; McAlpine, J.; Housty, M.; McCullum, M.; Gilks, C.B. Prophylactic salpingectomy and delayed oophorectomy as an alternative for BRCA mutation carriers. Obstet. Gynecol. 2013, 121, 14–24. [Google Scholar] [CrossRef]
- Müller, D.; Danner, M.; Rhiem, K.; Stollenwerk, B.; Engel, C.; Rasche, L.; Borsi, L.; Schmutzler, R.; Stock, S. Cost-effectiveness of different strategies to prevent breast and ovarian cancer in German women with a BRCA 1 or 2 mutation. Eur. J. Health Econ. 2018, 19, 341–353. [Google Scholar] [CrossRef]
- Kwon, J.S.; Sun, C.C.; Peterson, S.K.; White, K.G.; Daniels, M.S.; Boyd-Rogers, S.G.; Lu, K.H. Cost-effectiveness analysis of prevention strategies for gynecologic cancers in Lynch syndrome. Cancer 2008, 113, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.Y.; Caughey, A.B.; Little, S.E.; Cheung, M.K.; Chen, L.M. A cost-effectiveness analysis of prophylactic surgery versus gynecologic surveillance for women from hereditary non-polyposis colorectal cancer (HNPCC) Families. Fam. Cancer 2011, 10, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, P.; Eccleston, A.; Hurry, M.; Dyer, M. Targeted surgical prevention of epithelial ovarian cancer is cost effective and saves money in BRCA mutation carrying family members of women with epithelial ovarian cancer. A Canadian model. Gynecol. Oncol. 2019, 153, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.; Danner, M.; Schmutzler, R.; Engel, C.; Wassermann, K.; Stollenwerk, B.; Stock, S.; Rhiem, K. Economic modeling of risk-adapted screen-and-treat strategies in women at high risk for breast or ovarian cancer. Eur. J. Health Econ. 2019, 20, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Grann, V.R.; Whang, W.; Jacobson, J.S.; Heitjan, D.F.; Antman, K.H.; Neugut, A.I. Benefits and costs of screening Ashkenazi Jewish women for BRCA1 and BRCA2. J. Clin. Oncol. 1999, 17, 494–500. [Google Scholar] [CrossRef]
- Rubinstein, W.S.; Jiang, H.; Dellefave, L.; Rademaker, A.W. Cost-effectiveness of population-based BRCA1/2 testing and ovarian cancer prevention for Ashkenazi Jews: A call for dialogue. Genet. Med. 2009, 11, 629–639. [Google Scholar] [CrossRef]
- Manchanda, R.; Legood, R.; Burnell, M.; McGuire, A.; Raikou, M.; Loggenberg, K.; Wardle, J.; Sanderson, S.; Gessler, S.; Side, L.; et al. Cost-effectiveness of population screening for BRCA mutations in Ashkenazi Jewish women compared with family history-based testing. J. Natl. Cancer Inst. 2014, 107, 380. [Google Scholar] [CrossRef]
- Manchanda, R.; Patel, S.; Antoniou, A.C.; Levy-Lahad, E.; Turnbull, C.; Evans, D.G.; Hopper, J.L.; Macinnis, R.J.; Menon, U.; Jacobs, I.; et al. Cost-effectiveness of population based BRCA testing with varying Ashkenazi Jewish ancestry. Am. J. Obstet. Gynecol. 2017, 217, 578.e1–578.e12. [Google Scholar] [CrossRef]
- Patel, S.; Legood, R.; Evans, D.G.; Turnbull, C.; Antoniou, A.C.; Menon, U.; Jacobs, I.; Manchanda, R. Cost effectiveness of population based BRCA1 founder mutation testing in Sephardi Jewish women. Am. J. Obstet. Gynecol. 2018, 218, 431.e1–431.e12. [Google Scholar] [CrossRef]
| Organization | Year | Population | Recommendation | References | ||
|---|---|---|---|---|---|---|
| Screening | Risk-Reducing Intervention | |||||
| Chemoprevention | Surgery | |||||
| Society of Gynaecologic Oncology | 2015 | 1. HBOC 2. LS | - | Long-term COCP for HBOC | HBOC—RRBSO at 35 to 40 years of age; RRBS if RRBSO declined | [21,22] |
| European Society of Medical Oncology | 2016 | HBOC | 6 monthly MMS commencing from 30 years of age | Long-term COCP | RRBSO at 35 to 40 years of age | [23] |
| American College of Obstetrics and Gynaecologists | 1. 2017, 2. 2014 | 1. HBOC 2. LS | MMS as short-term surveillance (not screening) in HBOC at 30 to 35 years prior to RRBSO | - | 1. HBOC—RRBSO in BRCA1 variant 35–40 years and BRCA2 variant 40 to 45 years 2. LS—RRBSO + RRH around 40 years | [24,25] |
| Manchester International Consensus Group | 2019 | LS | Multigene panels using NGS technology involving BRCA and LS-susceptible genes | Long-term COCP | RRBSO + RRH at 35–40 years following childbearing | [26] |
| United States Preventive Services Task Force | 2019 | HBOC | Familial risk assessment screening | - | - | [27] |
| American Society of Clinical Oncology | 2020 | HBOC | Germline GT for all women diagnosed with EOC | - | - | [28] |
| National Comprehensive Cancer Network | 2021 | 1. HBOC 2. LFS | MMS in HBOC at 30 to 35 years if RRBSO declined | - | HBOC-RRBSO in BRCA1 variant 35–40 years and BRCA2 variant 40 to 45 years | [29] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghose, A.; Bolina, A.; Mahajan, I.; Raza, S.A.; Clarke, M.; Pal, A.; Sanchez, E.; Rallis, K.S.; Boussios, S. Hereditary Ovarian Cancer: Towards a Cost-Effective Prevention Strategy. Int. J. Environ. Res. Public Health 2022, 19, 12057. https://doi.org/10.3390/ijerph191912057
Ghose A, Bolina A, Mahajan I, Raza SA, Clarke M, Pal A, Sanchez E, Rallis KS, Boussios S. Hereditary Ovarian Cancer: Towards a Cost-Effective Prevention Strategy. International Journal of Environmental Research and Public Health. 2022; 19(19):12057. https://doi.org/10.3390/ijerph191912057
Chicago/Turabian StyleGhose, Aruni, Anita Bolina, Ishika Mahajan, Syed Ahmer Raza, Miranda Clarke, Abhinanda Pal, Elisabet Sanchez, Kathrine Sofia Rallis, and Stergios Boussios. 2022. "Hereditary Ovarian Cancer: Towards a Cost-Effective Prevention Strategy" International Journal of Environmental Research and Public Health 19, no. 19: 12057. https://doi.org/10.3390/ijerph191912057
APA StyleGhose, A., Bolina, A., Mahajan, I., Raza, S. A., Clarke, M., Pal, A., Sanchez, E., Rallis, K. S., & Boussios, S. (2022). Hereditary Ovarian Cancer: Towards a Cost-Effective Prevention Strategy. International Journal of Environmental Research and Public Health, 19(19), 12057. https://doi.org/10.3390/ijerph191912057







