Pharmaceutical Incompatibility of Lubricating Gel Formulation Reduces Antibacterial Activity of Chlorhexidine Gluconate: In Vitro Study in Northern Thailand
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. In Vitro Incompatibility Study
2.3. In Vitro Antibacterial Activity
2.4. Statistical Analysis
3. Results
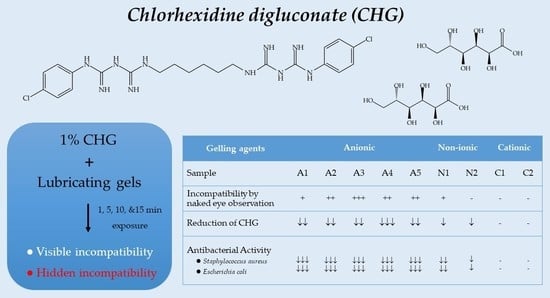
3.1. In Vitro Incompatibility Study
3.2. In Vitro Antibacterial Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef] [PubMed]
- Cheung, H.-Y.; Wong, M.M.-K.; Cheung, S.-H.; Liang, L.Y.; Lam, Y.-W.; Chiu, S.-K. Differential actions of chlorhexidine on the cell wall of Bacillus subtilis and Escherichia coli. PLoS ONE 2012, 7, e36659. [Google Scholar] [CrossRef] [PubMed]
- Hagi, A.; Iwata, K.; Nii, T.; Nakata, H.; Tsubotani, Y.; Inoue, Y. Bactericidal effects and mechanism of action of olanexidine gluconate, a new antiseptic. Antimicrob. Agents Chemother. 2015, 59, 4551–4559. [Google Scholar] [CrossRef] [PubMed]
- Samanth, S.A.; Varghese, S.S. The most effective concentration of chlorhexidine as a mouthwash-systematic review. J. Pharm. Sci. 2017, 9, 233–236. [Google Scholar]
- Kaiser, N.; Klein, D.; Karanja, P.; Greten, Z.; Newman, J. Inactivation of chlorhexidine gluconate on skin by incompatible alcohol hand sanitizing gels. Am. J. Infect. Control 2009, 37, 569–573. [Google Scholar] [CrossRef]
- Calogiuri, G.F.; Di Leo, E.; Trautmann, A.; Nettis, E.; Ferrannini, A.; Vacca, A. Chlorhexidine hypersensitivity: A critical and updated review. J. Allergy Ther. 2013, 4, 141. [Google Scholar]
- Al-Hameed, F.M.; Ahmed, G.R.; AlSaedi, A.A.; Bhutta, M.J.; Al-Hameed, F.F.; AlShamrani, M.M. Applying preventive measures leading to significant reduction of catheter-associated urinary tract infections in adult intensive care unit. Saudi Med. J. 2018, 39, 97–102. [Google Scholar] [CrossRef]
- Plantier, G.M.; Bosso, C.E.; Azevedo, B.N.; Correa, A.C.; Silva, A.L.; Raso, V. Effect of chlorhexidine and urinary catheter infection prevention in a Brazilian coronary ICU. Crit. Care 2015, 19, P80. [Google Scholar] [CrossRef]
- Mitchell, B.G.; Fasugba, O.; Cheng, A.C.; Gregory, V.; Koerner, J.; Collignon, P.; Gardner, A.; Graves, N. Chlorhexidine versus saline in reducing the risk of catheter associated urinary tract infection: A cost-effectiveness analysis. Int. J. Nurs. Stud. 2019, 97, 1–6. [Google Scholar] [CrossRef]
- Mitchell, B.G.; Fasugba, O.; Gardner, A.; Koerner, J.; Collignon, P.; Cheng, A.C.; Graves, N.; Morey, P.; Gregory, V. Reducing catheter-associated urinary tract infections in hospitals: Study protocol for a multi-site randomised controlled study. BMJ Open 2017, 7, e018871. [Google Scholar] [CrossRef]
- Fasugba, O.; Cheng, A.C.; Gregory, V.; Graves, N.; Koerner, J.; Collignon, P.; Gardner, A.; Mitchell, B.G. Chlorhexidine for meatal cleaning in reducing catheter-associated urinary tract infections: A multicentre stepped-wedge randomised controlled trial. Lancet Infect. Dis. 2019, 19, 611–619. [Google Scholar] [CrossRef]
- Ubaid, M.; Ilyas, S.; Mir, S.; Khan, A.K.; Rashid, R.; Khan, M.Z.U.; Kanwal, Z.G.; Nawaz, A.; Shah, A.; Murtaza, G. Formulation and in vitro evaluation of carbopol 934-based modified clotrimazole gel for topical application. An. Acad. Bras. Cienc. 2016, 88, 2303–2317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kkrishna Sailaja, A.; Supraja, R. An overall review on topical preparationgel. Innov. Int. J. Med. Pharm. Sci. 2016, 1, 17–20. [Google Scholar]
- Samala, M.; Sridevi, G. Role of polymers as gelling agents in the formulation of emulgels. Polym. Sci. 2016, 2, 67–98. [Google Scholar]
- Tran, G.; Huỳnh, T.T.; Bruins, F.; Ahmad, N.; Budris, W.; Posligua, A.; Hammel, J.; Nardone, B.; West, D. Evidence of incompatibility for topical anionic agents used in conjunction with chlorhexidine gluconate: A systematic review. J. Dermatol. Surg. 2016, 1, 66–70. [Google Scholar] [CrossRef]
- Webster, J.; Hood, R.H.; Burridge, C.A.; Doidge, M.L.; Phillips, K.M.; George, N. Water or antiseptic for periurethral cleaning before urinary catheterization: A randomized controlled trial. Am. J. Infect. Control 2001, 29, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.; Leung, P.; Wong, Y.C.; To, O.K.; Yeung, Y.F.; Chan, M.W.; Yip, Y.L.; Kwok, C.W. Water versus antiseptic periurethral cleansing before catheterization among home care patients: A randomized controlled trial. Am. J. Infect. Control 2008, 36, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Al-Farsi, S.; Oliva, M.; Davidson, R.; Richardson, S.E.; Ratnapalan, S. Periurethral cleaning prior to urinary catheterization in children: Sterile water versus 10% povidone-iodine. Clin. Pediatr. 2009, 48, 656–660. [Google Scholar] [CrossRef]
- Prevention of catheter-associated urinary tract infections in adults. Crit. Care Nurse 2016, 36, e9–e11. [CrossRef]
- Septimus, E.J.; Moody, J. Prevention of Device-Related Healthcare-Associated Infections. F1000Research 2016, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Karpanen, T.J.; Casey, A.L.; Whitehouse, T.; Nightingale, P.; Das, I.; Elliott, T.S. Clinical evaluation of a chlorhexidine intravascular catheter gel dressing on short-term central venous catheters. Am. J. Infect. Control 2016, 44, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Begum, S.; Reddy, Y.; Divya, B.; Komali, P.K.; Sushmitha, K.; Ruksar, S. Pharmaceutical incompatibilites: A review. Asian J. Pharm. Res. Dev. 2018, 6, 56–61. [Google Scholar] [CrossRef]
- Havlíková, L.; Matysová, L.; Nováková, L.; Hájková, R.; Solich, P. HPLC determination of chlorhexidine gluconate and p-chloroaniline in topical ointment. J. Pharm. Biomed. Anal. 2007, 43, 1169–1173. [Google Scholar] [CrossRef] [PubMed]
- Pelyuntha, W.; Chaiyasut, C.; Kantachote, D.; Sirilun, S. Cell-free supernatants from cultures of lactic acid bacteria isolated from fermented grape as biocontrol against Salmonella Typhi and Salmonella Typhimurium virulence via autoinducer-2 and biofilm interference. PeerJ 2019, 7, e7555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monegro, A.F.; Muppidi, V.; Regunath, H. Hospital acquired infections. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Ahoyo, T.A.; Bankolé, H.S.; Adéoti, F.M.; Gbohoun, A.A.; Assavèdo, S.; Amoussou-Guénou, M.; Kindé-Gazard, D.A.; Pittet, D. Prevalence of nosocomial infections and anti-infective therapy in Benin: Results of the first nationwide survey in 2012. Antimicrob. Resist. Infect. Control 2014, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Pezhman, B.; Fatemeh, R.; Amir, R.; Mahboobeh, R.; Mohammad, F. Nosocomial infections in an Iranian educational hospital: An evaluation study of the Iranian nosocomial infection surveillance system. BMC Infect. Dis. 2021, 21, 1256. [Google Scholar] [CrossRef] [PubMed]
- Boev, C.; Kiss, E. Hospital-acquired infections: Current trends and prevention. Crit. Care Nurs. Clin. N. Am. 2017, 29, 51–65. [Google Scholar] [CrossRef]
- Tolera, M.; Abate, D.; Dheresa, M.; Marami, D. Bacterial Nosocomial Infections and Antimicrobial Susceptibility Pattern among Patients Admitted at Hiwot Fana Specialized University Hospital, Eastern Ethiopia. Adv. Med. 2018, 2018, 2127814. [Google Scholar] [CrossRef]
- Yang, X.; Guo, R.; Xie, B.; Lai, Q.; Xu, J.; Hu, N.; Wan, L.; Dai, M.; Zhang, B. Drug resistance of pathogens causing nosocomial infection in orthopedics from 2012 to 2017: A 6-year retrospective study. J. Orthop. Surg. Res. 2021, 16, 100. [Google Scholar] [CrossRef]
- Szabó, S.; Feier, B.; Capatina, D.; Tertis, M.; Cristea, C.; Popa, A. An Overview of Healthcare Associated Infections and Their Detection Methods Caused by Pathogen Bacteria in Romania and Europe. J. Clin. Med. 2022, 11, 3204. [Google Scholar] [CrossRef]
- Ayobami, O.; Brinkwirth, S.; Eckmanns, T.; Markwart, R. Antibiotic resistance in hospital-acquired ESKAPE-E infections in low- and lower-middle-income countries: A systematic review and meta-analysis. Emerg. Microbes Infect. 2022, 11, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Thaprawat, P.; Greene, M.T.; Saint, S.; Kasatpibal, N.; Fowler, K.E.; Apisarnthanarak, A. Status of Hospital Infection Prevention Practices in Thailand in the Era of COVID-19: Results from a National Survey. Am. J. Infect. Control 2022, 50, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Apisarnthanarak, A.; Ratz, D.; Greene, M.T.; Khawcharoenporn, T.; Weber, D.J.; Saint, S. National survey of practices to prevent health care-associated infections in Thailand: The role of prevention bundles. Am. J. Infect. Control 2017, 45, 805–810. [Google Scholar] [CrossRef]
- Kotikula, I.; Chaiwarith, R. Epidemiology of catheter-associated urinary tract infections at maharaj nakorn chiang mai hospital, northern thailand. Southeast Asian J. Trop. Med. Public Health 2018, 49, 113–122. [Google Scholar]
- Abreu, A.C.; Tavares, R.R.; Borges, A.; Mergulhão, F.; Simões, M. Current and emergent strategies for disinfection of hospital environments. J. Antimicrob. Chemother. 2013, 68, 2718–2732. [Google Scholar] [CrossRef]
- Amini Tapouk, F.; Nabizadeh, R.; Mirzaei, N.; Hosseini Jazani, N.; Yousefi, M.; Valizade Hasanloei, M.A. Comparative efficacy of hospital disinfectants against nosocomial infection pathogens. Antimicrob. Resist. Infect. Control 2020, 9, 115. [Google Scholar] [CrossRef]
- Betchen, M.; Giovinco, H.M.; Curry, M.; Luu, J.; Fraimow, H.; Carabetta, V.J.; Nahra, R. Evaluating the effectiveness of hospital antiseptics on multidrug-resistant Acinetobacter baumannii: Understanding the relationship between microbicide and antibiotic resistance. Antibiotics 2022, 11, 614. [Google Scholar] [CrossRef]
- Dhama, K.; Patel, S.K.; Kumar, R.; Masand, R.; Rana, J.; Yatoo, M.I.; Tiwari, R.; Sharun, K.; Mohapatra, R.K.; Natesan, S.; et al. The role of disinfectants and sanitizers during COVID-19 pandemic: Advantages and deleterious effects on humans and the environment. Environ. Sci. Pollut. Res. 2021, 28, 34211–34228. [Google Scholar] [CrossRef]
- Leshem, T.; Gilron, S.; Azrad, M.; Peretz, A. Characterization of reduced susceptibility to chlorhexidine among Gram-negative bacteria. Microb. Infect. 2022, 24, 104891. [Google Scholar] [CrossRef]
- Zeng, P.; Rao, A.; Wiedmann, T.S.; Bowles, W. Solubility properties of chlorhexidine salts. Drug Dev. Ind. Pharm. 2009, 35, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Owen, S.C. Handbook of Pharmaceutical Excipients, 5th ed.; Rowe, R.C., Paul, J.S., Owen, S.C., Eds.; Pharmaceutical Press: London, UK, 2006; pp. 163–167. [Google Scholar]
- Rasimick, B.J.; Nekich, M.; Hladek, M.M.; Musikant, B.L.; Deutsch, A.S. Interaction between chlorhexidine digluconate and EDTA. J. Endod. 2008, 34, 1521–1523. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento, F.; del Rio, J.M.; Prieto, G.; Attwood, D.; Jones, M.N.; Mosquera, V. Thermodynamics of Micelle Formation of Chlorhexidine Digluconate. J. Phys. Chem. A 1995, 99, 17628–17631. [Google Scholar] [CrossRef]





| No. | Sample | Gelling Agents/Thickeners |
|---|---|---|
| 1 | A1 | Acrylates/C10-30 Alkyl Acrylate Cross-polymer |
| 2 | A2 | Carbopol 941 |
| 3 | A3 | Carbopol 940 |
| 4 | A4 | Sodium acrylate polymers |
| 5 | A5 | Carbopol 940 |
| 6 | N1 | Hydroxypropyl methylcellulose (HPMC) |
| 7 | N2 | Hydroxyethyl cellulose (HEC) |
| 8 | C1 | Positively-charged polysaccharide |
| 9 | C2 | Positively-charged polysaccharide |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pattananandecha, T.; Sirilun, S.; Apichai, S.; Ouirungroj, T.; Uirungroj, P.; Ogata, F.; Kawasaki, N.; Saenjum, C. Pharmaceutical Incompatibility of Lubricating Gel Formulation Reduces Antibacterial Activity of Chlorhexidine Gluconate: In Vitro Study in Northern Thailand. Int. J. Environ. Res. Public Health 2022, 19, 12285. https://doi.org/10.3390/ijerph191912285
Pattananandecha T, Sirilun S, Apichai S, Ouirungroj T, Uirungroj P, Ogata F, Kawasaki N, Saenjum C. Pharmaceutical Incompatibility of Lubricating Gel Formulation Reduces Antibacterial Activity of Chlorhexidine Gluconate: In Vitro Study in Northern Thailand. International Journal of Environmental Research and Public Health. 2022; 19(19):12285. https://doi.org/10.3390/ijerph191912285
Chicago/Turabian StylePattananandecha, Thanawat, Sasithorn Sirilun, Sutasinee Apichai, Teerapat Ouirungroj, Phisit Uirungroj, Fumihiko Ogata, Naohito Kawasaki, and Chalermpong Saenjum. 2022. "Pharmaceutical Incompatibility of Lubricating Gel Formulation Reduces Antibacterial Activity of Chlorhexidine Gluconate: In Vitro Study in Northern Thailand" International Journal of Environmental Research and Public Health 19, no. 19: 12285. https://doi.org/10.3390/ijerph191912285
APA StylePattananandecha, T., Sirilun, S., Apichai, S., Ouirungroj, T., Uirungroj, P., Ogata, F., Kawasaki, N., & Saenjum, C. (2022). Pharmaceutical Incompatibility of Lubricating Gel Formulation Reduces Antibacterial Activity of Chlorhexidine Gluconate: In Vitro Study in Northern Thailand. International Journal of Environmental Research and Public Health, 19(19), 12285. https://doi.org/10.3390/ijerph191912285