Effective Electro-Activation Process of Hydrogen Peroxide/Peroxydisulfate Induced by Atomic Hydrogen for Rapid Oxidation of Norfloxacin over the Carbon-Based Pd Nanocatalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of the Catalyst
2.3. Characterization
2.4. Electrocatalytic Activity and Electrochemical Analysis
3. Results and Discussion
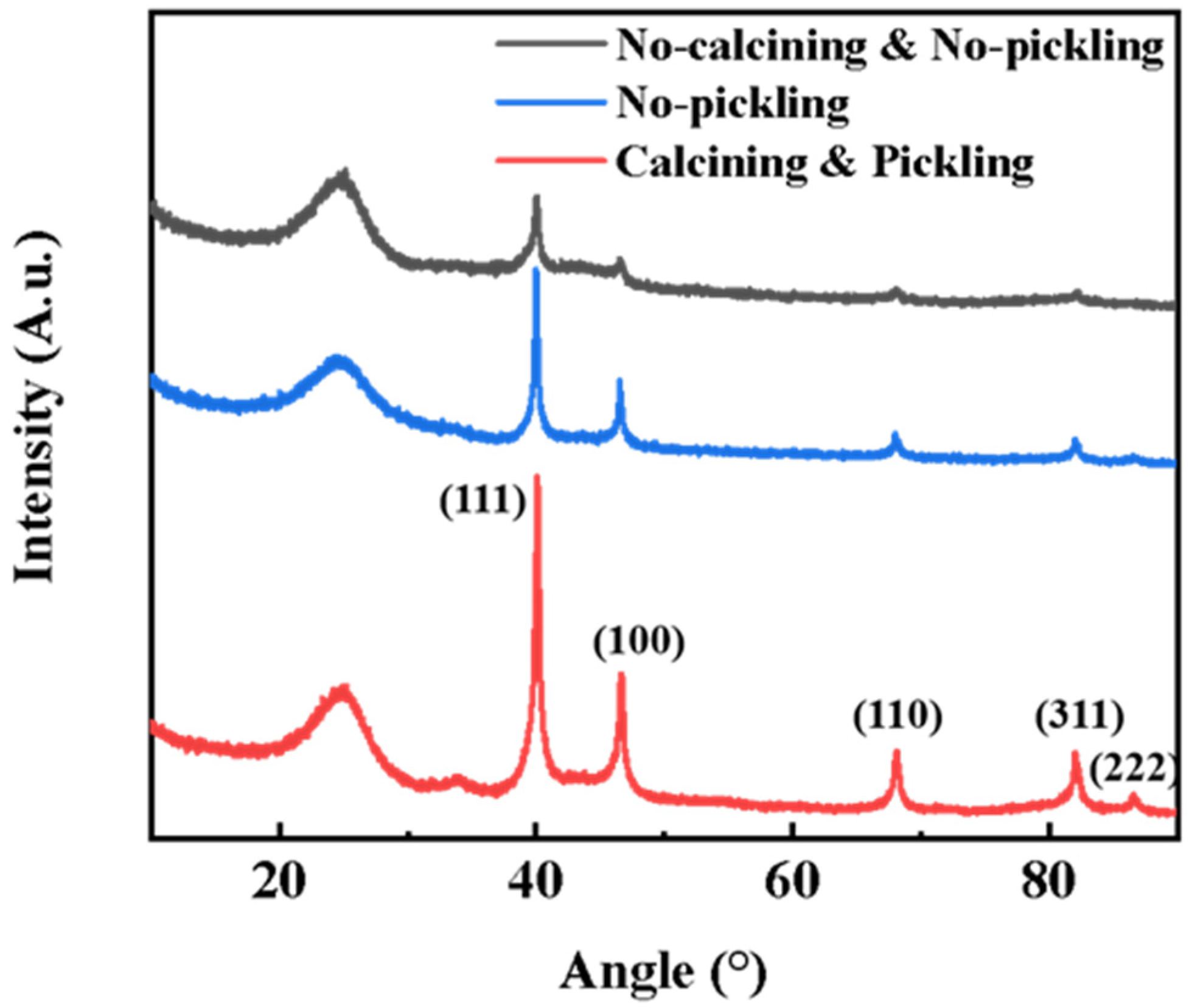
3.1. Characterization of Pd/C Catalyst
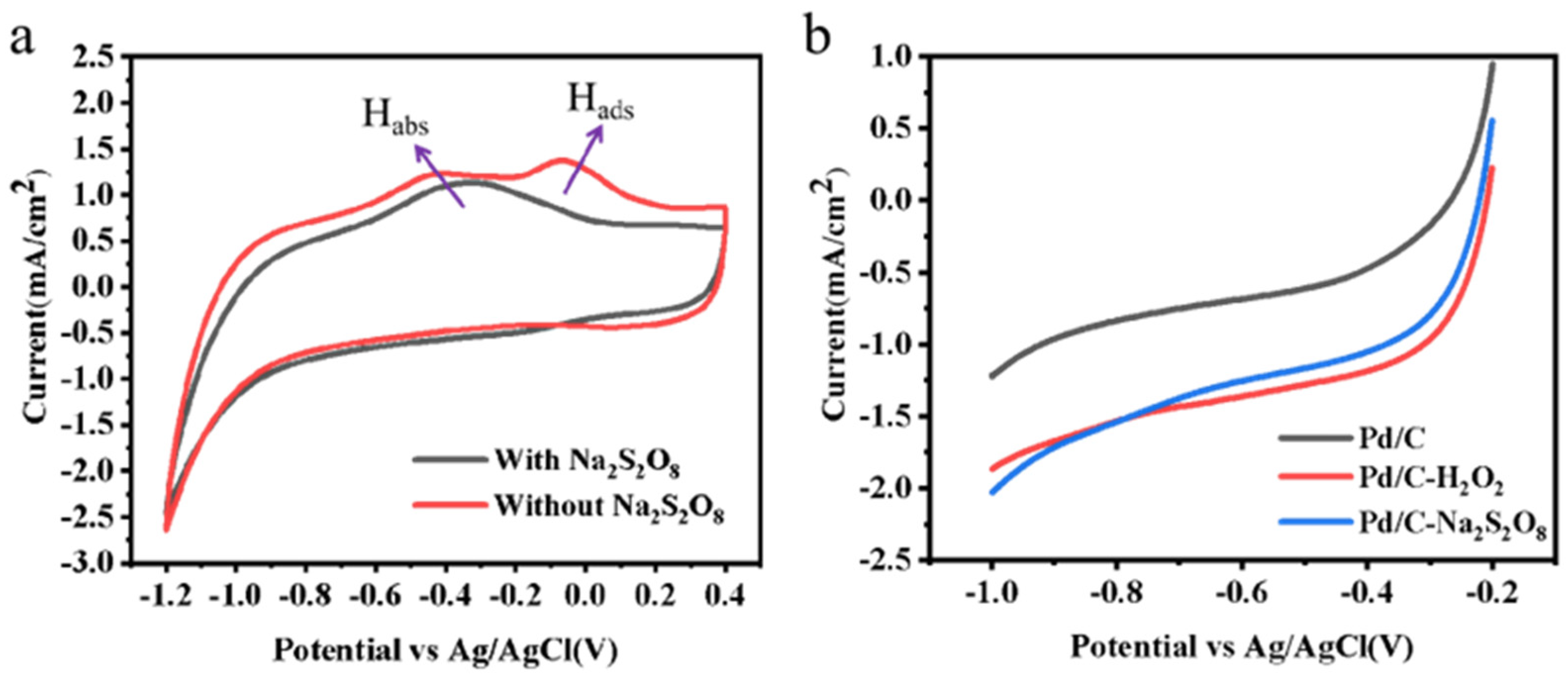
3.2. Electrochemical Analysis
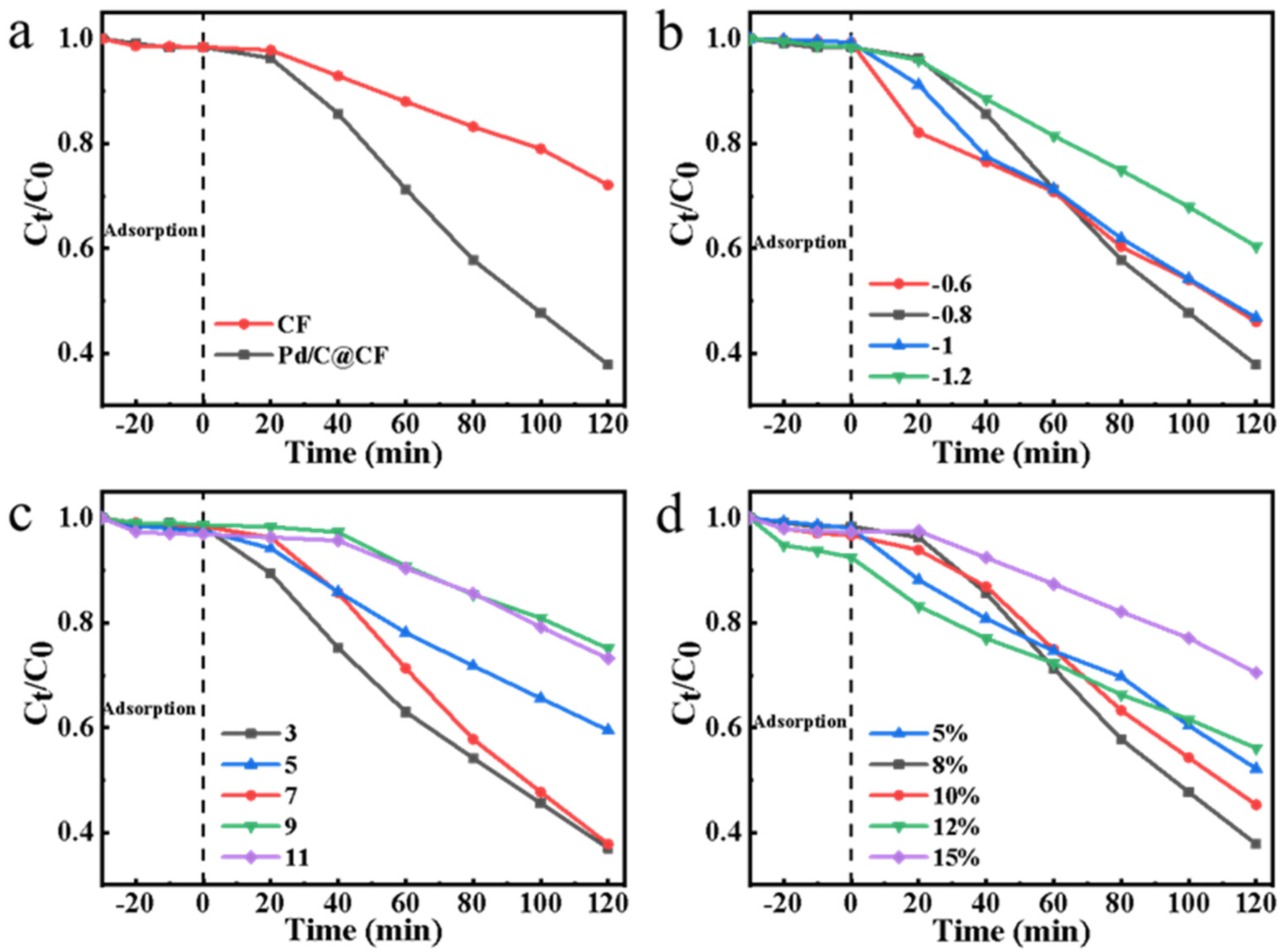
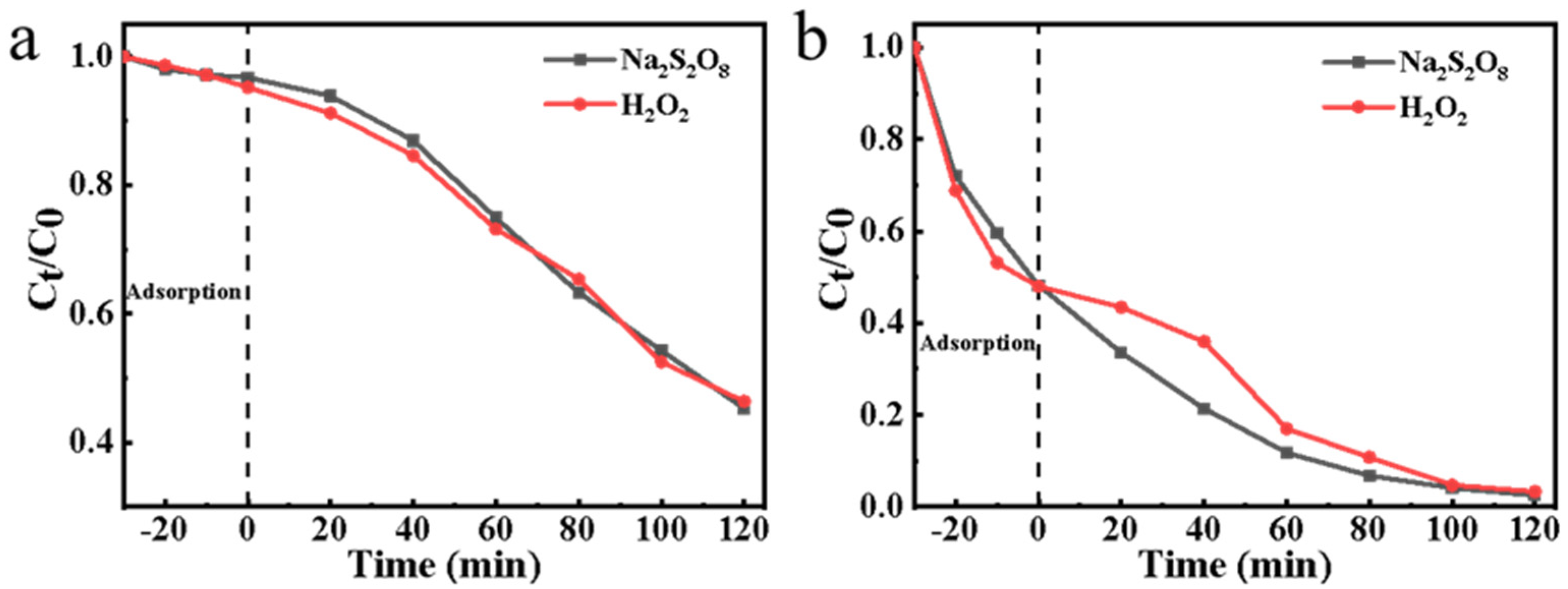
3.3. Electrocatalytic Activity
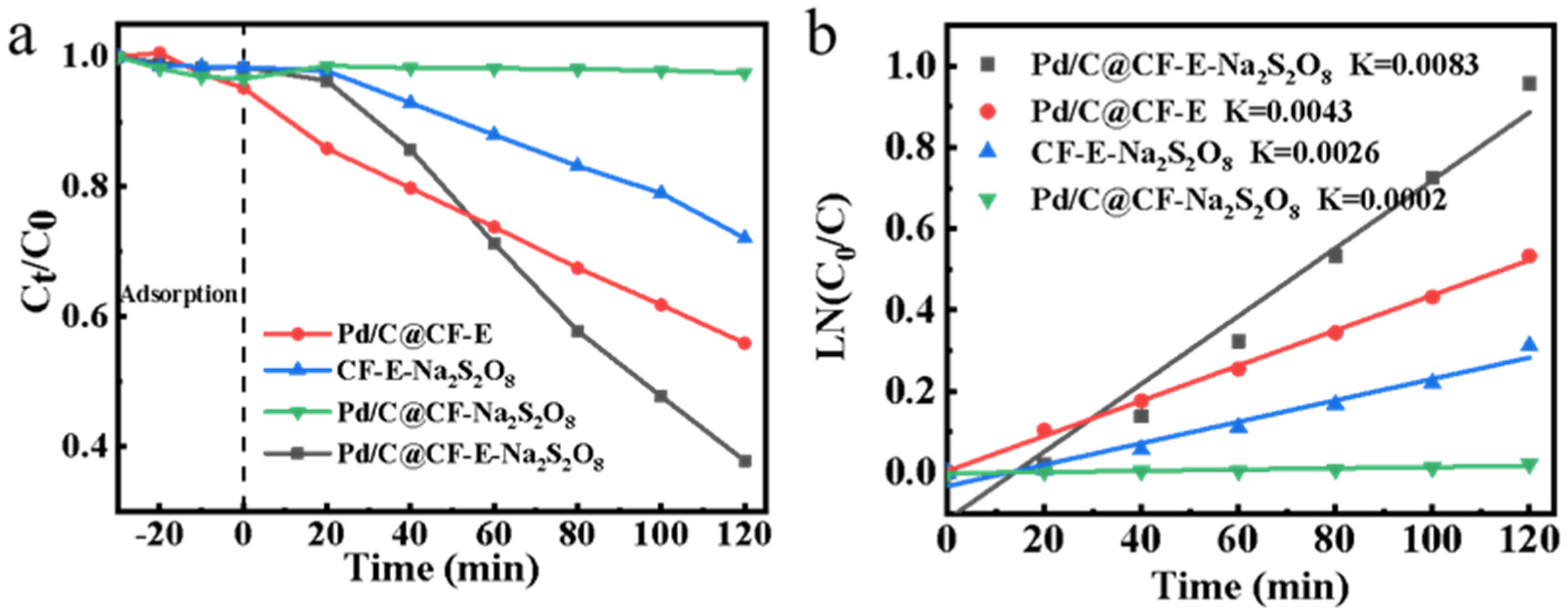
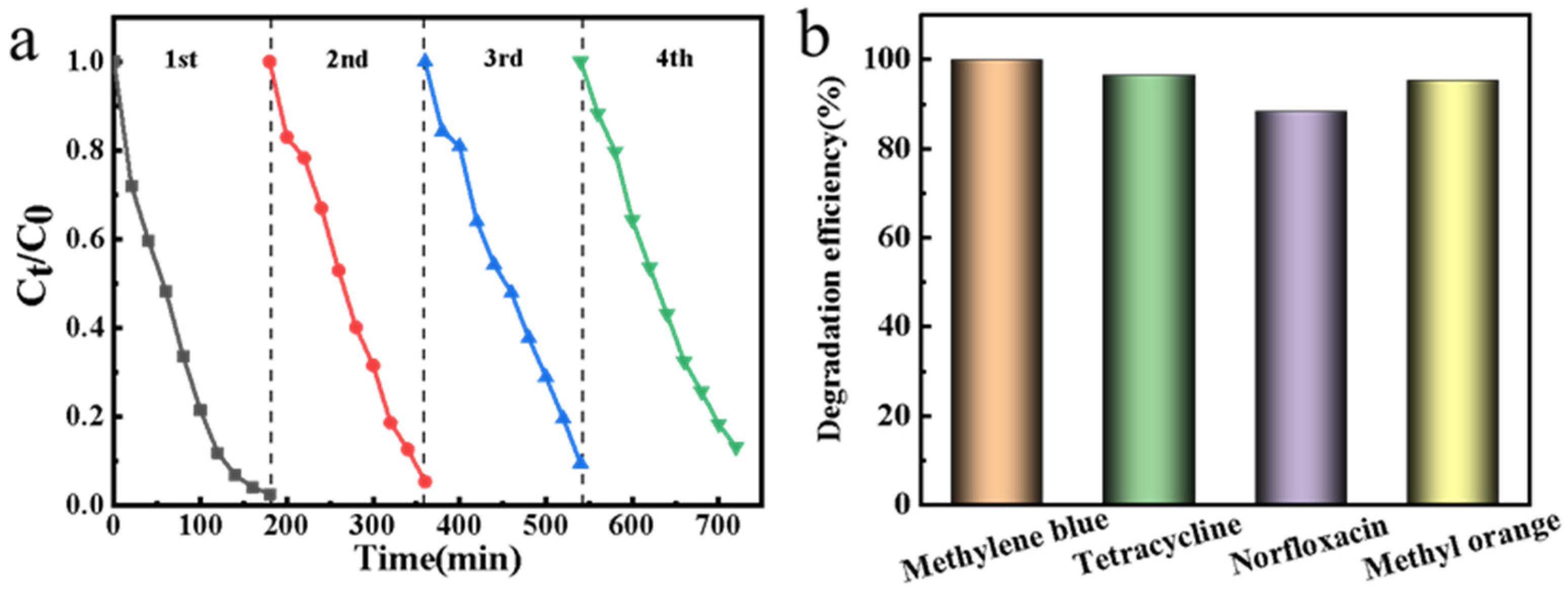
3.4. Catalyst Reusability and Application Potential
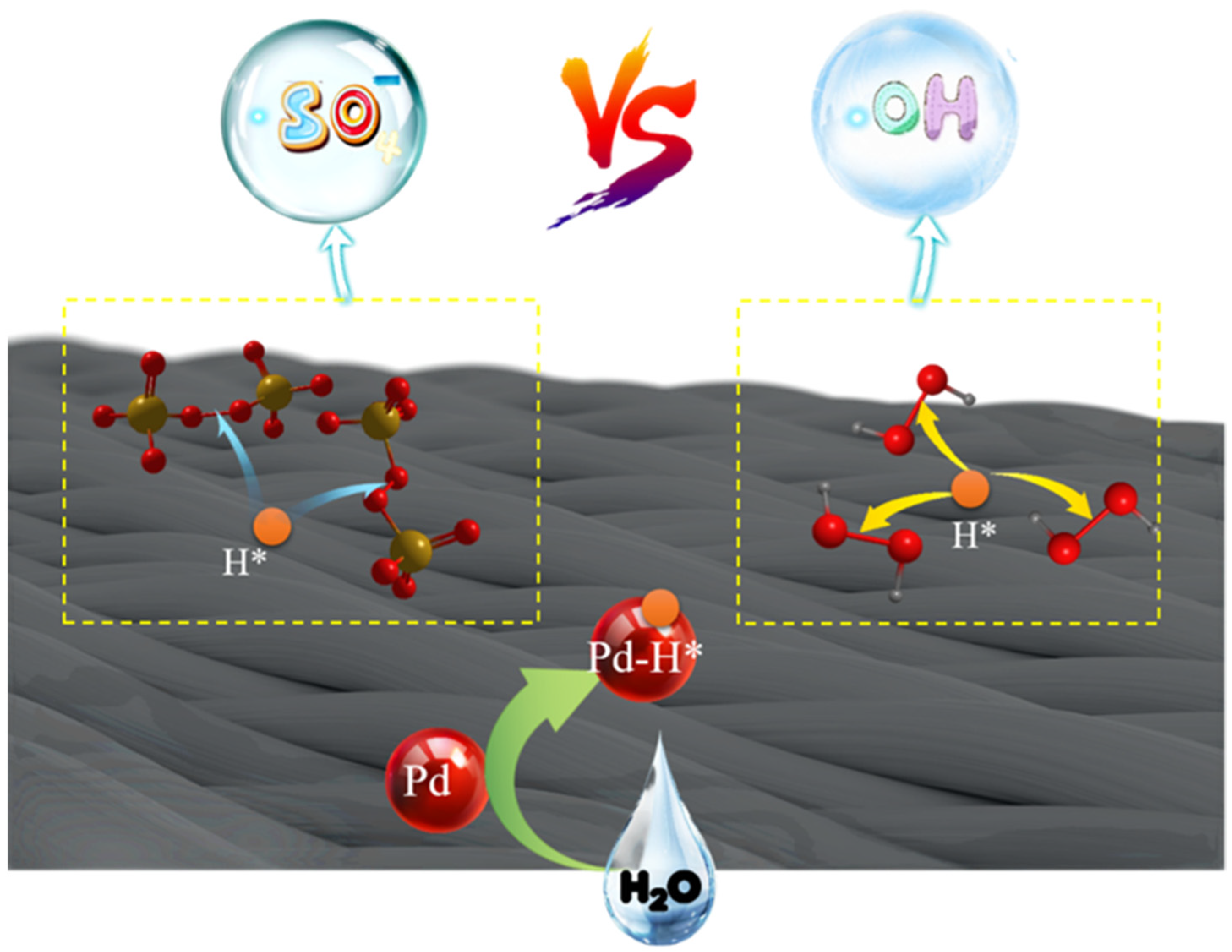
3.5. Catalytic Mechanism
- (1)
- Basic performance: E0(SO4•−/SO42−) is slightly larger than E0(•OH/OH−), and the lifetime of SO4•− radical is longer than •OH.
- (2)
- Scope of application: AOP-SO4•− process pH adaptability better than AOP-•OH, and can be applied in a wider range of pH values. This is determined by the reaction mechanism between two free radicals and organic pollutants. AOP-•OH mainly reacts with organic pollutants through the extraction of hydrogen or the addition of hydroxyl [52], while SO4•− tends to react with organic pollutants through electron transfer [53]. Therefore, SO4•− has higher activity under neutral and alkaline conditions, and the AOP-SO4•− system has less stringent pH requirements than the AOP-•OH system.
- (3)
- The practical application: the persulfate used to produce SO4•− is a solid powder, which is easier to transport and store and more stable than H2O2, which is more conducive to its widespread use in experiments and engineering. However, H2O2 can be reduced to O2 through an ORR reaction, so the removal of pollutants can be realized without any additional drugs.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dewil, R.; Mantzavinos, D.; Poulios, I.; Rodrigo, M.A. New perspectives for advanced oxidation processes. J. Environ. Manag. 2017, 195, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhao, R. Advanced oxidation processes (AOPs) in wastewater treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- M’arimi, M.; Mecha, C.; Kiprop, A.K.; Ramkat, R. Recent trends in applications of advanced oxidation processes (AOPs) in bioenergy production. Renew. Sustain. Energy Rev. 2020, 121, 109669. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, X.; Sun, K.; Lin, C.; Ma, J.; He, M.; Ouyang, W. Persulfate-based advanced oxidation processes (AOPs) for organic-contaminated soil remediation: A review. Chem. Eng. J. 2019, 372, 836–851. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Reactive species in advanced oxidation processes: Formation, identification and reaction mechanism. Chem. Eng. J. 2020, 401, 126158. [Google Scholar] [CrossRef]
- Luo, H.; Fu, H.; Yin, H.; Lin, Q. Carbon materials in persulfate-based advanced oxidation processes: The roles and construction of active sites. J. Hazard. Mater. 2021, 426, 128044. [Google Scholar] [CrossRef]
- Lee, J.; von Gunten, U.; Kim, J.H. Persulfate-Based Advanced Oxidation: Critical Assessment of Opportunities and Roadblocks. Env. Sci. Technol. 2020, 54, 3064–3081. [Google Scholar] [CrossRef]
- Zhou, X.; Zeng, Z.; Zeng, G.; Lai, C.; Xiao, R.; Liu, S.; Huang, D.; Qin, L.; Liu, X.; Li, B.; et al. Persulfate activation by swine bone char-derived hierarchical porous carbon: Multiple mechanism system for organic pollutant degradation in aqueous media. Chem. Eng. J. 2020, 383, 123091. [Google Scholar] [CrossRef]
- Yang, L.; Xue, J.; He, L.; Wu, L.; Ma, Y.; Chen, H.; Li, H.; Peng, P.; Zhang, Z. Review on ultrasound assisted persulfate degradation of organic contaminants in wastewater: Influences, mechanisms and prospective. Chem. Eng. J. 2019, 378, 122146. [Google Scholar] [CrossRef]
- Tan, C.; Gao, N.; Deng, Y.; An, N.; Deng, J. Heat-activated persulfate oxidation of diuron in water. Chem. Eng. J. 2012, 203, 294–300. [Google Scholar] [CrossRef]
- Santos, A.; Fernandez, J.; Rodriguez, S.; Dominguez, C.; Lominchar, M.; Lorenzo, D.; Romero, A. Abatement of chlorinated compounds in groundwater contaminated by HCH wastes using ISCO with alkali activated persulfate. Sci. Total Environ. 2018, 615, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Ma, J.; Liu, W.; Zou, J.; Yue, S.; Li, X.; Wiesner, M.R.; Fang, J. Removal of 2-MIB and geosmin using UV/persulfate: Contributions of hydroxyl and sulfate radicals. Water Res. 2015, 69, 223–233. [Google Scholar] [CrossRef]
- Dhaka, S.; Kumar, R.; Khan, M.A.; Paeng, K.-J.; Kurade, M.B.; Kim, S.-J.; Jeon, B.-H. Aqueous phase degradation of methyl paraben using UV-activated persulfate method. Chem. Eng. J. 2017, 321, 11–19. [Google Scholar] [CrossRef]
- Chen, W.-S.; Huang, C.-P. Mineralization of aniline in aqueous solution by electrochemical activation of persulfate. Chemosphere 2015, 125, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhou, M.; Du, X.; Xu, X. Enhanced mechanism of 2, 4-dichlorophenoxyacetic acid degradation by electrochemical activation of persulfate on Blue-TiO2 nanotubes anode. Sep. Purif. Technol. 2021, 254, 117560. [Google Scholar] [CrossRef]
- Chen, X.; Oh, W.-D.; Lim, T.-T. Graphene-and CNTs-based carbocatalysts in persulfates activation: Material design and catalytic mechanisms. Chem. Eng. J. 2018, 354, 941–976. [Google Scholar] [CrossRef]
- Wei, Z.; Villamena, F.A.; Weavers, L.K. Kinetics and mechanism of ultrasonic activation of persulfate: An in situ EPR spin trapping study. Env. Sci Technol. 2017, 51, 3410–3417. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Bruton, T.A.; Li, W.; Buren, J.V.; Prasse, C.; Doyle, F.M.; Sedlak, D.L. Oxidation of benzene by persulfate in the presence of Fe (III)-and Mn (IV)-containing oxides: Stoichiometric efficiency and transformation products. Env. Sci. Technol. 2016, 50, 890–898. [Google Scholar] [CrossRef]
- Liu, R.; Chen, H.-m.; Fang, L.-p.; Xu, C.; He, Z.; Lai, Y.; Zhao, H.; Bekana, D.; Liu, J.-f. Au@ Pd bimetallic nanocatalyst for carbon–halogen bond cleavage: An old story with new insight into how the activity of Pd is influenced by Au. Env. Sci. Technol. 2018, 52, 4244–4255. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Zhang, G.; Ji, Q.; Liu, H.; Hua, X.; Xia, H.; Sillanpää, M.; Qu, J. pH-independent production of hydroxyl radical from atomic H*-mediated electrocatalytic H2O2 reduction: A green Fenton process without byproducts. Env. Sci. Technol. 2020, 54, 14725–14731. [Google Scholar] [CrossRef]
- Zeng, H.; Lan, H.; An, X.; Repo, E.; Park, Y.; Pastushok, O.; Liu, H.; Qu, J. Insight into electroreductive activation process of peroxydisulfate for eliminating organic pollution: Essential role of atomic hydrogen. Chem. Eng. J. 2021, 426, 128355. [Google Scholar] [CrossRef]
- Peng, Y.; Cui, M.; Zhang, Z.; Shu, S.; Shi, X.; Brosnahan, J.T.; Liu, C.; Zhang, Y.; Godbold, P.; Zhang, X. Bimetallic composition-promoted electrocatalytic hydrodechlorination reaction on silver–palladium alloy nanoparticles. ACS Catal. 2019, 9, 10803–10811. [Google Scholar] [CrossRef]
- Lee, S.R.; Cho, J.M.; Son, M.; Park, M.-J.; Kim, W.Y.; Kim, S.Y.; Bae, J.W. Selective hydrodechlorination of trichloromethane to dichloromethane over bimetallic Pt-Pd/KIT-6: Catalytic activity and reaction kinetics. Chem. Eng. J. 2018, 331, 556–569. [Google Scholar] [CrossRef]
- Li, Y.; Miller, C.J.; Wu, L.; Waite, T.D. Hydroxyl Radical Production via a Reaction of Electrochemically Generated Hydrogen Peroxide and Atomic Hydrogen: An Effective Process for Contaminant Oxidation? Environ. Sci. Technol. 2022, 56, 5820–5829. [Google Scholar] [CrossRef]
- Zheng, W.; You, S.; Yao, Y.; Jin, L.; Liu, Y. Development of atomic hydrogen-mediated electrocatalytic filtration system for peroxymonosulfate activation towards ultrafast degradation of emerging organic contaminants. Appl. Catal. B 2021, 298, 120593. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, Y.; Liu, F.; Wang, Y.; Ren, N.; You, S. Atomic Hydrogen in Electrocatalytic Systems: Generation, Identification, and Environmental Applications. Water Res. 2022, 223, 118994. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Lee, J.G.; Lee, S.-H.; Seo, M.; Piao, L.; Bae, J.H.; Lim, S.Y.; Park, Y.J.; Chung, T.D. Hydrogen-atom-mediated electrochemistry. Nat. Commun. 2013, 4, 1–8. [Google Scholar] [CrossRef]
- Pan, Y.; Su, H.; Zhu, Y.; Molamahmood, H.V.; Long, M. CaO2 based Fenton-like reaction at neutral pH: Accelerated reduction of ferric species and production of superoxide radicals. Water Res. 2018, 145, 731–740. [Google Scholar] [CrossRef]
- Chaplin, B.P.; Reinhard, M.; Schneider, W.F.; Schüth, C.; Shapley, J.R.; Strathmann, T.J.; Werth, C.J. Critical review of Pd-based catalytic treatment of priority contaminants in water. Environ. Sci. Technol. 2012, 46, 3655–3670. [Google Scholar] [CrossRef]
- Wang, G.; Liu, J.; Sui, Y.; Wang, M.; Qiao, L.; Du, F.; Zou, B. Palladium structure engineering induced by electrochemical H intercalation boosts hydrogen evolution catalysis. J. Mater. Chem A Mater. 2019, 7, 14876–14881. [Google Scholar] [CrossRef]
- Rajic, L.; Fallahpour, N.; Alshawabkeh, A.N. Impact of electrode sequence on electrochemical removal of trichloroethylene from aqueous solution. Appl. Catal. B 2015, 174, 427–434. [Google Scholar] [CrossRef]
- Jiang, G.; Wang, K.; Li, J.; Fu, W.; Zhang, Z.; Johnson, G.; Lv, X.; Zhang, Y.; Zhang, S.; Dong, F. Electrocatalytic hydrodechlorination of 2, 4-dichlorophenol over palladium nanoparticles and its pH-mediated tug-of-war with hydrogen evolution. Chem. Eng. J. 2018, 348, 26–34. [Google Scholar] [CrossRef]
- Trasatti, S. Work function, electronegativity, and electrochemical behaviour of metals: III. Electrolytic hydrogen evolution in acid solutions. J. Electroanal. Chem. Interfacial Electrochem. 1972, 39, 163–184. [Google Scholar] [CrossRef]
- Jiang, G.; Lan, M.; Zhang, Z.; Lv, X.; Lou, Z.; Xu, X.; Dong, F.; Zhang, S. Identification of active hydrogen species on palladium nanoparticles for an enhanced electrocatalytic hydrodechlorination of 2, 4-dichlorophenol in water. Environ. Sci. Technol. 2017, 51, 7599–7605. [Google Scholar] [CrossRef]
- Zhao, X.; Li, A.; Mao, R.; Liu, H.; Qu, J. Electrochemical removal of haloacetic acids in a three-dimensional electrochemical reactor with Pd-GAC particles as fixed filler and Pd-modified carbon paper as cathode. Water Res. 2014, 51, 134–143. [Google Scholar] [CrossRef]
- Matzek, L.W.; Carter, K.E. Activated persulfate for organic chemical degradation: A review. Chemosphere 2016, 151, 178–188. [Google Scholar] [CrossRef]
- Liang, C.; Lin, Y.-T.; Shih, W.-H. Treatment of trichloroethylene by adsorption and persulfate oxidation in batch studies. Ind. Eng. Chem. Res. 2009, 48, 8373–8380. [Google Scholar] [CrossRef]
- Yang, S.; Xiao, T.; Zhang, J.; Chen, Y.; Li, L. Activated carbon fiber as heterogeneous catalyst of peroxymonosulfate activation for efficient degradation of Acid Orange 7 in aqueous solution. Sep. Purif. Technol. 2015, 143, 19–26. [Google Scholar] [CrossRef]
- Yang, L.; Chen, C.; Bao, R.; Huang, Z.; Wang, W.; Zhang, C.; Xia, J.; Geng, J.; Li, H. Effective green electro-Fenton process induced by atomic hydrogen for rapid oxidation of organic pollutants over a highly active and reusable carbon based palladium nanocatalyst. Appl. Surf. Sci. 2022, 602, 154325. [Google Scholar] [CrossRef]
- Chen, C.; Bao, R.; Yang, L.; Tai, S.; Zhao, Y.; Wang, W.; Xia, J.; Li, H. Application of inorganic perovskite LaNiO3 partial substituted by Ce and Cu in absorbance and photocatalytic degradation of antibiotics. Appl. Surf. Sci. 2022, 579, 152026. [Google Scholar] [CrossRef]
- Liang, R.; Huang, R.; Ying, S.; Wang, X.; Yan, G.; Wu, L. Facile in situ growth of highly dispersed palladium on phosphotungstic-acid-encapsulated MIL-100 (Fe) for the degradation of pharmaceuticals and personal care products under visible light. Nano Res. 2018, 11, 1109–1123. [Google Scholar] [CrossRef]
- Ding, X.; Yao, Z.; Xu, Y.; Liu, B.; Liu, Q.; She, Y. Aqueous-phase hydrodechlorination of 4-chlorophenol on palladium nanocrystals: Identifying the catalytic sites and unraveling the reaction mechanism. J. Catal. 2018, 368, 336–344. [Google Scholar] [CrossRef]
- Quaino, P.; Santos, E. Hydrogen evolution reaction on palladium multilayers deposited on Au (111): A theoretical approach. Langmuir 2015, 31, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Qayum, A.; Peng, X.; Yuan, J.; Qu, Y.; Zhou, J.; Huang, Z.; Xia, H.; Liu, Z.; Tan, D.Q.; Chu, P.K. Highly Durable and Efficient Ni-FeOx/FeNi3 Electrocatalysts Synthesized by a Facile In Situ Combustion-Based Method for Overall Water Splitting with Large Current Densities. ACS Appl Mater. Interfaces 2022, 14, 27842–27853. [Google Scholar] [CrossRef]
- Shen, Y.; Tong, Y.; Xu, J.; Wang, S.; Wang, J.; Zeng, T.; He, Z.; Yang, W.; Song, S. Ni-based layered metal-organic frameworks with palladium for electrochemical dechlorination. Appl. Catal. B 2020, 264, 118505. [Google Scholar] [CrossRef]
- Cheng, L.; Jiang, T.; Yan, K.; Gong, J.; Zhang, J. A dual-cathode photoelectrocatalysis-electroenzymatic catalysis system by coupling BiVO4 photoanode with hemin/Cu and carbon cloth cathodes for degradation of tetracycline. Electrochim. Acta 2019, 298, 561–569. [Google Scholar] [CrossRef]
- Liang, C.; Su, H.-W. Identification of sulfate and hydroxyl radicals in thermally activated persulfate. Ind. Eng. Chem. Res. 2009, 48, 5558–5562. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Radical generation by the interaction of transition metals with common oxidants. Environ. Sci. Technol. 2004, 38, 3705–3712. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, G.; Liu, M.; Wang, Q.; Wang, P. Enhanced degradation of Bisphenol A (BPA) by peroxymonosulfate with Co3O4-Bi2O3 catalyst activation: Effects of pH, inorganic anions, and water matrix. Chem. Eng. J. 2018, 338, 300–310. [Google Scholar] [CrossRef]
- Lan, H.; Mao, R.; Tong, Y.; Liu, Y.; Liu, H.; An, X.; Liu, R. Enhanced electroreductive removal of bromate by a supported Pd–In Bimetallic catalyst: Kinetics and mechanism investigation. Environ. Sci. Technol. 2016, 50, 11872–11878. [Google Scholar] [CrossRef]
- Sopaj, F.; Oturan, N.; Pinson, J.; Podvorica, F.I.; Oturan, M.A. Effect of cathode material on electro-Fenton process efficiency for electrocatalytic mineralization of the antibiotic sulfamethazine. Chem. Eng. J. 2020, 384, 123249. [Google Scholar] [CrossRef]
- Minisci, F.; Citterio, A.; Giordano, C. Electron-transfer processes: Peroxydisulfate, a useful and versatile reagent in organic chemistry. Acc. Chem. Res. 1983, 16, 27–32. [Google Scholar] [CrossRef]
- Neta, P.; Madhavan, V.; Zemel, H.; Fessenden, R.W. Rate constants and mechanism of reaction of sulfate radical anion with aromatic compounds. J. Am. Chem. Soc. 1977, 99, 163–164. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Cui, M.; Cheng, S.; Zhang, S.; Li, Y.; Luo, T.; Zheng, T.; Li, H. Effective Electro-Activation Process of Hydrogen Peroxide/Peroxydisulfate Induced by Atomic Hydrogen for Rapid Oxidation of Norfloxacin over the Carbon-Based Pd Nanocatalyst. Int. J. Environ. Res. Public Health 2022, 19, 12332. https://doi.org/10.3390/ijerph191912332
Yang L, Cui M, Cheng S, Zhang S, Li Y, Luo T, Zheng T, Li H. Effective Electro-Activation Process of Hydrogen Peroxide/Peroxydisulfate Induced by Atomic Hydrogen for Rapid Oxidation of Norfloxacin over the Carbon-Based Pd Nanocatalyst. International Journal of Environmental Research and Public Health. 2022; 19(19):12332. https://doi.org/10.3390/ijerph191912332
Chicago/Turabian StyleYang, Ling, Mengmeng Cui, Shiyu Cheng, Shaoqi Zhang, Ying Li, Te Luo, Tianyu Zheng, and Hua Li. 2022. "Effective Electro-Activation Process of Hydrogen Peroxide/Peroxydisulfate Induced by Atomic Hydrogen for Rapid Oxidation of Norfloxacin over the Carbon-Based Pd Nanocatalyst" International Journal of Environmental Research and Public Health 19, no. 19: 12332. https://doi.org/10.3390/ijerph191912332
APA StyleYang, L., Cui, M., Cheng, S., Zhang, S., Li, Y., Luo, T., Zheng, T., & Li, H. (2022). Effective Electro-Activation Process of Hydrogen Peroxide/Peroxydisulfate Induced by Atomic Hydrogen for Rapid Oxidation of Norfloxacin over the Carbon-Based Pd Nanocatalyst. International Journal of Environmental Research and Public Health, 19(19), 12332. https://doi.org/10.3390/ijerph191912332





