Early Detection of SARS-CoV-2 Epidemic Waves: Lessons from the Syndromic Surveillance in Lombardy, Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting
2.2. Data Mining
2.3. Statistical Methods
2.3.1. Statistical Process Control (SPC)
2.3.2. Autoregressive Moving Average (ARMA)
2.3.3. The Exponentially Weighted Moving Average (EWMA) Chart
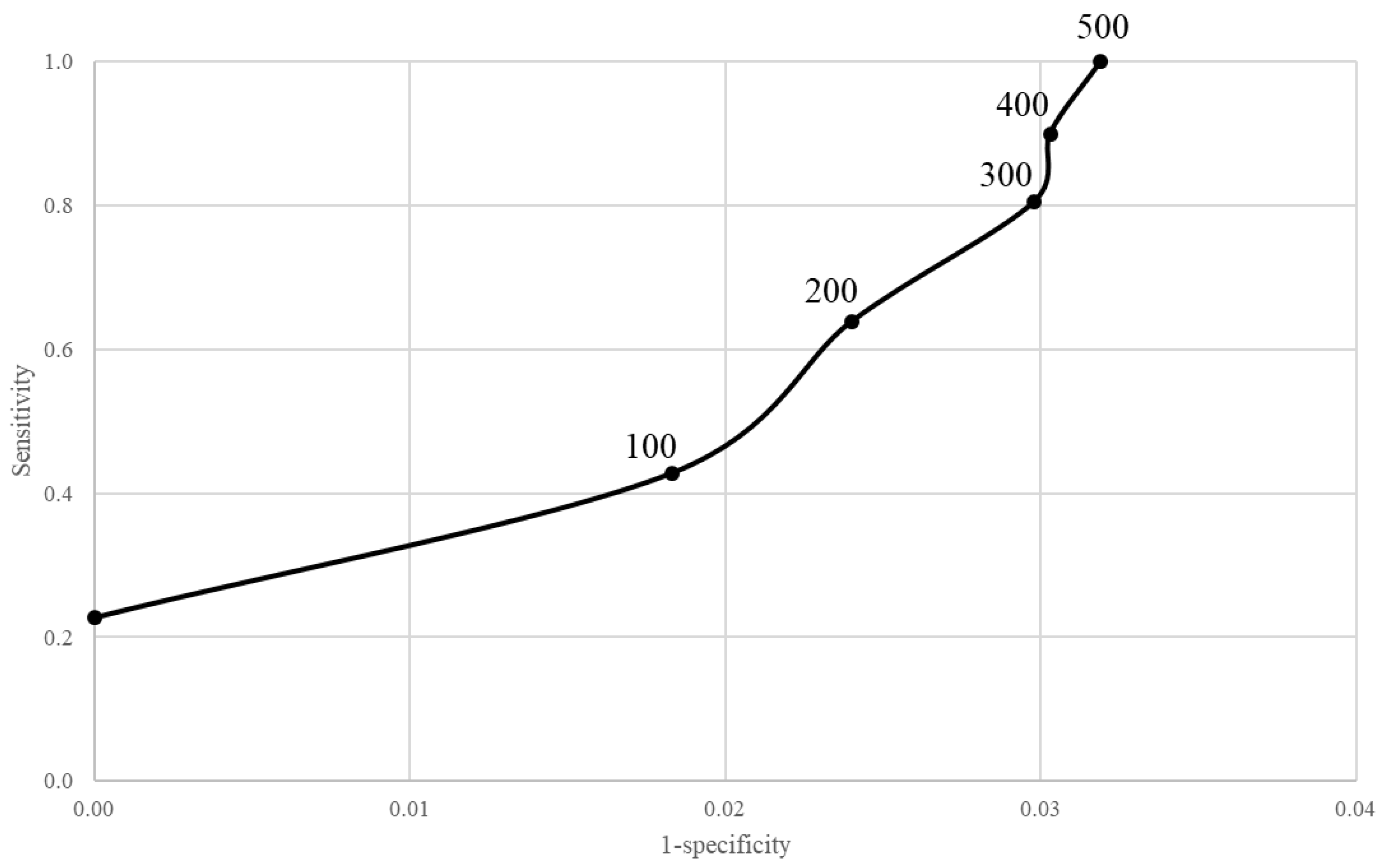
2.4. Model Performance
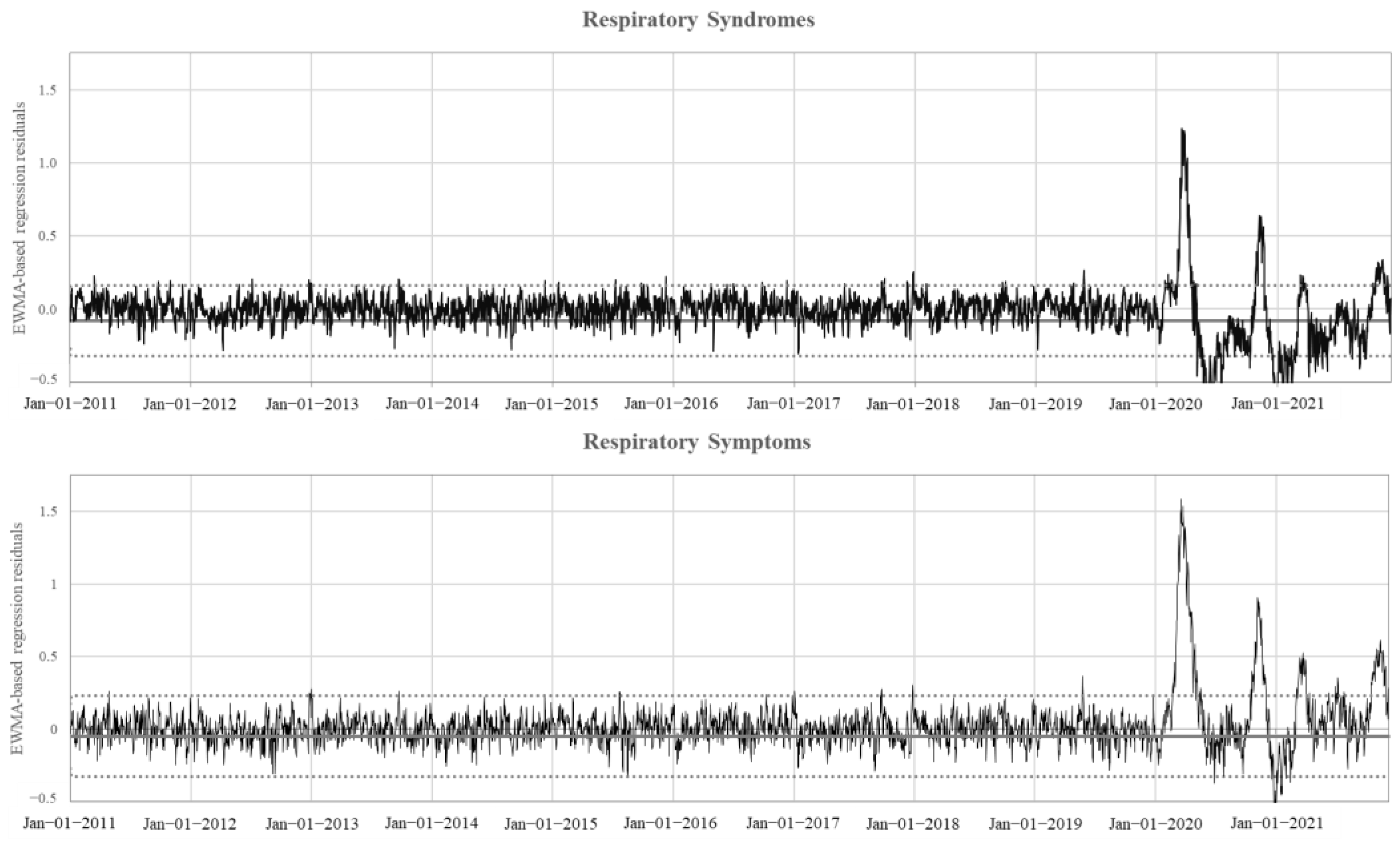
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Noji, E.K.; Toole, M.J. The Historical Development of Public Health Responses to Disasters. Disasters 1997, 21, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Van Bavel, B.J.P.; Curtis, D.R. Better Understanding Disasters by Better Using History. Int. J. Mass Emerg. Disasters 2016, 34, 143–169. [Google Scholar]
- Conti, A.A. Historical and methodological highlights of quarantine measures: From ancient plague epidemics to current coronavirus disease (COVID-19) pandemic. Acta Biomed. 2020, 91, 226–229. [Google Scholar] [PubMed]
- Lazebnik, T.; Bunimovich-Mendrazitsky, S. Generic approach for mathematical model of multi-strain pandemics. PLoS ONE 2022, 17, e0260683. [Google Scholar] [CrossRef]
- Our World in Data. Statistics and Research. Coronavirus Pandemic (COVID-19). Available online: https://ourworldindata.org/coronavirus (accessed on 16 September 2022).
- Lazebnik, T.; Bunimovich-Mendrazitsky, S.; Labib, S. Pandemic management by a spatio–temporal mathematical model. Int. J. Nonlinear Sci. Numer. Simul. 2021. [Google Scholar] [CrossRef]
- Brodeur, A.; Gray, D.; Islam, A.; Bhuiyan, S. A literature review of the economics of COVID-19. J. Econ. Surv. 2021, 35, 1007–1044. [Google Scholar] [CrossRef]
- Khyar, O.; Allali, K. Global dynamics of a multi-strain SEIR epidemic model with general incidence rates: Application to COVID-19 pandemic. Nonlinear Dyn. 2020, 102, 489–509. [Google Scholar] [CrossRef]
- Lazebnik, T.; Blumrosen, G. Advanced multi-mutation with intervention policies pandemic model. IEEE Access 2022, 10, 22769–22781. [Google Scholar] [CrossRef]
- Arruda, E.F.; Das, S.S.; Dias, C.M.; Pastore, D.H. Modelling and optimal control of multi strain epidemics, with application to COVID-19. PLoS ONE 2021, 16, e0257512. [Google Scholar] [CrossRef]
- Yan, P.; Chen, H.; Zeng, D. Syndromic surveillance systems. Ann. Rev. Inf. Sci. Technol. 2008, 42, 425–495. [Google Scholar] [CrossRef]
- Henning, K.J. Overview of syndromic surveillance: What is syndromic surveillance? Morb. Mortal. Wkly. Rep. 2004, 53, 5–11. [Google Scholar]
- Griffin, B.A.; Jain, A.K.; Davies-Cole, J.; Glymph, C.; Lum, G.; Washington, S.C.; A Stoto, M. Early detection of influenza outbreaks using the DC Department of Health’s syndromic surveillance system. BMC Public Health 2009, 9, 483. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Huang, C. The use of CUSUM residual chart to monitor respiratory syndromic data. IIE Trans. 2014, 46, 790–797. [Google Scholar] [CrossRef]
- Oud, M.A.; Almuqrin, M. On the early detecting of the COVID-19 outbreak. J. Infect. Dev. Ctries. 2021, 15, 1625–1629. [Google Scholar] [CrossRef] [PubMed]
- Szarka, J.L., 3rd; Gan, L.; Woodall, W.H. Comparison of the early aberration reporting system (EARS) W2 methods to an adaptive threshold method. Stat. Med. 2011, 30, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, A.S. The EWMA control chart: Properties and comparison with other quality-control procedures by computer simulation. Clin. Chem. 1997, 43, 594–601. [Google Scholar] [CrossRef]
- Albarracin, O.Y.E.; Alencar, A.P.; Ho, L.L. Effect of neglecting autocorrelation in regression EWMA charts for monitoring count time series. Qual. Reliab. Eng. Int. 2018, 34, 1752–1762. [Google Scholar] [CrossRef]
- Lall, R.; Abdelnabi, J.; Ngai, S.; Parton, H.B.; Saunders, K.; Sell, J.; Wahnich, A.; Weiss, D.; Mathes, R.W. Advancing the use of emergency department syndromic surveillance data, New York City, 2012–2016. Public Health Rep. 2017, 132 (Suppl. 1), 23S–30S. [Google Scholar] [CrossRef]
- Brunekreef, T.E.; Otten, H.G.; van den Bosch, S.C.; Hoefer, I.E.; Laar, J.M.; Limper, M.; Haitjema, S. Text mining of electronic health records can accurately identify and characterize patients with systemic lupus erythematosus. ACR Open Rheumatol. 2021, 3, 65–71. [Google Scholar] [CrossRef]
- Chakraborty, G.; Pagolu, M.; Garla, S. Text Mining and Analysis Practical Methods, Examples, and Case Studies Using SAS®; SAS Institute Inc.: Cary, NC, USA, 2013. [Google Scholar]
- Schat, E.; Ceulemans, E. The Exponentially Weighted Moving Average Procedure for Detecting Changes in Intensive Longitudinal Data in Psychological Research in Real-Time: A Tutorial Showcasing Potential Applications. Assessment 2022, 10731911221086985. [Google Scholar] [CrossRef]
- Roberts, S.W. Control chart tests based on geometric moving averages. Technometrics 1959, 1, 239–250. [Google Scholar] [CrossRef]
- Montgomery, D.C. Introduction to Statistical Quality Control, 8th ed.; John Wiley and Sons, Inc.: New York, NY, USA, 2019. [Google Scholar]
- Schat, E.; Tuerlinckx, F.; Smit, A.C.; De Ketelaere, B.; Ceulemans, E. Detecting mean changes in experience sampling data in real time: A comparison of univariate and multivariate statistical process control methods. Psychol. Methods 2021. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Mohinuddin, S.; Arif, M. Spatiotemporal dynamics of temperature and precipitation with reference to COVID-19 pandemic lockdown: Perspective from Indian subcontinent. Environ. Dev. Sustain. 2021, 29, 13778–13818. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.gov.uk/government/publications/uk-covid-19-alert-level-methodology-an-overview/uk-covid-19-alert-level-methodology-an-overview (accessed on 20 September 2022).
- Available online: https://preventepidemics.org/wp-content/uploads/2020/05/Annex-2_Example-of-an-alert-level-system_US_FINAL.pdf (accessed on 20 September 2022).
- Brandal, L.T.; MacDonald, E.; Veneti, L.; Ravlo, T.; Lange, H.; Naseer, U.; Feruglio, S.; Bragstad, K.; Hungnes, O.; Ødeskaug, L.; et al. Outbreak caused by the SARS-CoV-2 Omicron variant in Norway, November to December 2021. Eurosurveillance 2021, 26, 2101147. [Google Scholar] [CrossRef] [PubMed]
- Supharakonsakun, Y.; Areepong, Y.; Sukparungsee, S. The performance of a modified EWMA control chart for monitoring autocorrelated PM2.5 and carbon monoxide air pollution data. PeerJ 2020, 8, e10467. [Google Scholar] [CrossRef] [PubMed]
- Baldewijns, G.; Luca, S.; Vanrumste, B.; Croonenborghs, T. Developing a system that can automatically detect health changes using transfer times of older adults. BMC Med. Res. Methodol. 2016, 16, 23. [Google Scholar] [CrossRef]
- Eren-Oruklu, M.; Cinar, A.; Quinn, L. Hypoglycemia prediction with subject-specific recursive time-series models. J. Diabetes Sci. Technol. 2010, 4, 25–33. [Google Scholar] [CrossRef]
- Liu, L.; Yue, J.; Lai, X.; Huang, J.; Zhang, J. Multivariate nonparametric chart for influenza epidemic monitoring. Sci. Rep. 2019, 9, 17472. [Google Scholar] [CrossRef]
- Steiner, S.H.; Grant, K.; Coory, M.; Kelly, H.A. Detecting the start of an influenza outbreak using exponentially weighted moving average charts. BMC Med. Inform. Decis. Mak. 2010, 10, 37. [Google Scholar] [CrossRef]
- Carson, P.K.; Yeh, A.B. Exponentially weighted moving average (EWMA) control charts for monitoring an analytical process. Ind. Eng. Chem. Res. 2008, 47, 405–411. [Google Scholar] [CrossRef]
- Lipsitch, M.; Hayden, F.G.; Cowling, B.J.; Leung, G.M. How to maintain surveillance for novel influenza A H1N1 when there are too many cases to count. Lancet 2009, 374, 1209–1211. [Google Scholar] [CrossRef]
- Lucas, J.M.; Saccucci, M.S. Exponentially Weighted Moving Average Control Schemes: Properties and Enhancements. Technometrics 2012, 32, 1990. [Google Scholar]
- Corominas, L.; Villez, K.; Aguado, D.; Rieger, L.; Rosén, C.; Vanrolleghem, P.A. Performance evaluation of fault detection methods for wastewater treatment processes. Biotechnol. Bioeng. 2011, 108, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Ip, D.K.; Liao, Q.; Wu, P.; Gao, Z.; Cao, B.; Feng, L.; Xu, X.; Jiang, H.; Li, M.; Bao, J.; et al. Detection of mild to moderate influenza A/H7N9 infection by China’s national sentinel surveillance system for influenza-like illness: Case series. BMJ 2013, 346, f3693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- TRIPLE S. Syndromic Surveillance System in Europe. Guidelines for designing and implementing a syndromic surveillance system. Available online: https://webgate.ec.europa.eu/chafea_pdb/assets/files/pdb/20091112/20091112_d08_giss_en_ps.pdf (accessed on 16 May 2022).


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagarella, G.; Maistrello, M.; Minoja, M.; Leoni, O.; Bortolan, F.; Cereda, D.; Corrao, G. Early Detection of SARS-CoV-2 Epidemic Waves: Lessons from the Syndromic Surveillance in Lombardy, Italy. Int. J. Environ. Res. Public Health 2022, 19, 12375. https://doi.org/10.3390/ijerph191912375
Bagarella G, Maistrello M, Minoja M, Leoni O, Bortolan F, Cereda D, Corrao G. Early Detection of SARS-CoV-2 Epidemic Waves: Lessons from the Syndromic Surveillance in Lombardy, Italy. International Journal of Environmental Research and Public Health. 2022; 19(19):12375. https://doi.org/10.3390/ijerph191912375
Chicago/Turabian StyleBagarella, Giorgio, Mauro Maistrello, Maddalena Minoja, Olivia Leoni, Francesco Bortolan, Danilo Cereda, and Giovanni Corrao. 2022. "Early Detection of SARS-CoV-2 Epidemic Waves: Lessons from the Syndromic Surveillance in Lombardy, Italy" International Journal of Environmental Research and Public Health 19, no. 19: 12375. https://doi.org/10.3390/ijerph191912375
APA StyleBagarella, G., Maistrello, M., Minoja, M., Leoni, O., Bortolan, F., Cereda, D., & Corrao, G. (2022). Early Detection of SARS-CoV-2 Epidemic Waves: Lessons from the Syndromic Surveillance in Lombardy, Italy. International Journal of Environmental Research and Public Health, 19(19), 12375. https://doi.org/10.3390/ijerph191912375





