Does Multi-Strain Probiotic Supplementation Impact the Effort Capacity of Competitive Road Cyclists?
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. The Level of Cyclists’ Capacity
3.2. Analysis of Body Composition of Cyclists during the Experiment
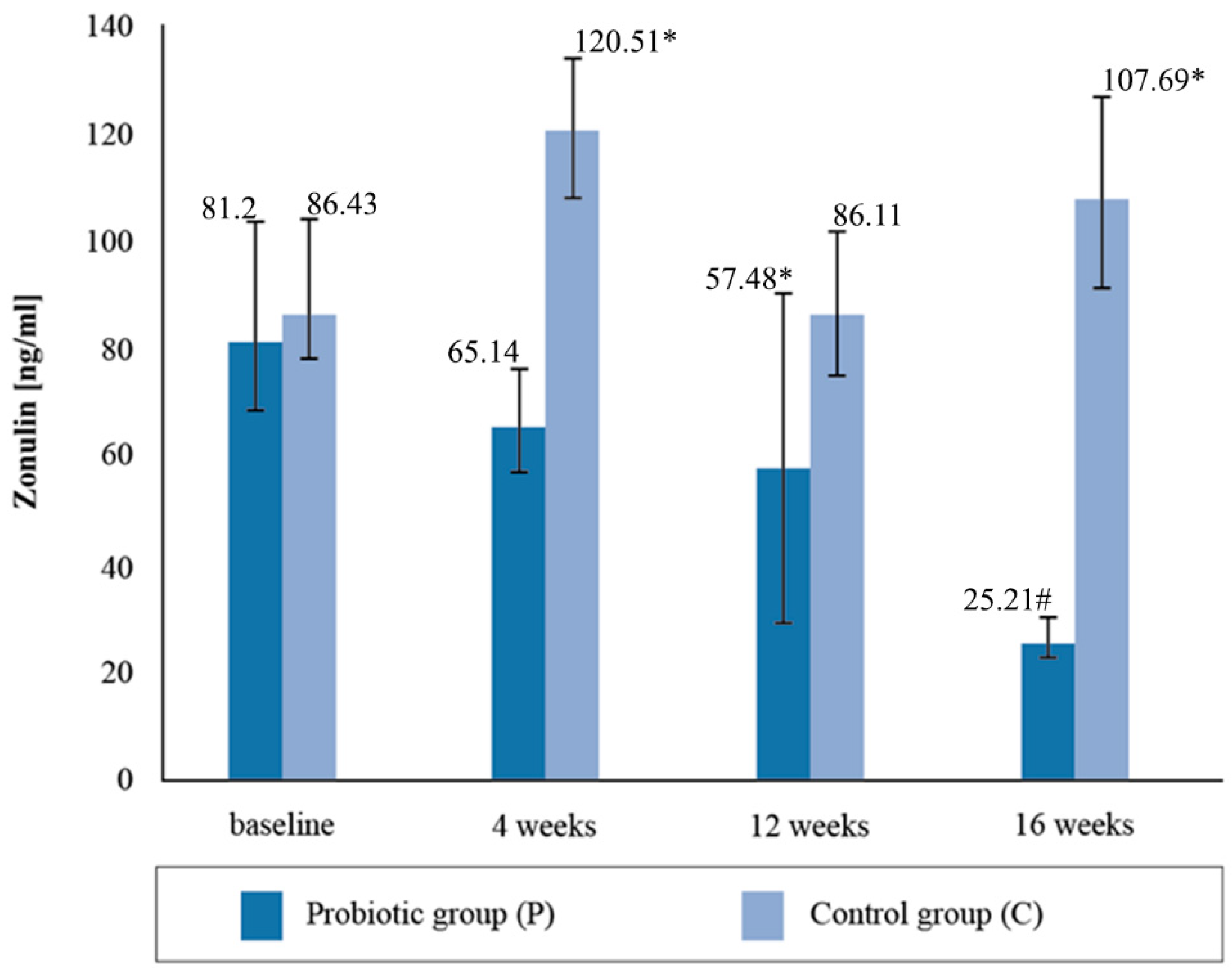
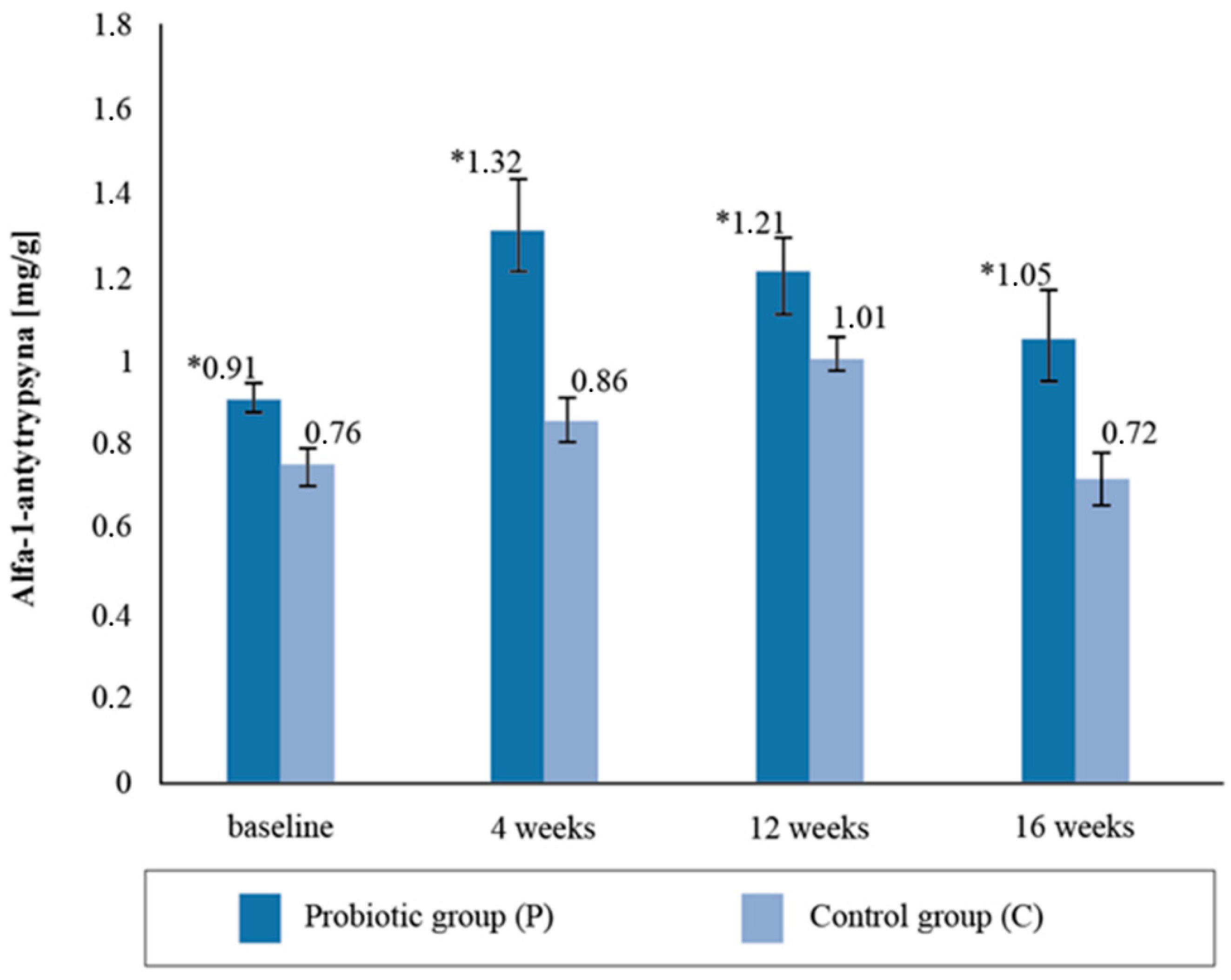
3.3. Analysis of Selected Markers of Intestinal Membrane Permeability
3.4. Analysis of the Concentration of Immunoglobulin A and Pro-Inflammatory (TNF-α, Il-1β, Il-6, and Il-8) and Anti-Inflammatory Cytokines (Il-10)
3.5. Analysis of the Pro-Oxidative and Antioxidant Potential of Cyclists’ Blood
4. Discussion
5. Conclusions
- The probiotic supplementation resulted in increased levels of aerobic capacity, assessed by an increase in the relative magnitude of maximal oxygen uptake, an increase in the duration of exercise to failure, an increase in the load on the ergometer, a decrease in the participants’ heart rates, and a feeling of less discomfort during the exercise test, confirming the beneficial effect of probiotics on the cyclists’ exercise capacity.
- There were no effects of probiotic supplementation on the levels of maximal aerobic power, maximal anaerobic power per kilogram of body weight, power per revolution, and the time to achieve and maintain MAP.
- With respect to athletes training in competitive road cycling, supplementation with multi-strain probiotics did not affect most body composition parameters, except for muscle mass content, which increased slightly.
- As a result of the undertaken probiotic therapy, the frequency and intensity of the cyclists’ gastrointestinal complaints decreased, the concentration of zonulin in the stool mass decreased, and α1 antitrypsin was maintained at a similar level during the experiment, indicating a sealing of the intestinal barrier and beneficial changes in the cyclists’ intestinal function.
- The probiotic supplementation resulted in a reduction in the concentrations of tumor necrosis factor TNF-α measured after the aerobic and anaerobic tests, IL-6 before and after the anaerobic test, IL-10 before the anaerobic test, total oxidative status (TOS) of the blood plasma before and after the anaerobic test, and the maintenance of IgA immunoglobulin levels throughout the experiment, as evidenced by the beneficial effect of the probiotic supplementation on the pro-oxidative and antioxidant status of the participants and their levels of immunity.
- Due to the observed positive effects of probiotics with respect to reducing inflammation, sealing the intestinal barrier, increasing aerobic capacity, decreasing lactate concentration post-exercise, and alleviating the feeling of heaviness in all the exercise tests, it is justified to recommend probiotic supplementation to cyclists and to pay attention to the consumption of food products that are a source of probiotics.
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jäger, R.; Purpura, M.; Stone, J.D.; Turner, S.M. Probiotic Streptococcus thermophilus FP4 and Bifidobacterium breve BR03 supplementation attenuates performance and range-of-motion decrements following muscle damaging exercise. Nutrients 2016, 8, 642. [Google Scholar] [CrossRef]
- Jäger, R.; Shields, K.A.; Lowery, R.P.; De Souza, E.O. Probiotic Bacillus coagulants GBI-30, 6086 reduces exercise-induced muscle damage and increases recovery. Peer J. 2016, 4, e2276. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Mohr, A.E.; Carpenter, K.C.; Kerksick, C.M.; Purpura, M. International Society of Sports Nutrition Position Stand: Probiotics. J. Int. Soc. Sports Nutr. 2019, 16, 62. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pastén, A.; Pérez-Hernández, N.; Añorve-Morga, J.; Jiménez-Alvarado, R.; Cariño-Cortés, R.; Sosa-Lozada, T.; Fernández-Martínez, E. The Activity of Prebiotics and Probiotics in Hepatogastrointestinal Disorders and Diseases Associated with Metabolic Syndrome. Int. J. Mol. Sci. 2022, 23, 7229. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewska, Z.; Zawartka, A.; Schab, M.; Martyniak, A.; Skoczen, S.; Tomasik, P.J.; Wedrychowicz, A. Prebiotics, Probiotics, and Postbiotics in the Prevention and Treatment of Anemia. Microorganisms 2022, 10, 1330. [Google Scholar] [CrossRef]
- Peters, H.; Vanberge-Henegouwen, G.; de Vries, W.R. Potential benefits and hazards of physical activity and exercise on the gastrointestinal tract. Gut 2001, 48, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Marlicz, W. Metabolic Disorders Forum. Via Med. 2014, 5, 129–140. [Google Scholar]
- Marlicz, W.; Starzyńska, T.; Marlicz, K. Patient with irritable bowel syndrome in the practice of a gastroenterologist. Gastroenterol. Pol. 2013, 20, 61–68. [Google Scholar]
- Mazur-Kurach, P.; Soboń, T.; Klimek, A.T.; Frączek, B.; Klimek-Jelonek, A. Effects of probiotic supplementation on selected health indices among a group of competitive cyclists (effect of probiotic therapy on health indices). JKES 2021, 90, 33–44. [Google Scholar] [CrossRef]
- ter Steege, R.W.; Van der Palen, J.; Kolkman, J.J. Prevalence of gastrointestinal complaints in runners competing in a long-distance run: An internet-based observational study in 1281 subjects. Scand. J. Gastroenterol. 2008, 43, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Isolauri, E.; Laitinen, K.; Salminen, S. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am. J. Clin. Nutr. 2008, 88, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Mazzawi, T. Gut Microbiota Manipulation in Irritable Bowel Syndrome. Microorganisms 2022, 10, 1332. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Bishop, N.C.; Oliveira, M. Daily probiotic’s (Lactobacillus casei Shirota) reduction of infection incidence in athletes. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Kekkonen, R.A.; Vasankari, T.J.; Vuorimaa, T.; Haahtela, T.; Julkunen, I.; Korpela, R. The effect of probiotics on respiratory infections and gastrointestinal symptoms during training in marathon runners. Int. J. Sport Nutr. Exerc. Metab. 2007, 17, 352–363. [Google Scholar] [CrossRef]
- Mengheri, E. Health, probiotics, and inflammation. J. Clin. Gastroenterol. 2008, 42, 177–178. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H. Obesity, metabolic syndrome and microbiota: Multiple interactions. J. Clin. Gastroenterol. 2010, 44, 16–18. [Google Scholar] [CrossRef]
- Fuller, R. What is a probiotic? Biologist 2004, 51, 232. [Google Scholar]
- Ng, S.C.; Hart, A.L.; Kamm, M.A.; Stagg, A.J.; Knight, S.C. Mechanisms of action of probiotics: Recent advances. Inflamm. Bowel Dis. 2009, 15, 300–310. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Gomez-Llorente, C.; Fontana, L.; Gil, A. Modulation of immunity and inflammatory gene expression in the gut, in inflammatory diseases of the gut and in the liver by probiotics. World J. Gastroenterol. 2014, 20, 15632–15649. [Google Scholar] [CrossRef]
- Miettinen, M.; Vuopio-Varkila, J.; Varkila, K. Production of human tumor necrosis factor alpha, interleukin-6, and interleukin-10 is induced by lactic acid bacteria. Infect. Immun. 1996, 64, 5403–5405. [Google Scholar] [CrossRef]
- Nazemian, V.; Shadnoush, M.; Manaheji, H.; Zaringhalam, J. Probiotics and inflammatory pain: A literature review study. Middle East J. Rehab. Health 2016, 3, e36087. [Google Scholar] [CrossRef]
- Ohland, C.L.; Macnaughton, W.K. Probiotic bacteria and intestinal epithelial barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G807–G819. [Google Scholar] [CrossRef] [PubMed]
- Korpela, K.; Salonen, A.; Vepsäläinen, O.; Suomalainen, M.; Kolmeder, C.; Varjosalo, M.; Miettinen, S.; Kukkonen, K.; Savilahti, E.; Kuitunen, M. Probiotic supplementation restores normal microbiota composition and function in antibiotic-treated and in caesarean-born infants. Microbiome 2018, 6, 182. [Google Scholar] [CrossRef] [PubMed]
- Hibberd, A.; Yde, C.; Ziegler, M.; Honoré, A.H.; Saarinen, M.T.; Lahtinen, S.; Stahl, B.; Jensen, H.; Stenman, L. Probiotic or synbiotic alters the gut microbiota and metabolism in a randomised controlled trial of weight management in overweight adults. Benef. Microbes 2019, 10, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Eutamene, H.; Bueno, L. Role of probiotics in correcting abnormalities of colonic flora induced by stress. Gut 2007, 56, 1495–1497. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Kim, N.; Yun, M.; Oh, Y.J.; Choi, H.J. Mind-altering with the gut: Modulation of the gut-brain axis with probiotics. J. Microbiol. 2018, 56, 172–182. [Google Scholar] [CrossRef]
- Santosa, S.; Farnworth, E.; Jones, P.J. Probiotics and their potential health claims. Nutrients 2019, 11, 18–23. [Google Scholar] [CrossRef]
- Kuebutornye, F.K.A.; Abarike, E.D.; Lu, Y. A review on the application of Bacillus as probiotics in aquaculture. Fish Shellfish Immunol. 2019, 87, 820–828. [Google Scholar] [CrossRef]
- Aya, V.; Flórez, A.; Perez, L.; Ramírez, J.D. Association between physical activity and changes in intestinal microbiota composition: A systematic review. PLoS ONE 2021, 16, e0247039. [Google Scholar] [CrossRef]
- Clancy, R.L.; Gleeson, M.; Cox, A.; Callister, R.; Dorrington, M.; D’Este, C.; Pang, G.; Pyne, D.; Fricker, P.; Henriksson, A. Reversal in fatigued athletes of a defect in interferon csecretion after administration of Lactobacillus acidophilus. Br. J. Sports Med. 2006, 40, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Pyne, D.; West, N.; Cox, A.; Cripps, A. Probiotics supplementation for athletes—Clinical and physiological effects. Eur. J. Sport Sci. 2015, 15, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Salarkia, N.; Ghadamli, L.; Zaeri, F.; Sabaghian Rad, L. Effects of probiotic yogurt on performance, respiratory and digestive systems of young adult female endurance swimmers: A randomized controlled trial. Med. J. Islam. Repub. Iran 2013, 27, 141–146. [Google Scholar]
- Salehzadeh, K. The effects of probiotic yogurt drink on lipid profile, CRP, and record changes in aerobic athletes. Life Sci. 2015, 9, 32–37. [Google Scholar] [CrossRef]
- Pugh, J.N.; Fearn, R.; Morton, J.P.; Close, G.L. Gastrointestinal symptoms in elite athletes: Time to recognize the problem? Br. J. Sports Med. 2018, 52, 487–488. [Google Scholar] [CrossRef] [PubMed]
- Klaenhammer, T.R.; Kleerebezem, M.; Kopp, M.V.; Rescigno, M. The impact of probiotics and prebiotics on the immune system. Nat. Rev. Immunol. 2012, 12, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Sang, L.X.; Chang, B.; Zhang, W.L.; Wu, X.M.; Li, X.H.; Jiang, M. Remission induction and maintenance effect of probiotics on ulcerative colitis: A meta-analysis. World J. Gastroenterol. 2010, 16, 1908–1915. [Google Scholar] [CrossRef]
- Lucía, A.; Rivero, J.L.; Pérez, M.; Serrano, A.L.; Calbet, J.A.; Santalla, A.; Chicharro, J.L. Determinants of VO(2) kinetics at high power outputs during a ramp exercise protocol. Med. Sci. Sports Exerc. 2002, 34, 326–331. [Google Scholar]
- Calero, C.D.Q.; Rincón, E.O.; Marqueta, P.M. Probiotics, prebiotics and synbiotics: Useful for athletes and active individuals? A systematic review. Benef. Microbes 2020, 11, 135–149. [Google Scholar] [CrossRef]
- O’Brien, K.V.; Stewart, L.K.; Forney, L.A.; Aryana, K.J.; Prinyawiwatkul, W.; Boeneke, C.A. The effects of postexercise consumption of a kefir beverage on performance and recovery during intensive endurance training. J. Dairy Sci. 2015, 98, 7446–7449. [Google Scholar] [CrossRef]
- Martarelli, D.; Verdenelli, M.C.; Scuri, S.; Cocchioni, M.; Silvi, S.; Cecchini, C.; Pompei, P. Effect of a probiotic intake on oxidant and antioxidant parameters in plasma of athletes during intense exercise training. Curr. Microbiol. 2011, 62, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Shing, C.M.; Peake, J.M.; Leong, C.; David, L.; Neil, B.; Fortes, M.B.; Vitetta, L. Effects of probiotics supplementation on gastrointestinal permeability, inflammation and exercise performance in the heat. Eur. J. Appl. Physiol. 2013, 114, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Marshall, H.; Catherine, B.; Chrismas, R.; Suckling, C.A.; Roberts, J.D.; Foster, J.; Taylor, L. Chronic probiotic supplementation with or without glutamine does not influence the eHsp72 response to a multi-day ultra-endurance exercise event. Appl. Physiol. Nutr. Metab. 2017, 42, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, M.; Frauwallner, A. Exercise, intestinal barrier dysfunction and probiotic supplementation. Acute Top. Sport Nutr. 2012, 59, 47–56. [Google Scholar]
- Cairns, S.P. Lactic Acid and Exercise Performance. Sports Med. 2006, 36, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.S.; Muhamad, A.S.; Ooi, F.K.; Meor-Osman, J.; Chen, C.K. The effects of combined probiotic ingestion and circuit training on muscular strength and power and cytokine responses in young males. Appl. Physiol. Nutr. Metab. 2017, 43, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Marinkovic, D.; Minic, R.; Dikic, N.; Andjelkovic, M.; Kostic-Vucicevic, M.; Stojmenovic, T.; Djordjevic, B. Lactobacillus helveticus Lafti L10 supplementation reduces respiratory infection duration in a cohort of elite athletes: A randomized, double-blind, placebo-controlled trial. Appl. Physiol. Nutr. Metab. 2016, 41, 782–789. [Google Scholar]
- Buśko, K. Influence of two high-intensity intermittent training programmes on anaerobic capacity in humans. Biol. Sport 2011, 28, 23–30. [Google Scholar] [CrossRef]
- Popadic-Gacesa, J.Z.; Barak, O.F.; Grujic, N.G. Maximal anaerobic power test in athletes of different sport disciplines. J. Strength Cond. Res. 2009, 23, 751–755. [Google Scholar] [CrossRef]
- Winter, E.M.; MacLaren, D.P. Assessment of Maximal Intensity Exercise. In Kinanthropometry and Exercise Physiology Laboratory Manual: Tests, Procedures and Data: Volume Two: Physiology, 3rd ed.; Eston, R., Reilly, T., Eds.; Routledge: Abingdon, UK, 2009; pp. 307–334. [Google Scholar]
- Zupan, M.F.; Arata, A.W.; Dawson, L.H.; Wile, A.L.; Payn, T.L.; Hannon, M.E. Wingate Anaerobic Test peak power and anaerobic capacity classifications for men and women intercollegiate athletes. J. Strength Cond. Res. 2009, 23, 2598–2604. [Google Scholar] [CrossRef]
- Yu, Y.; Deck, J.A.; Hunsaker, L.A.; Deck, L.M.; Royer, R.E.; Goldberg, E.; Vander Jagt, D.L. Selective active site inhibitors of human lactate dehydrogenases A4, B4, and C4. Biochem. Pharmacol. 2001, 62, 81–89. [Google Scholar] [CrossRef]
- Fasano, A. Zonulin and ist regulation of intestinal barrier function: The biological door to inflammation, autoimmunity and cancer. Physiol. Rev. 2011, 91, 151–175. [Google Scholar] [CrossRef] [PubMed]
- Sapone, A.; de Magistris, L.; Pietzak, M.; Clemente, M.G.; Tripathi, A.; Cucca, F.; Lampis, R.; Kryszak, D.; Carteni, M.; Generoso, M.; et al. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes 2006, 55, 1443–1449. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Uzzau, S.; Goldblum, S.E.; Fasano, A. Human zonulin, a potential modulator of intestinal tight junctions. J. Cell Sci. 2000, 113, 4435–4440. [Google Scholar] [CrossRef]
- Viljanen, M.; Kuitunen, M.; Haahtela, T.; Junnteunen-Backman, K.; Korpela, R. Probiotic effects on faecal inflammatory markers and on faecal IgA in food allergic atopic egzema/dermatitis syndrome infants. Pediatr. Allergy Immunol. 2005, 16, 65–71. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Oliveira, M.; McCauley, T.; Tauler, P.; Lawrence, C. Effects of a Lactobacillus salivarius probiotic intervention on infection, cold symptom duration and severity and mucosal immunity in endurance athletes. Int. J. Sport Nutr. Exerc. Metab. 2012, 2, 235–245. [Google Scholar] [CrossRef]
- Cox, A.J.; Pyne, D.B.; Saunders, P.U.; Fricker, P.A. Oral administration of the probiotic Lactobacillus fermentum VRI-003 and mucosal immunity in endurance athletes. Br. J. Sports Med. 2010, 44, 222–226. [Google Scholar] [CrossRef]
- Foster, S.L.; Hargreaves, D.C.; Medzhitov, R. Gene-specific control of inflammation by TLR-induced chromatin modification. Nature 2007, 447, 972–978. [Google Scholar] [CrossRef]
- Round, J.L.; Lee, S.M.; Li, J.; Jabri, B.; Chatila, T.A.; Mazmanian, S.K. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 2011, 332, 974–977. [Google Scholar] [CrossRef]
- West, N.P.; Pyne, D.B.; Cripps, A.W.; Christophersen, C.T.; Michael, A.; Fricker, P.A. Gut Balance, a symbiotic supplement, increases fecal Lactobacillus paracasei but has little effect on immunity in healthy physically active individuals. Gut Microbes 2012, 3, 221–227. [Google Scholar] [CrossRef]
- Hughes, R.L.; Holscher, H.D. Fueling Gut Microbes: A Review of the Interaction between Diet, Exercise, and the Gut Microbiota in Athletes. Adv. Nutr. 2021, 12, 2190–2215. [Google Scholar] [CrossRef] [PubMed]
- West, N.P.; Pyne, D.B.; Cripps, A.W.; Hopkins, W.G.; Eskesen, D.C.; Jairath, A. Lactobacillus fermentum (PCCs) supplementation and gastrointestinal and respiratory-tract illness symptoms: A randomised control trial in athletes. Nutr. J. 2011, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.; Kekkonen, R.; Korpela, R.; Delgado, L.; Haahtela, T. Allergy in marathon runners and effect of Lactobacillus GG supplementation on allergic inflammatory markers. Respir. Med. 2007, 101, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Brand, A. Passenger leukocytes, cytokines, and transfusion reactions. Engl. J. Med. 1994, 331, 670–671. [Google Scholar] [CrossRef] [PubMed]
- Heddle, N.M. Pathophysiology of febrile nonhemolytic transfusion reactions. Curr. Opin. Hematol. 1999, 6, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.; Anwar, A.; Fragen, M.; Rananto, C.; Johnson, R.; Holbert, D. Cytokines and cell adhesion molecules associated with high-intensity eccentric exercise. Eur. J. Appl. Physiol. 2000, 82, 61–67. [Google Scholar] [CrossRef]
- Hirose, L.; Nosaka, K.; Newton, M.; Laveder, A.; Kano, M.; Peake, J.; Suzuki, K. Changes in inflammatory mediators following eccentric exercise of the elbow flexors. Exerc. Immunol. Rev. 2004, 10, 20. [Google Scholar]
- Willoughby, D.; McFarlin, B.; Bois, C. Interleukin-6 expression after repeated bouts of eccentric exercise. Int. J. Sports Med. 2003, 24, 15–21. [Google Scholar] [CrossRef]
- Thomas, L.V.; Ockhuizen, T. New insights into the impact of the intestinal microbiota on health and disease: A symposium report. Br. J. Nutr. 2012, 1, 1–13. [Google Scholar] [CrossRef]
- Gill, H.S.; Teixeira, S.K.; Rosado, A.M.; Cox, F.; Costa, M. High-dose probiotic supplementation containing Lactobacillus casei for 7 days does not enhance salivary antimicrobial protein responses to exertional heat stress compared with placebo. Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 150–160. [Google Scholar] [CrossRef]
- Tiollier, E. Effect of a Probiotics Supplementation on Respiratory Infections and Immune and Hormonal Parameters during Intense Military Training Guarantor. Mil. Med. 2007, 172, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.S.; Tzeng, C.H.; Hu, H.Y. Cytokine release in febrile non-haemolytic red cell transfusion reactions. Vox Sang 2002, 82, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Watson, T.A.; Callister, R.; Taylor, R.D.; Sibbritt, D.W.; MacDonald-Wicks, L.K.; Garg, M.L. Antioxidant restriction and oxidative stress in short-duration exhaustive exercise. Med. Sci. Sports Exerc. 2005, 37, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Fisher-Wellman, K.; Bloomer, R.J. Acute exercise and oxidative stress a 30-year history. Dyn. Med. 2009, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- McArdle, A.; Jackson, M.J. Exercise, oxidative stress and ageing. J. Anat. 2000, 197, 539–541. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, M.G.; Kyparos, A.; Hadziioannou, M.; Panou, N.; Samaras, I.; Jamurtas, A.Z.; Kouretas, D. Acute exercise markedly increases blood oxidative stress in boys and girls. Appl. Physiol. Nutr. Metab. 2007, 32, 197–206. [Google Scholar] [CrossRef]
- Shing, C.M.; Peak, J.M.; Ahern, S.M.; Strobel, N.A.; Wilson, G.; Jenkins, D.G.; Coombes, J.S. The effect of consecutive days of exercise on markers of oxidative stress. Appl. Physiol. Nutr. Metab. 2007, 32, 677. [Google Scholar] [CrossRef]
- Charkhi, S.A.; Ghram, A.; Soori, R.; Akbarnejad, A.; Azizi Ghuchan, F.; Zare, M. Purslane supplementation lowers oxidative stress, inflammatory and muscle damage biomarkers after high-intensity intermittent exercise in female runners. Balt. J. Health Phys. Activ. 2021, 13, 17–27. [Google Scholar] [CrossRef]


| Indicator/Measurement | Probiotic Group (P) | Control Group (C) | p | |||||
|---|---|---|---|---|---|---|---|---|
| x | SD | SE | x | SD | SE | |||
| VO2max [mL/kg/min] | baseline | 65.28 * | 6.00 | 1.66 | 58.03 * | 0.52 | 2.92 | p = 0.1336 |
| 4-weeks | 63.62 * | 5.79 | 1.60 | 58.45 * | 7.99 | 2.22 | p = 0.0069 | |
| 12-weeks | 65.03 | 5.77 | 1.60 | 60.36 * | 8.38 | 2.33 | p = 0.45 | |
| 16-weeks | 69.18 *# | 5.69 | 1.58 | 57.03 | 6.69 | 1.86 | p = 0.006 | |
| t [min] | baseline | 14.35 | 1.80 | 0.50 | 14.92 | 1.12 | 0.31 | p = 1.0000 |
| 4-weeks | 15.00 | 1.15 | 0.32 | 14.88 | 1.75 | 0.48 | p = 0.9483 | |
| 12-weeks | 15.12 | 2.11 | 0.58 | 15.04 | 2.14 | 0.59 | p = 0.7 | |
| 16-weeks | 15.65 # | 2.02 | 0.56 | 15.23 | 1.89 | 0.53 | p = 1.0000 | |
| Pmax [W/kg] | baseline | 5.11 | 0.68 | 0.19 | 4.83 | 0.71 | 0.20 | p = 0.3531 |
| 4-weeks | 5.29 | 0.47 | 0.13 | 4.88 | 0.81 | 0.23 | p = 0.70 | |
| 12-weeks | 5.38 | 0.68 | 0.19 | 4.91 | 0.85 | 0.24 | p = 0.9323 | |
| 16-weeks | 5.36 | 0.60 | 0.17 | 4.86 | 0.79 | 0.22 | p = 0.7595 | |
| HRmax [bpm] | baseline | 193.3 * | 3.01 | 0.84 | 182.8 * | 7.74 | 2.15 | p = 0.0031 |
| 4-weeks | 193.8 * | 4.24 | 1.18 | 182.8 * | 7.69 | 2.13 | p = 0.003 | |
| 12-weeks | 192.0 * | 6.76 | 1.87 | 184.0 * | 0.47 | 2.90 | p = 0.003 | |
| 16-weeks | 188.6 *# | 7.08 | 1.96 | 182.9 * | 9.36 | 2.60 | p = 0.01 | |
| Indicator/Measurement | Probiotic Group (P) | Control Group (C) | |||||
|---|---|---|---|---|---|---|---|
| x | SD | SE | x | SD | SE | ||
| LA [mmol/L] pre-exercise | baseline | 2.33 | 0.48 | 0.13 | 1.78 | 0.38 | 0.11 |
| 4-weeks | 3.09 | 1.80 | 0.50 | 2.14 | 0.39 | 0.11 | |
| 12-weeks | 2.71 | 1.80 | 0.50 | 1.84 | 0.24 | 0.07 | |
| 16-weeks | 2.34 | 0.87 | 0.24 | 2.57 | 0.64 | 0.18 | |
| LA [mmol/L] 3 min post-exercise | baseline | 13.42 | 1.39 | 0.38 | 12.38 | 2.50 | 0.69 |
| 4-weeks | 14.36 | 1.97 | 0.55 | 13.44 | 3.44 | 0.95 | |
| 12-weeks | 12.39 | 2.51 | 0.70 | 12.95 | 3.03 | 0.84 | |
| 16-weeks | 12.36 | 2.94 | 0.81 | 12.24 | 2.61 | 0.72 | |
| LA [mmol/L] 10 min post-exercise | baseline | 11.12 | 1.25 | 0.35 | 10.26 | 2.74 | 0.76 |
| 4-weeks | 11.67 | 1.74 | 0.48 | 10.03 | 2.65 | 0.73 | |
| 12-weeks | 9.95 | 2.87 | 0.80 | 9.48 | 3.49 | 0.97 | |
| 16-weeks | 8.90 # | 2.76 | 0.76 | 10.22 | 2.86 | 0.79 | |
| LA [mmol/L] 20 min post-exercise | baseline | 7.16 | 1.53 | 0.43 | 6.53 | 2.54 | 0.70 |
| 4-weeks | 8.55 | 2.15 | 0.60 | 7.11 | 2.22 | 0.62 | |
| 12-weeks | 6.14 | 1.85 | 0.51 | 6.02 | 2.86 | 0.79 | |
| 16-weeks | 6.13 # | 2.16 | 0.60 | 6.82 | 2.43 | 0.67 | |
| Measurement | Probiotic Group (P) | Control Group (C) | p | ||||
|---|---|---|---|---|---|---|---|
| x | SD | SE | x | SD | SE | ||
| baseline | 19.38 | 0.48 | 0.13 | 19.30 | 0.72 | 0.20 | p = 0.9542 |
| 4-weeks | 19.30 | 0.6 | 0.16 | 19.53 | 0.49 | 0.13 | p = 0.3674 |
| 12-weeks | 19.00 | 0.55 | 0.15 | 19.07 | 0.88 | 0.22 | p = 0.78 |
| 16-weeks | 18.43 * | 0.6 | 0.16 | 19.38 * | 0.73 | 0.20 | p = 0.0026 |
| Indicator/Measurement | Probiotic Group (P) | Control Group (C) | p | |||||
|---|---|---|---|---|---|---|---|---|
| x | SD | SE | x | SD | SE | |||
| Wglob. [kJ] | baseline | 12.59 | 1.18 | 0.33 | 13.84 | 1.49 | 0.41 | p = 0.1 |
| 4-weeks | 12.69 | 1.10 | 0.31 | 13.91 | 1.50 | 0.42 | p = 0.08 | |
| 12-weeks | 12.78 | 0.98 | 0.27 | 13.63 | 1.74 | 0.48 | p = 0.82 | |
| 16-weeks | 12.83 | 0.07 | 0.30 | 13.64 | 1.69 | 0.47 | p = 0.94 | |
| MAP [W] | baseline | 742.7 | 72.31 | 2 0.05 | 816.6 | 99.44 | 27.58 | p = 0.07 |
| 4-weeks | 753.0 | 69.46 | 19.27 | 817.6 | 102.8 | 28.51 | p = 0.22 | |
| 12-weeks | 746.1 | 61.83 | 17.15 | 791.6 | 108.5 | 30.08 | p = 0.71 | |
| 16-weeks | 742.0 | 65.52 | 18.17 | 801.1 | 115.4 | 22.01 | p = 0.94 | |
| MAP [W·kg−1] | baseline | 11.69 | 1.09 | 0.30 | 11.67 | 2.56 | 0.10 | p = 0.94 |
| 4-weeks | 11.71 | 1.05 | 0.2 | 11.61 | 0.5 | 0.24 | p = 0.472 | |
| 12-weeks | 11.60 | 0.79 | 0.22 | 11.77 | 2.60 | 0.31 | p = 0.472 | |
| 16-weeks | 11.66 | 0.76 | 0.21 | 11.84 | 2.61 | 0.29 | p = 0.474 | |
| P [W] | baseline | 629.5 | 58.96 | 16.35 | 692.1 | 74.44 | 20.65 | p = 0.06 |
| 4-weeks | 634.4 | 55.40 | 15.37 | 695.6 | 75.07 | 20.82 | p = 0.08 | |
| 12-weeks | 638.7 | 48.90 | 13.56 | 680.4 | 85.50 | 23.71 | p = 0.06 | |
| 16-weeks | 641.5 | 53.66 | 14.88 | 682.5 | 85.97 | 23.85 | p = 0.655 | |
| P [W·kg−1] | baseline | 9.92 | 0.93 | 0.26 | 9.75 | 0.46 | 0.13 | p = 0.4 |
| 4-weeks | 9.87 | 0.85 | 0.23 | 9.89 | 0.51 | 0.14 | p = 0.06 | |
| 12-weeks | 9.93 | 0.63 | 0.17 | 9.55 | 0.69 | 0.19 | p = 0.26 | |
| 16-weeks | 10.00 | 0.66 | 0.18 | 9.66 | 0.63 | 0.17 | p = 0.261 | |
| to [s] | baseline | 4.26 | 0.76 | 0.21 | 3.83 | 0.48 | 0.13 | p = 0.31 |
| 4-weeks | 3.94 | 0.60 | 0.17 | 3.78 | 0.25 | 0.07 | p = 0.062 | |
| 12-weeks | 3.91 | 0.45 | 0.13 | 3.59 | 0.33 | 0.09 | p = 0.472 | |
| 16-weeks | 4.01 | 0.58 | 0.16 | 3.67 | 0.27 | 0.08 | p = 0.65 | |
| tu [s] | baseline | 3.39 | 0.87 | 0.24 | 3.31 | 0.43 | 0.12 | p = 0.7 |
| 4-weeks | 3.16 | 0.67 | 0.19 | 3.16 | 0.30 | 0.08 | p = 1.00 | |
| 12-weeks | 3.18 | 0.52 | 0.14 | 3.36 | 0.46 | 0.13 | p = 0.089 | |
| 16-weeks | 3.42 | 0.45 | 0.12 | 3.04 | 0.67 | 0.19 | p = 0.08 | |
| Indicator/Measurement | Probiotic Group (P) | Control Group (C) | |||||
|---|---|---|---|---|---|---|---|
| x | SD | SE | x | SD | SE | ||
| LA [mmol/L] pre-exercise | baseline | 2.18 | 0.31 | 0.09 | 1.92 | 0.52 | 0.14 |
| 4-weeks | 2.06 | 0.41 | 0.11 | 2.09 | 0.53 | 0.15 | |
| 12-weeks | 2.13 | 0.49 | 0.13 | 2.12 | 0.44 | 0.12 | |
| 16-weeks | 1.76 | 0.44 | 0.12 | 2.03 | 0.43 | 0.12 | |
| LA [mmol/L] 3 min post-exercise | baseline | 11.14 | 1.86 | 0.52 | 11.59 | 1.98 | 0.55 |
| 4-weeks | 13.16 | 2.11 | 0.59 | 11.93 | 3.01 | 0.83 | |
| 12-weeks | 10.43 | 1.63 | 0.45 | 10.21 | 2.26 | 0.63 | |
| 16-weeks | 10.05 # | 0.87 | 0.24 | 11.52 | 2.28 | 0.63 | |
| LA [mmol/L] 10 min post-exercise | baseline | 11.23 | 1.88 | 0.52 | 11.71 | 2.46 | 0.68 |
| 4-weeks | 11.51 | 1.81 | 0.50 | 10.45 | 3.32 | 0.92 | |
| 12-weeks | 9.34 | 1.50 | 0.42 | 8.76 | 2.10 | 0.58 | |
| 16-weeks | 9.55 # | 1.63 | 0.45 | 9.30 | 2.09 | 0.58 | |
| LA [mmol/L] 20 min post-exercise t | baseline | 7.55 | 1.85 | 0.51 | 8.26 | 1.60 | 0.44 |
| 4-weeks | 7.16 | 2.09 | 0.58 | 7.80 | 2.17 | 0.60 | |
| 12-weeks | 6.60 | 1.25 | 0.35 | 7.45 | 2.13 | 0.59 | |
| 16-weeks | 5.67 # | 1.22 | 0.34 | 7.65 | 1.91 | 0.53 | |
| Indicator/Measurement | Probiotic Group (P) | Control Group (C) | |||||
|---|---|---|---|---|---|---|---|
| x | SD | SE | x | SD | SE | ||
| Body weight [kg] | baseline | 63.52 | 3.77 | 1.05 | 65.17 | 8.44 | 2.34 |
| 4-weeks | 63.97 | 4.26 | 1.18 | 64.14 | 7.55 | 2.09 | |
| 12-weeks | 64.14 | 4.41 | 1.22 | 65.92 | 7.35 | 2.04 | |
| 16-weeks | 63.92 | 4.15 | 1.15 | 66.52 | 7.45 | 2.07 | |
| BMI [kg/m2] | baseline | 20.80 | 1.11 | 0.31 | 21.79 | 2.04 | 0.57 |
| 4-weeks | 20.95 | 1.33 | 0.37 | 21.18 | 1.90 | 0.53 | |
| 12-weeks | 20.92 | 1.20 | 0.33 | 21.77 | 1.47 | 0.41 | |
| 16-weeks | 21.06 | 1.03 | 0.29 | 21.97 | 1.26 | 0.35 | |
| FFM [%] | baseline | 92.15 | 2.60 | 0.72 | 90.59 | 5.17 | 1.43 |
| 4-weeks | 92.63 | 3.75 | 1.04 | 91.00 | 5.86 | 1.63 | |
| 12-weeks | 93.00 | 13.33 | 3.70 | 89.60 | 6.47 | 1.79 | |
| 16-weeks | 96.10 | 3.27 | 0.91 | 90.11 | 6.12 | 1.70 | |
| FM [%] | baseline | 7.85 | 2.60 | 0.72 | 9.41 | 5.17 | 1.43 |
| 4-weeks | 7.37 | 3.75 | 1.04 | 9.00 | 5.97 | 1.66 | |
| 12-weeks | 7.00 | 3.95 | 1.10 | 9.40 | 6.47 | 1.79 | |
| 16-weeks | 5.9 | 2.82 | 0.78 | 9.89 | 6.12 | 1.70 | |
| MM [%] | baseline | 51.95 * | 2.28 | 0.63 | 47.78 * | 8.66 | 2.40 |
| 4-weeks | 53.40 * | 2.82 | 0.78 | 48.95 * | 9.33 | 2.59 | |
| 12-weeks | 55.00 * | 4.06 | 1.13 | 53.09 * | 4.39 | 1.22 | |
| 16-weeks | 58.68 *# | 6.19 | 1.72 | 52.83 * | 4.02 | 1.12 | |
| Indicator/Measurement | Probiotic Group (P) | Control Group (C) | p | |||||
|---|---|---|---|---|---|---|---|---|
| x | SD | SE | x | SD | SE | |||
| Ig A [mg/mL] | baseline | 1.85 | 1.13 | 0.31 | 1.59 | 0.59 | 0.16 | p = 0.31 |
| 4-weeks | 1.88 | 1.01 | 0.28 | 1.58 | 0.58 | 0.16 | p = 0.38 | |
| 12-weeks | 1.95 | 1.00 | 0.28 | 1.58 | 0.60 | 0.17 | p = 0.3583 | |
| 16-weeks | 2.03 # | 0.98 | 0.27 | 1.61 | 0.60 | 0.17 | p = 0.9539 | |
| Indicator/Measurement | Probiotic Group (P) | Control Group (C) | p | |||||
|---|---|---|---|---|---|---|---|---|
| x | SD | SE | x | SD | SE | |||
| TNF-α before aerobic test | baseline | 9.65 * | 5.84 | 1.62 | 14.26 * | 5.32 | 1.47 | p = 0.03 |
| 4-weeks | 14.29 * | 7.71 | 2.14 | 20.59 * | 5.60 | 1.55 | p = 0.0001 | |
| 12-weeks | 9.54 * | 4.08 | 1.13 | 15.26 * | 7.58 | 2.10 | p = 0.0204 | |
| 16-weeks | 10.15 * | 2.76 | 0.77 | 11.72 * | 8.11 | 2.25 | p = 0.0471 | |
| TNF-α after aerobic test | baseline | 13.88 * | 5.90 | 1.64 | 11.28 * | 4.21 | 1.17 | p = 0.0018 |
| 4-weeks | 11.39 *# | 6.01 | 1.67 | 25.32 * | 10.51 | 2.92 | p = 0.0001 | |
| 12-weeks | 9.75 *# | 3.89 | 1.08 | 20.62 * | 10.96 | 3.04 | p = 0.0175 | |
| 16-weeks | 9.84 *# | 4.30 | 1.19 | 14.74 * | 10.36 | 2.87 | p = 0.031 | |
| TNF-α before anaerobic test | baseline | 7.34 | 3.05 | 0.85 | 13.49 | 8.65 | 2.40 | p = 0.2719 |
| 4-weeks | 10.71 | 5.08 | 1.41 | 10.35 | 1.91 | 0.53 | p = 0.9326 | |
| 12-weeks | 8.85 | 4.12 | 1.14 | 10.55 | 2.81 | 0.78 | p = 0.7893 | |
| 16-weeks | 9.42 | 5.09 | 1.41 | 9.32 | 3.43 | 0.95 | p = 0.742 | |
| TNF-α after anaerobic test | baseline | 8.54 * | 6.37 | 1.77 | 21.71 * | 7.26 | 2.01 | p = 0.0035 |
| 4-weeks | 10.48 * | 6.77 | 1.88 | 16.81 * | 8.96 | 2.48 | p = 0.0445 | |
| 12-weeks | 7.78 *# | 2.89 | 0.80 | 14.74 * | 9.97 | 2.76 | p = 0.0282 | |
| 16-weeks | 6.8 *# | 3.41 | 0.95 | 13.53 * | 11.02 | 3.06 | p = 0.0204 | |
| Indicator/Measurement | Probiotic Group (P) | Control Group (C) | |||||
|---|---|---|---|---|---|---|---|
| x | SD | SE | x | SD | SE | ||
| IL-1β [pg/mL] before aerobic test | baseline | 0.065 | 0.041 | 0.022 | 0.109 | 0.021 | 0.041 |
| 16 weeks | 0.094 | 0.056 | 0.033 | 0.103 | 0.060 | 0.039 | |
| IL-1β [pg/mL] after aerobic test | baseline | 0.084 | 0.024 | 0.029 | 0.083 | 0.052 | 0.031 |
| 16 weeks | 0.110 | 0.064 | 0.038 | 0.082 | 0.228 | 0.031 | |
| IL-1β [pg/mL] before anaerobic test | baseline | 0.107 | 0.051 | 0.037 | 0.066 | 0.030 | 0.025 |
| 16 weeks | 0.094 | 0.056 | 0.033 | 0.075 | 0.015 | 0.028 | |
| IL-1β [pg/mL] after anaerobic test | baseline | 0.097 | 0.029 | 0.034 | 0.071 | 0.002 | 0.026 |
| 16 weeks | 0.110 | 0.064 | 0.038 | 0.099 | 0.060 | 0.037 | |
| Indicator/Measurement | Probiotic Group (P) | Control Group (C) | p | |||||
|---|---|---|---|---|---|---|---|---|
| x | SD | SE | x | SD | SE | |||
| IL-6 [pg/mL] before aerobic test | baseline | 1.18 | 0.41 | 0.11 | 1.09 | 0.17 | 0.05 | p = 0.11 |
| 16-weeks | 0.80 | 0.27 | 0.08 | 1.23 | 0.46 | 0.13 | p = 0.0801 | |
| IL-6 [pg/mL] after aerobic test | baseline | 1.71 | 0.72 | 0.20 | 1.14 | 0.18 | 0.05 | p = 0.123 |
| 16-weeks | 1.76 | 1.00 | 0.28 | 1.65 | 0.24 | 0.07 | p = 0.4227 | |
| IL-6 [pg/mL] before anaerobic test | baseline | 1.20 * | 0.37 | 0.10 | 2.61 * | 2.26 | 0.63 | p = 0.0272 |
| 16-weeks | 0.86 * | 0.19 | 0.05 | 1.06 *# | 0.21 | 0.06 | p = 0.036 | |
| IL-6 [pg/mL] after anaerobic test | baseline | 1.47 | 0.45 | 0.12 | 1.57 | 0.63 | 0.17 | p = 0.5577 |
| 16-weeks | 0.97 # | 0.27 | 0.08 | 1.05 # | 0.31 | 0.09 | p = 0.9154 | |
| Indicator/Measurement | Probiotic Group (P) | Control Group (C) | p | |||||
|---|---|---|---|---|---|---|---|---|
| x | SD | SE | x | SD | SE | |||
| IL-8 [pg/mL] before aerobic test | baseline | 6.75 * | 1.12 | 0.31 | 4.70 * | 1.40 | 0.39 | p = 0.0086 |
| 16-weeks | 7.68 | 1.80 | 0.50 | 7.10 # | 1.10 | 0.30 | p = 0.1814 | |
| IL-8 [pg/mL] after aerobic test | baseline | 7.48 | 3.20 | 0.89 | 5.41 | 1.23 | 0.34 | p = 0.2334 |
| 16-weeks | 8.48 | 1.36 | 0.38 | 8.73 # | 2.50 | 0.69 | p = 0.1814 | |
| IL-8 [pg/mL] before anaerobic test | baseline | 7.38 | 2.65 | 0.73 | 6.70 | 1.00 | 0.28 | p = 0.9442 |
| 16-weeks | 6.87 | 1.39 | 0.39 | 6.57 | 1.03 | 0.28 | p = 0.7893 | |
| IL-8 [pg/mL] after anaerobic test | baseline | 6.71 | 1.39 | 0.38 | 6.11 | 0.41 | 0.11 | p = 0.9442 |
| 16-weeks | 6.65 | 2.02 | 0.56 | 5.48 | 2.38 | 0.66 | p = 0.7893 | |
| Indicator/Measurement | Probiotic Group (P) | Control Group (C) | p | |||||
|---|---|---|---|---|---|---|---|---|
| x | SD | SE | x | SD | SE | |||
| IL-10 [pg/mL] (before aerobic test) | baseline | 0.48 | 0.37 | 0.10 | 0.54 | 0.25 | 0.07 | p = 0.85 |
| 16-weeks | 0.45 | 0.28 | 0.08 | 0.36 | 0.19 | 0.05 | p = 0.115 | |
| IL-10 [pg/mL] (after aerobic test) | baseline | 0.76 | 0.72 | 0.20 | 0.57 | 0.35 | 0.10 | p = 0.266 |
| 16-weeks | 0.47 *# | 0.56 | 0.15 | 0.29 * | 0.11 | 0.03 | p = 0.023 | |
| IL-10 [pg/mL] (before anaerobic test) | baseline | 0.70 * | 0.43 | 0.12 | 0.39 * | 0.18 | 0.05 | p = 0.047 |
| 16-weeks | 0.44 | 0.25 | 0.07 | 0.35 | 0.19 | 0.05 | p = 0.12 | |
| IL-10 [pg/mL] (after anaerobic test) | baseline | 0.54 * | 0.33 | 0.09 | 0.31 * | 0.18 | 0.05 | p = 0.025 |
| 16-weeks | 0.48 * | 0.34 | 0.09 | 0.29 * | 0.14 | 0.04 | p = 0.023 | |
| Indicator/Measurement | Probiotic Group (P) | Control Group (C) | p | |||||
|---|---|---|---|---|---|---|---|---|
| x | SD | SE | x | SD | SE | |||
| TOS—before aerobic test | baseline | 352.9 | 226.6 | 62.85 | 562.9 | 406.4 | 112.7 | p = 0.11 |
| 4-weeks | 536.6 | 266.9 | 74.02 | 624.13 | 1786 | 495.4 | p = 0.19 | |
| 12-weeks | 492.4 | 192.3 | 53.34 | 480.9 | 40.30 | 11.18 | p = 0.833 | |
| 16-weeks | 498.6 | 242.4 | 67.23 | 625.1 | 365.6 | 101.4 | p = 0.309 | |
| TOS—after aerobic test | baseline | 546.9 | 284.7 | 78.96 | 757.1 | 197.7 | 54.83 | p = 0.22 |
| 4-weeks | 616.0 | 318.5 | 88.34 | 620.13 | 340.0 | 94.29 | p = 0.45 | |
| 12-weeks | 1023 | 722.7 | 200.4 | 957.3 | 370.2 | 102.7 | p = 0.772 | |
| 16-weeks | 692.1 | 470.9 | 130.6 | 770.3 | 153.8 | 42.65 | p = 0.574 | |
| TOS—before anaerobic test | baseline | 663.7 * | 433.3 | 120.2 | 783.9 * | 272.3 | 75.52 | p = 0.04 |
| 4-weeks | 497.7 * | 266.7 | 73.97 | 711.2 * | 280.0 | 77.67 | p = 0.048 | |
| 12-weeks | 536.8 * | 247.7 | 68.69 | 652.5 * | 189.9 | 52.66 | p = 0.019 | |
| 16-weeks | 484.6 *# | 235.8 | 65.41 | 918.1 * | 295.6 | 81.98 | p = 0.024 | |
| TOS—after anaerobic test | baseline | 643.1 * | 391.5 | 108.6 | 740.9 * | 245.3 | 68.04 | p = 0.045 |
| 4-weeks | 402.8 * | 191.2 | 53.03 | 1635 * | 1426 | 395.7 | p = 0.005 | |
| 12-weeks | 625.1 | 568.7 | 157.7 | 662.6 | 271.6 | 75.32 | p = 0.83 | |
| 16-weeks | 435.9 *# | 256.2 | 71.06 | 751.4 * | 292.6 | 81.14 | p = 0.034 | |
| Indicator/Measurement | Probiotic Group (P) | Control Group (C) | p | |||||
|---|---|---|---|---|---|---|---|---|
| x | SD | SE | x | SD | SE | |||
| TAS—before aerobic test | baseline | 302.3 | 36.39 | 10.09 | 319.6 | 34.95 | 9.69 | p = 0.229 |
| 4-weeks | 305.7 | 30.29 | 8.40 | 321.2 | 28.91 | 8.02 | p = 0.196 | |
| 12-weeks | 302.0 | 50.68 | 14.06 | 313.7 | 27.77 | 7.70 | p = 0.470 | |
| 16-weeks | 303.7 | 29.58 | 8.20 | 289.1 | 31.91 | 8.85 | p = 0.237 | |
| TAS—after aerobic test | baseline | 331.3 | 39.04 | 10.83 | 326.5 | 33.13 | 9.19 | p = 0.738 |
| 4-weeks | 307.1 | 38.83 | 10.77 | 294.8 | 36.01 | 9.99 | p = 0.41 | |
| 12-weeks | 338.4 | 51.21 | 14.20 | 290.9 | 27.28 | 7.56 | p = 0.686 | |
| 16-weeks | 299.0 | 47.75 | 13.24 | 304.5 | 9.71 | 2.69 | p = 0.68 | |
| TAS—before anaerobic test | baseline | 302.8 | 34.02 | 9.44 | 343.4 | 22.03 | 6.11 | p = 0.23 |
| 4-weeks | 308.9 | 40.29 | 11.17 | 309.4 | 37.06 | 10.28 | p = 0.970 | |
| 12-weeks | 291.0 | 45.77 | 12.69 | 298.2 | 51.16 | 14.19 | p = 0.708 | |
| 16-weeks | 279.8 | 44.12 | 12.24 | 292.7 | 52.30 | 14.51 | p = 0.11 | |
| TAS—after anaerobic test | baseline | 328.7 | 59.28 | 16.44 | 329.7 | 57.11 | 15.84 | p = 0.42 |
| 4-weeks | 314.5 | 40.79 | 11.31 | 363.3 | 22.66 | 6.28 | p = 0.06 | |
| 12-weeks | 308.3 | 56.69 | 15.72 | 304.7 | 45.20 | 12.54 | p = 0.860 | |
| 16-weeks | 301.2 | 14.71 | 4.08 | 299.9 | 44.11 | 12.23 | p = 0.925 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazur-Kurach, P.; Frączek, B.; Klimek, A.T. Does Multi-Strain Probiotic Supplementation Impact the Effort Capacity of Competitive Road Cyclists? Int. J. Environ. Res. Public Health 2022, 19, 12205. https://doi.org/10.3390/ijerph191912205
Mazur-Kurach P, Frączek B, Klimek AT. Does Multi-Strain Probiotic Supplementation Impact the Effort Capacity of Competitive Road Cyclists? International Journal of Environmental Research and Public Health. 2022; 19(19):12205. https://doi.org/10.3390/ijerph191912205
Chicago/Turabian StyleMazur-Kurach, Paulina, Barbara Frączek, and Andrzej T. Klimek. 2022. "Does Multi-Strain Probiotic Supplementation Impact the Effort Capacity of Competitive Road Cyclists?" International Journal of Environmental Research and Public Health 19, no. 19: 12205. https://doi.org/10.3390/ijerph191912205
APA StyleMazur-Kurach, P., Frączek, B., & Klimek, A. T. (2022). Does Multi-Strain Probiotic Supplementation Impact the Effort Capacity of Competitive Road Cyclists? International Journal of Environmental Research and Public Health, 19(19), 12205. https://doi.org/10.3390/ijerph191912205








