Effect of COVID-19 Vaccines on Reducing the Risk of Long COVID in the Real World: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Registration and Search Strategy
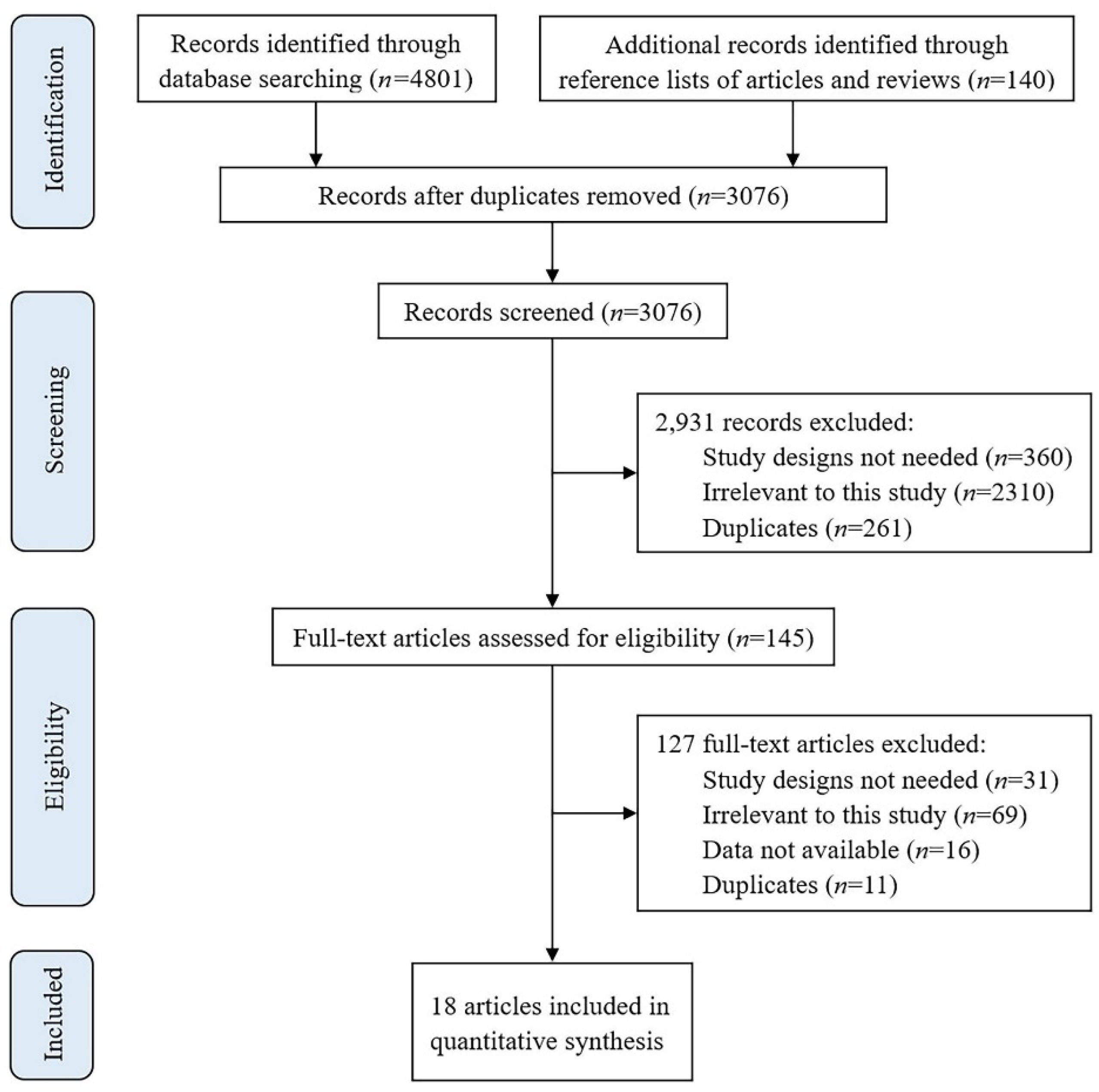
2.2. Study Selection
2.3. Data Extraction
2.4. Quality Assessment of Included Studies
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Included Studies
3.2. Primary Meta-Analysis and Sensitivity Analysis
3.3. Subgroup Analysis
3.4. Meta-Analysis for Long COVID Symptoms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. WHO Coronavirus (COVID-19) Dashboard. 31 August 2022. Available online: https://covid19.who.int (accessed on 1 September 2022).
- Blomberg, B.; Mohn, K.G.-I.; Brokstad, K.A.; Zhou, F.; Linchausen, D.W.; Hansen, B.-A.; Lartey, S.; Onyango, T.B.; Kuwelker, K.; Sævik, M.; et al. Long COVID in a prospective cohort of home-isolated patients. Nat. Med. 2021, 27, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Selvaskandan, H.; Nimmo, A.; Savino, M.; Afuwape, S.; Brand, S.; Graham-Brown, M.; Medcalf, J.; Cockwell, P.; Beckwith, H. Burnout and long COVID among the UK nephrology workforce: Results from a national survey investigating the impact of COVID-19 on working lives. Clin. Kidney J. 2021, 15, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Weldetsadik, A.Y.; Abayneh, M.; Abraha, M.; Betizazu, S.S.; Bekele, D. Clinical Characteristics and Outcome of Pediatric COVID-19 Patients in Ethiopia During the Early COVID-19 Pandemic: A Prospective Cohort Study. Pediatr. Health Med. Ther. 2022, 13, 165–174. [Google Scholar] [CrossRef] [PubMed]
- De Sire, A.; Moggio, L.; Marotta, N.; Agostini, F.; Tasselli, A.; Ferrante, V.D.; Curci, C.; Calafiore, D.; Ferraro, F.; Bernetti, A.; et al. Impact of Rehabilitation on Fatigue in Post-COVID-19 Patients: A Systematic Review and Meta-Analysis. Appl. Sci. 2022, 12, 8593. [Google Scholar] [CrossRef]
- WHO. Coronavirus Disease (COVID-19): Post COVID-19 Condition. 16 December 2021. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/coronavirus-disease-(covid-19)-post-covid-19-condition (accessed on 1 September 2022).
- Ayoubkhani, D.; Bosworth, M.L.; King, S.; Pouwels, K.B.; Glickman, M.; Nafilyan, V.; Zaccardi, F.; Khunti, K.; Alwan, N.A.; Walker, A.S. Risk of Long COVID in people infected with SARS-CoV-2 after two doses of a COVID-19 vaccine: Community-based, matched cohort study. medRxiv, 2022; preprints. [Google Scholar] [CrossRef]
- WHO. A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus, 6 October 2021. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 (accessed on 1 September 2022).
- Raveendran, A.V.; Jayadevan, R.; Sashidharan, S. Long COVID: An overview. Diabetes Metab. Syndr. 2021, 15, 869–875. [Google Scholar] [CrossRef]
- Emecen, A.N.; Keskin, S.; Turunc, O.; Suner, A.F.; Siyve, N.; Sensoy, E.B.; Dinc, F.; Kilinc, O.; Oguz, V.A.; Bayrak, S.; et al. The presence of symptoms within 6 months after COVID-19: A single-center longitudinal study. Ir. J. Med. Sci. 2022. [Google Scholar] [CrossRef]
- Ssentongo, P.; Ssentongo, A.E.; Voleti, N.; Groff, D.; Sun, A.; Ba, D.M.; Nunez, J.; Parent, L.J.; Chinchilli, V.M.; Paules, C.I. SARS-CoV-2 vaccine effectiveness against infection, symptomatic and severe COVID-19: A systematic review and meta-analysis. BMC Infect. Dis. 2022, 22, 439. [Google Scholar] [CrossRef]
- Zheng, C.; Shao, W.; Chen, X.; Zhang, B.; Wang, G.; Zhang, W. Real-world effectiveness of COVID-19 vaccines: A literature review and meta-analysis. Int. J. Infect. Dis. 2021, 114, 252–260. [Google Scholar] [CrossRef]
- Gao, P.; Cai, S.; Liu, Q.; Du, M.; Liu, J.; Liu, M. Effectiveness and Safety of SARS-CoV-2 Vaccines among Children and Adolescents: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 421. [Google Scholar] [CrossRef]
- Mohr, N.M.; Plumb, I.D.; Harland, K.K.; Pilishvili, T.; Flemming-Dutra, K.E.; Krishnadasan, A.; Hoth, K.F.; Saydah, S.H.; Mankoff, Z.; Haran, J.P.; et al. Presence of Symptoms 6 Weeks after COVID-19 among Vaccinated and Unvaccinated U.S. Healthcare Personnel. medRxiv, 2022; preprints. [Google Scholar]
- Azzolini, E.; Levi, R.; Sarti, R.; Pozzi, C.; Mollura, M.; Mantovani, A.; Rescigno, M. Association Between BNT162b2 Vaccination and Long COVID After Infections Not Requiring Hospitalization in Health Care Workers. JAMA 2022, 328, 676–678. [Google Scholar] [CrossRef]
- Wynberg, E.; Han, A.X.; Boyd, A.; van Willigen, H.D.; Verveen, A.; Lebbink, R.; van der Straten, K.; Kootstra, N.; van Gils, M.J.; Russell, C.; et al. The effect of SARS-CoV-2 vaccination on post-acute sequelae of COVID-19 (PASC): A prospective cohort study. Vaccine 2022, 40, 4424–4431. [Google Scholar] [CrossRef] [PubMed]
- Byambasuren, O.; Stehlik, P.; Clark, J.; Alcorn, K.; Glasziou, P. Impact of COVID-19 vaccination on long COVID: A systematic review and meta-analysis. medRxiv, 2022; preprints. [Google Scholar] [CrossRef]
- Notarte, K.I.; Catahay, J.A.; Velasco, J.V.; Pastrana, A.; Ver, A.T.; Pangilinan, F.C.; Peligro, P.J.; Casimiro, M.; Guerrero, J.J.; Gellaco, M.M.L.; et al. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: A systematic review. EClinicalMedicine 2022, 53, 101624. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: https://www.ohri.ca//programs/clinical_epidemiology/oxford.asp (accessed on 30 June 2022).
- Rostom, A.; Dubé, C.; Cranney, A.; Saloojee, N.; Sy, R.; Mack, D.; Patel, D.; McNeal, J.; Moher, D.; Garrity, C.; et al. Celiac Disease. Rockville (MD): Agency for Healthcare Research and Quality (US); 2004 Sep. (Evidence Reports/Technology Assessments, No. 104.) Appendix D. Quality Assessment Forms. 2004. Available online: https://www.ncbi.nlm.nih.gov/books/NBK35156/ (accessed on 24 May 2022).
- Nehme, M.; Braillard, O.; Salamun, J.; Jacquerioz, F.; Courvoisier, D.S.; Spechbach, H.; Guessous, I. Symptoms After COVID-19 Vaccination in Patients with Post-Acute Sequelae of SARS-CoV-2. J. Gen. Intern. Med. 2022, 37, 1585–1588. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, H.Y.; Alrashed, A.M.; Jawhari, A.M.; Abdel-Moneim, A.S. Neuropsychiatric symptoms in post-COVID-19 long haulers. Acta Neuropsychiatr. 2022; in press. [Google Scholar] [CrossRef]
- El Otmani, H.; Nabili, S.; Berrada, M.; Bellakhdar, S.; El Moutawakil, B.; Abdoh Rafai, M. Prevalence, characteristics and risk factors in a Moroccan cohort of Long-COVID-19. Neurol. Sci. 2022, 43, 5175–5180. [Google Scholar] [CrossRef]
- Taquet, M.; Dercon, Q.; Harrison, P.J. Six-month sequelae of post-vaccination SARS-CoV-2 infection: A retrospective cohort study of 10,024 breakthrough infections. Brain Behav. Immun. 2022, 103, 154–162. [Google Scholar] [CrossRef] [PubMed]
- De Arriba Fernández, A.; Alonso Bilbao, J.L.; Espiñeira Francés, A.; Cabeza Mora, A.; Gutiérrez Pérez, Á.; Díaz Barreiros, M.Á.; Serra Majem, L. Assessment of SARS-CoV-2 Infection According to Previous Metabolic Status and Its Association with Mortality and Post-Acute COVID-19. Nutrients 2022, 14, 2925. [Google Scholar] [CrossRef]
- Messiah, S.E.; Hao, T.; DeSantis, S.M.; Swartz, M.D.; Talebi, Y.; Kohl, H.W., III; Zhang, S.; Valerio-Shewmaker, M.; Yaseen, A.; Kelder, S.H.; et al. Comparison of Persistent Symptoms Following SARS-CoV-2 Infection by Antibody Status in Nonhospitalized Children and Adolescents. Pediatr. Infect. Dis. J. 2022; in press. [Google Scholar] [CrossRef]
- Meza-Torres, B.; Delanerolle, G.; Okusi, C.; Mayer, N.; Anand, S.; McCartney, J.; Gatenby, P.; Glampson, B.; Chapman, M.; Curcin, V.; et al. Differences in clinical presentation with long COVID following community and hospital infection, and associations with all-cause mortality: English sentinel network database study (Preprint). JMIR Public Health Surveill. 2022, 8, e37668. [Google Scholar] [CrossRef]
- Peghin, M.; De Martino, M.; Palese, A.; Gerussi, V.; Bontempo, G.; Graziano, E.; Visintini, E.; D’Elia, D.; Dellai, F.; Marrella, F.; et al. Post–COVID-19 syndrome and humoral response association after 1 year in vaccinated and unvaccinated patients. Clin. Microbiol. Infect. 2022, 28, 1140–1148. [Google Scholar] [CrossRef]
- Pinato, D.J.; Ferrante, D.; Aguilar-Company, J.; Bower, M.; Salazar, R.; Mirallas, O.; Sureda, A.; Bertuzzi, A.; Brunet, J.; Lambertini, M.; et al. Vaccination against SARS-CoV-2 protects from morbidity, mortality and sequelae from COVID19 in patients with cancer. Eur. J. Cancer 2022, 171, 64–74. [Google Scholar] [CrossRef]
- Zisis, S.N.; Durieux, J.C.; Mouchati, C.; Perez, J.A.; McComsey, G.A. The Protective Effect of Coronavirus Disease 2019 (COVID-19) Vaccination on Postacute Sequelae of COVID-19: A Multicenter Study From a Large National Health Research Network. Open Forum Infect. Dis. 2022, 9, ofac228. [Google Scholar] [CrossRef]
- Hajjaji, N.; Lepoutre, K.; Lakhdar, S.; Bécourt, S.; Bellier, C.; Kaczmarek, E.; Broyelle, A.; Giscard, S.; Lartigau, E. 16 Months Follow Up of Patients’ Behavior and Mild COVID-19 Patterns in a Large Cohort of Cancer Patients During the Pandemic. Front. Oncol. 2022, 12, 901426. [Google Scholar] [CrossRef] [PubMed]
- Kuodi, P.; Gorelik, Y.; Zayyad, H.; Wertheim, O.; Wiegler, K.B.; Abu Jabal, K.; Dror, A.A.; Nazzal, S.; Glikman, D.; Edelstein, M. Association between BNT162b2 vaccination and reported incidence of post-COVID-19 symptoms: Cross-sectional study 2020-21, Israel. npj Vaccines 2022, 7, 101. [Google Scholar] [CrossRef] [PubMed]
- Al-Aly, Z.; Bowe, B.; Xie, Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat. Med. 2022, 28, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.A.; Luginbuhl, R.D.; Parker, R. Reduced incidence of long-COVID symptoms related to administration of COVID-19 vaccines both before COVID-19 diagnosis and up to 12 weeks after. medRxiv 2021. preprints. [Google Scholar] [CrossRef]
- Budhiraja, S.; Indrayan, A.; Mahajan, M. Effect of COVID-19 vaccine on long-COVID: A 2-year followup observational study from hospitals in north India. medRxiv, 2022; preprints. [Google Scholar] [CrossRef]
- NICE. COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19. 11 November 2021. Available online: https://www.nice.org.uk/guidance/ng188 (accessed on 24 September 2022).
- Liu, Q.; Qin, C.; Liu, M.; Liu, J. Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: A systematic review and meta-analysis. Infect. Dis. Poverty 2021, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- Kow, C.S.; Hasan, S.S. Real-world effectiveness of BNT162b2 mRNA vaccine: A meta-analysis of large observational studies. Inflammopharmacology 2021, 29, 1075–1090. [Google Scholar] [CrossRef] [PubMed]
- Nasreen, S.; Chung, H.; He, S.; Brown, K.A.; Gubbay, J.B.; Buchan, S.A.; Fell, D.B.; Austin, P.C.; Schwartz, K.L.; Sundaram, M.E.; et al. Effectiveness of COVID-19 vaccines against symptomatic SARS-CoV-2 infection and severe outcomes with variants of concern in Ontario. Nat. Microbiol. 2022, 7, 379–385. [Google Scholar] [CrossRef]
- Ayoubkhani, D.; Bermingham, C.; Pouwels, K.B.; Glickman, M.; Nafilyan, V.; Zaccardi, F.; Khunti, K.; Alwan, N.A.; Walker, A.S. Trajectory of long COVID symptoms after COVID-19 vaccination: Community based cohort study. BMJ 2022, 377, e069676. [Google Scholar] [CrossRef]
- Jacobs, L.G.; Paleoudis, E.G.; Bari, D.L.-D.; Nyirenda, T.; Friedman, T.; Gupta, A.; Rasouli, L.; Zetkulic, M.; Balani, B.; Ogedegbe, C.; et al. Persistence of symptoms and quality of life at 35 days after hospitalization for COVID-19 infection. PLoS ONE 2020, 15, e0243882. [Google Scholar] [CrossRef]
- Ziauddeen, N.; Gurdasani, D.; O’Hara, M.E.; Hastie, C.; Roderick, P.; Yao, G.; Alwan, N.A. Characteristics and impact of Long COVID: Findings from an online survey. PLoS ONE 2022, 17, e0264331. [Google Scholar] [CrossRef]
- Suyanto, S.; Kandel, S.; Kemal, R.A.; Arfianti, A. The Quality of Life of Coronavirus Disease Survivors Living in Rural and Urban Area of Riau Province, Indonesia. Infect. Dis. Rep. 2022, 14, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Boaventura, P.; Macedo, S.; Ribeiro, F.; Jaconiano, S.; Soares, P. Post-COVID-19 Condition: Where Are We Now? Life 2022, 12, 517. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Kream, R.M.; Stefano, G.B. Long-Term Respiratory and Neurological Sequelae of COVID-19. Med. Sci. Monit. 2020, 26, e928996. [Google Scholar] [CrossRef] [PubMed]
- Castanares-Zapatero, D.; Chalon, P.; Kohn, L.; Dauvrin, M.; Detollenaere, J.; de Noordhout, C.M.; Jong, C.P.-D.; Cleemput, I.; Heede, K.V.D. Pathophysiology and mechanism of long COVID: A comprehensive review. Ann. Med. 2022, 54, 1473–1487. [Google Scholar] [CrossRef]
- Sivan, M.; Greenhalgh, T.; Milne, R.; Delaney, B. Are vaccines a potential treatment for long covid? BMJ 2022, 377, o988. [Google Scholar] [CrossRef]
- Maglietta, G.; Diodati, F.; Puntoni, M.; Lazzarelli, S.; Marcomini, B.; Patrizi, L.; Caminiti, C. Prognostic Factors for Post-COVID-19 Syndrome: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 1541. [Google Scholar] [CrossRef] [PubMed]
- Yaksi, N.; Teker, A.G.; Imre, A. Long COVID in Hospitalized COVID-19 Patients: A Retrospective Cohort Study. Iran. J. Public Health 2022, 51, 88–95. [Google Scholar] [CrossRef]
- Scherlinger, M.; Felten, R.; Gallais, F.; Nazon, C.; Chatelus, E.; Pijnenburg, L.; Mengin, A.; Gras, A.; Vidailhet, P.; Arnould-Michel, R.; et al. Refining “Long-COVID” by a Prospective Multimodal Evaluation of Patients with Long-Term Symptoms Attributed to SARS-CoV-2 Infection. Infect. Dis. Ther. 2021, 10, 1747–1763. [Google Scholar] [CrossRef]
- Venkatesan, P. Do vaccines protect from long COVID? Lancet Respir. Med. 2022, 10, E30. [Google Scholar] [CrossRef]


| Study ID | Study Design | Nationality of Population | Age (Mean ± SD or Range) (Years) | Vaccination Time | Type of Vaccine | Definition of Long COVID * | Sample Size for Meta-Analysis | Quality Assessment |
|---|---|---|---|---|---|---|---|---|
| Nehme 2022 [21] | Cross-sectional study | Switzerland | 43.5 ± 13.7 | After SARS-CoV-2 infection | mRNA-1273, BNT162b2 | Presence of fatigue, difficulty concentrating or memory loss, loss of or change in smell, loss of or change in taste, shortness of breath, and headache more than 6 months after an infection | 1596 | Low risk |
| Ayoubkhani 2022 [7] | Cohort study | UK | 18–69 | Before SARS-CoV-2 infection | ChAdOx1 nCoV-19, BNT162b2, mRNA-1273 | Presence of symptoms more than 4 weeks after the first having COVID-19, that are not explained by something else | 6180 | Moderate risk |
| Kuodi 2022 [32] | Cross-sectional study | Israel | ≥19 | Before and after SARS-CoV-2 infection | Mainly BNT162b2 | No clear definition | 951 | Low risk |
| Alghamdi 2022 [22] | Cross-sectional study | Saudi Arabia | 12–70 | NA | ChAdOx1 nCoV-19, BNT162b2 | No clear definition | 2218 | Moderate risk |
| Simon 2021 [34] | Cohort study | USA | NA | Before and after COVID-19 diagnosis | NA | Presence of one or more COVID-associated symptoms between 12 and 20 weeks after the initial COVID-19 diagnosis | 240,648 | Low risk |
| Taquet 2022 [24] | Cohort study | USA | 57.0 ± 17.9 | Before SARS-CoV-2 infection | BNT162b2, mRNA-1273, Ad26.COV2.S, other COVID-19 vaccines | Presence of chest/throat pain, abnormal breathing, abdominal symptoms, fatigue/malaise, anxiety/depression, pain, headache, cognitive dysfunction, and myalgia between 90 and 120 days after COVID-19 diagnosis | 9953 | Low risk |
| Otmani 2022 [23] | Case-control study | Morocco | NA | After contracting the COVID-19 infection | NA | Guideline published by the NICE | 118 | Low risk |
| Azzolini 2022 [15] | Cohort study | Italy | 44.3 ± 10.7 (with long COVID); 41.2 ± 11.4 (without long COVID) | Before SARS-CoCV-2 infection | BNT162b2 | Prescence at least 1 SARS-CoV-2-related symptom with a duration of more than 4 weeks | 739 | Moderate risk |
| Wynberg 2022 [16] | Cohort study | Netherlands | 53.5 (IQR: 41.0–64.0) | After SARS-CoV-2 infection | BNT162b2, mRNA-1273, ChAdOx1 nCoV-19, Ad26.COV2.S | Criteria published by the WHO | 315 | Low risk |
| Al-Aly 2022 [33] | Cohort study | USA | 66.63 ± 13.84 | Before SARS-CoV-2 infection | BNT162b2, mRNA-1273, Ad26.COV2.S | The symptoms starting from 30 days after the first positive SARS-CoV-2 test | 147,414 | Low risk |
| Fernández 2022 [25] | Cohort study | Spain | 41.0 ± 16.8 | Before or after COVID-19 diagnosis | BNT162b2, mRNA-1273, ChAdOx1 nCoV-19, Ad26.COV2.S | Prescence of symptoms that persisted for more than 3 weeks after the initial infection and cannot be explained by other causes | 110,726 | Low risk |
| Messiah 2022 [26] | Cohort study | USA | 5–19 | NA | NA | Guideline published by the NICE | 1748 | Low risk |
| Meza-Torres 2022 [27] | Cohort study | UK | 44.5 ± 21.77 | Before or after COVID-19 diagnosis | NA | Presence of fatigue, breathlessness, cognitive dysfunction, and a variety of other symptoms occurring more than 28 days after COVID-19 infection | 408,882 | Low risk |
| Peghin 2022 [28] | Cohort study | Italy | ≥18 | After COVID-19 diagnosis | BNT162b2, mRNA-1273, ChAdOx1 nCoV-19, Ad26.COV2.S | Guideline published by the NICE | 479 | Low risk |
| Pinato 2022 [29] | Cohort study | UK, Italy, Spain | ≥18 | Before SARS-CoV-2 infection | BNT162b2, mRNA-1273, ChAdOx1 nCoV-19, Ad26.COV2.S | Presence of long-term effects start at least 4 weeks after infection | 1228 | Low risk |
| Zisis 2022 [30] | Cohort study | USA | ≥18 | After COVID-19 diagnosis | NA | Prescence of new, continuing, or recurrent symptoms that occur 4 or more weeks after the initial SARS-CoV-2 infection | 50,450 | Low risk |
| Budhiraja 2022 [35] | Cross-sectional study | India | <18-≥75 | Before COVID-19 diagnosis | ChAdOx1nCoV-19, a whole-virion inactivated vero cell derived vaccine (available as Covaxin in India) | Presence of any symptoms after discharge from the hospital | 5529 | Low risk |
| Hajjaji 2022 [31] | Cross-sectional study | France | ≥18 | NA | NA | Persistent symptoms of SARS-CoV-2 infection lasting more than 6 months | 168 | Moderate risk |
| Subgroups | The Number of Studies | The Number of People | I2 (%) | RR (95% CI) | p Value of Meta-analysis |
|---|---|---|---|---|---|
| The number of vaccine doses | |||||
| 1 dose | 6 | 655,962 | 99 | 0.83 (0.65–1.07) | 0.14 |
| 2 doses | 7 | 420,402 | 90 | 0.83 (0.74–0.94) | <0.01 |
| Age | |||||
| <60 years | 3 | 12,415 | 89 | 0.76 (0.54–1.06) | 0.11 |
| ≥60 years | 2 | 9509 | 55 | 0.87 (0.60–1.24) | 0.43 |
| Vaccination time | |||||
| Before SARS-CoV-2 infection/COVID-19 | 6 | 180,996 | 97 | 0.82 (0.74–0.91) | <0.01 |
| After SARS-CoV-2 infection/COVID-19 | 4 | 2508 | 24 | 0.83 (0.74–0.92) | <0.01 |
| Definition of long COVID | |||||
| Presence of symptoms more than 4 weeks after SARS-CoV-2 infection/COVID-19 diagnisis * | 7 | 419,374 | 87 | 0.68 (0.53–0.87) | <0.01 |
| Other definitions | 8 | 526,302 | 99 | 0.75 (0.64–0.88) | <0.01 |
| Long COVID Symptom | The Number of Studies | The number of People | I2 (%) | RR (95% CI) | p Value of Meta-Analysis |
|---|---|---|---|---|---|
| Anxiety and/or depression | 4 | 28,604 | 70 | 0.83 (0.67–1.03) | 0.08 |
| Chest or throat pain | 3 | 26,386 | 0 | 1.01 (0.95–1.08) | 0.67 |
| Cognitive dysfunction/symptoms | 2 | 22,124 | 8 | 0.89 (0.83–0.96) | <0.01 |
| Fatigue | 6 | 225,478 | 97 | 0.77 (0.58–1.02) | 0.07 |
| Hair loss | 2 | 6480 | 50 | 0.86 (0.62–1.19) | 0.37 |
| Headache/migraine | 4 | 76,836 | 99 | 0.95 (0.50–1.79) | 0.87 |
| Kidney diseases/problems | 2 | 148,365 | 0 | 0.68 (0.64–0.73) | <0.01 |
| Loss of concentration | 2 | 6480 | 71 | 0.65 (0.35–1.19) | 0.16 |
| Loss of smell | 3 | 8698 | 75 | 0.67 (0.36–1.26) | 0.21 |
| Loss of taste | 3 | 8698 | 68 | 0.71 (0.48–1.07) | 0.10 |
| Myalgia | 2 | 25,435 | 15 | 0.68 (0.62–0.74) | <0.01 |
| Nausea and/or vomiting | 2 | 6480 | 87 | 0.80 (0.31–2.02) | 0.63 |
| Respiratory symptoms/sequelae | 5 | 78,064 | 98 | 0.91 (0.60–1.40) | 0.68 |
| Sleeping disorders/problem sleeping | 3 | 8698 | 25 | 0.74 (0.64–0.86) | <0.01 |
| Weight loss | 2 | 6480 | 95 | 1.24 (0.22–7.05) | 0.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, P.; Liu, J.; Liu, M. Effect of COVID-19 Vaccines on Reducing the Risk of Long COVID in the Real World: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 12422. https://doi.org/10.3390/ijerph191912422
Gao P, Liu J, Liu M. Effect of COVID-19 Vaccines on Reducing the Risk of Long COVID in the Real World: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2022; 19(19):12422. https://doi.org/10.3390/ijerph191912422
Chicago/Turabian StyleGao, Peng, Jue Liu, and Min Liu. 2022. "Effect of COVID-19 Vaccines on Reducing the Risk of Long COVID in the Real World: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 19, no. 19: 12422. https://doi.org/10.3390/ijerph191912422
APA StyleGao, P., Liu, J., & Liu, M. (2022). Effect of COVID-19 Vaccines on Reducing the Risk of Long COVID in the Real World: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 19(19), 12422. https://doi.org/10.3390/ijerph191912422









