Transition from Laser to Intravitreal Injections for Diabetic Retinopathy: Hospital Utilization and Costs from an Extended Healthcare Perspective
Abstract
1. Introduction
2. Methods
2.1. Study Design and Patient Population
2.2. Costs
2.3. Analyses and Outcomes
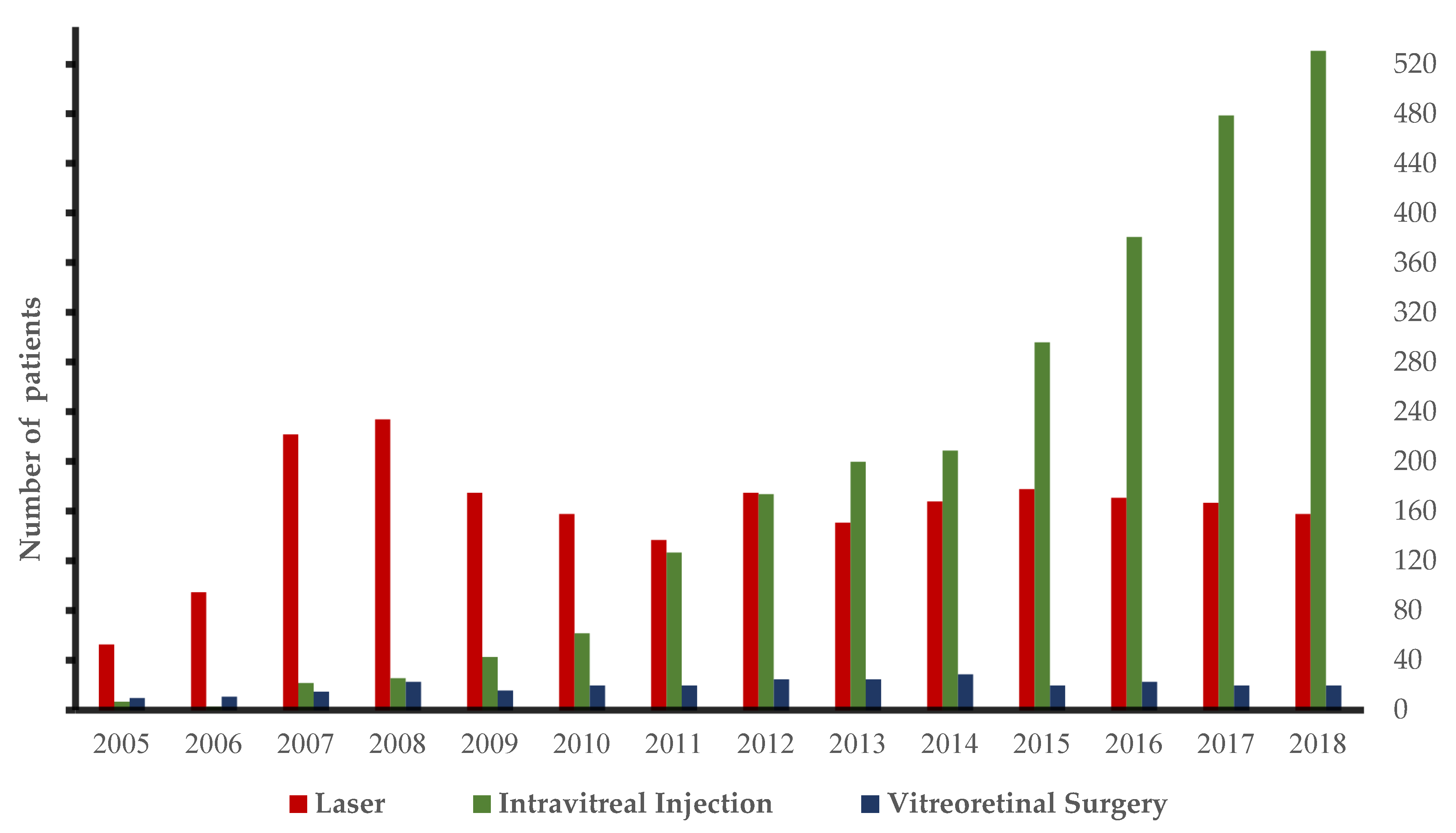
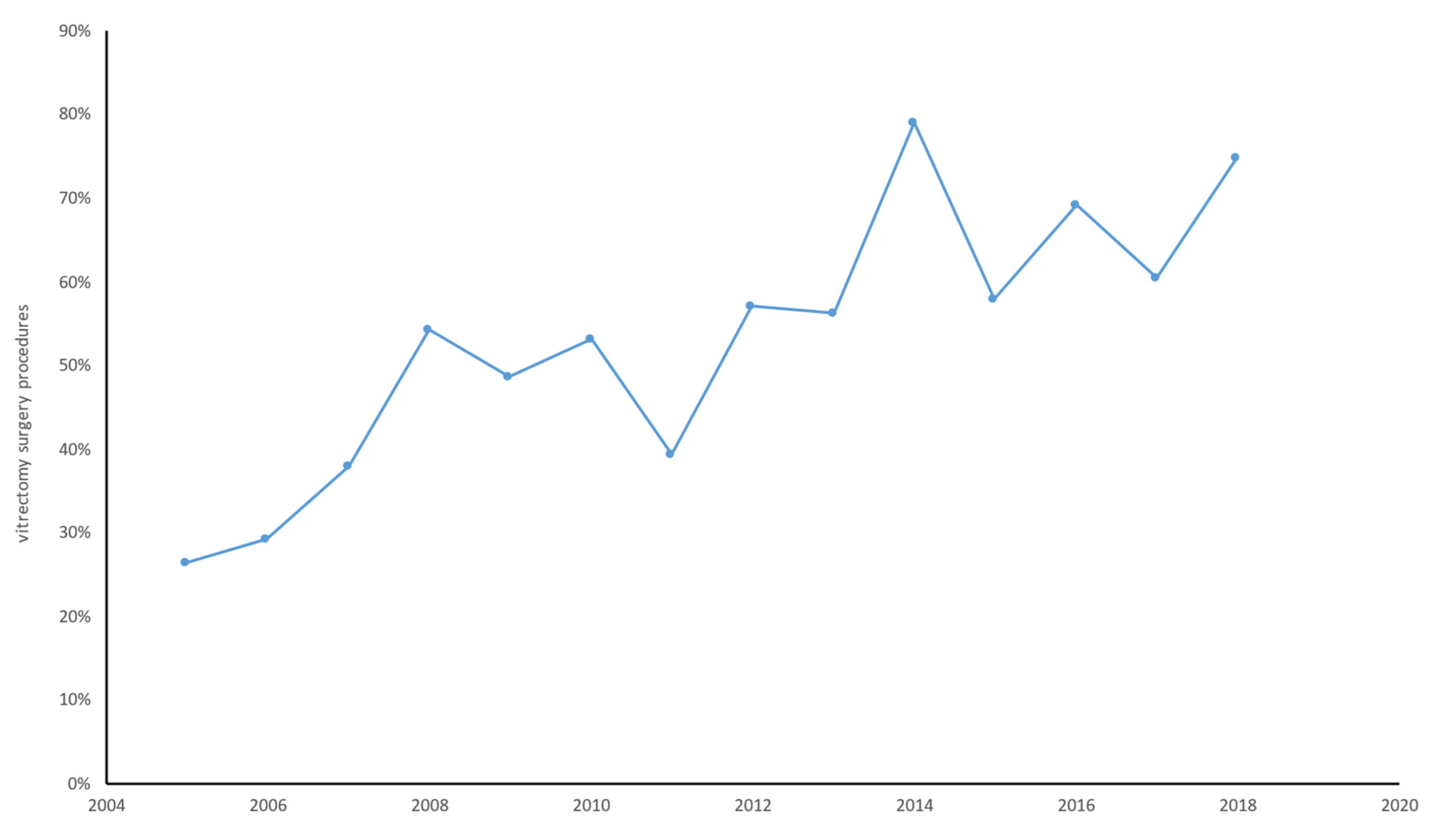
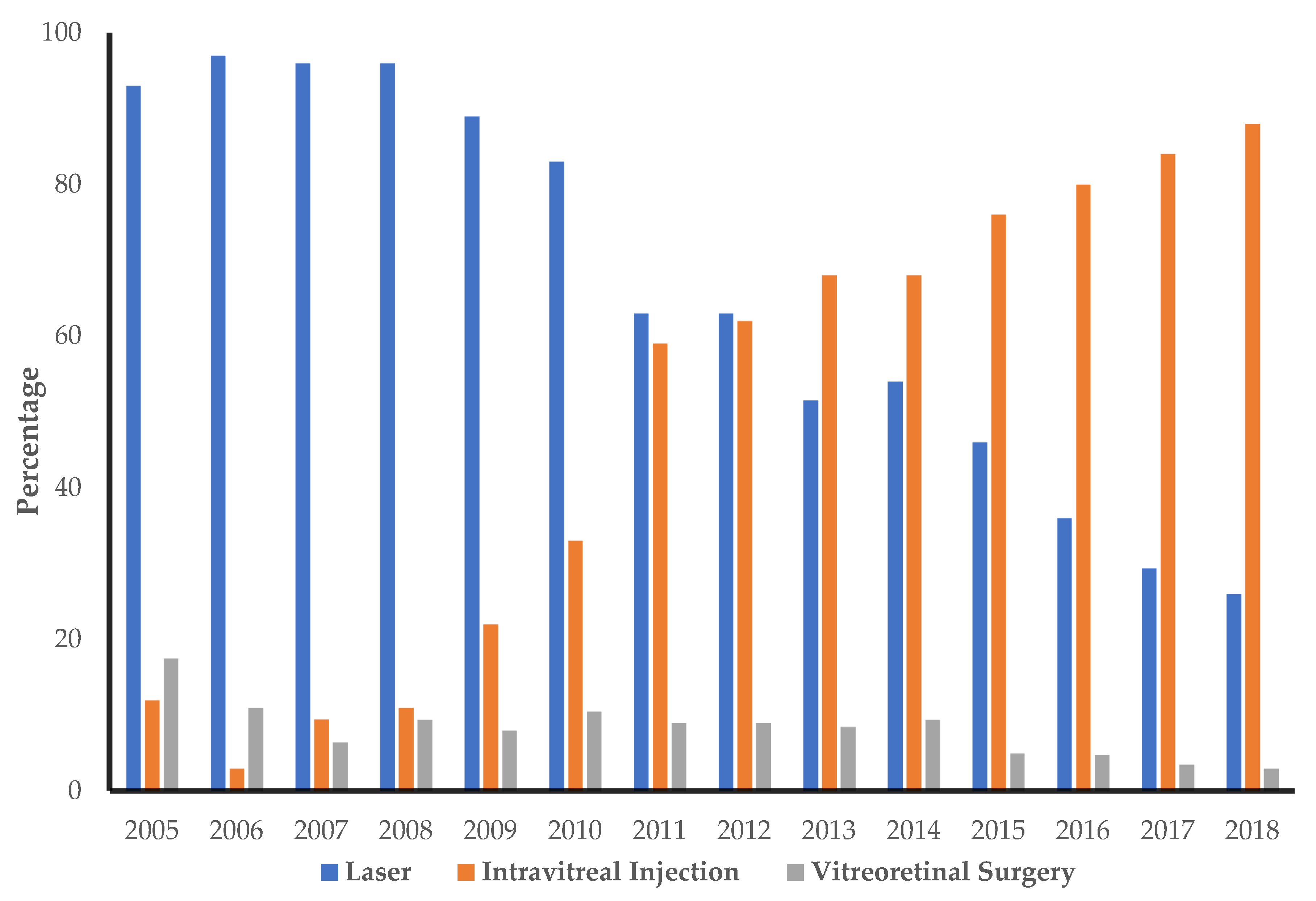
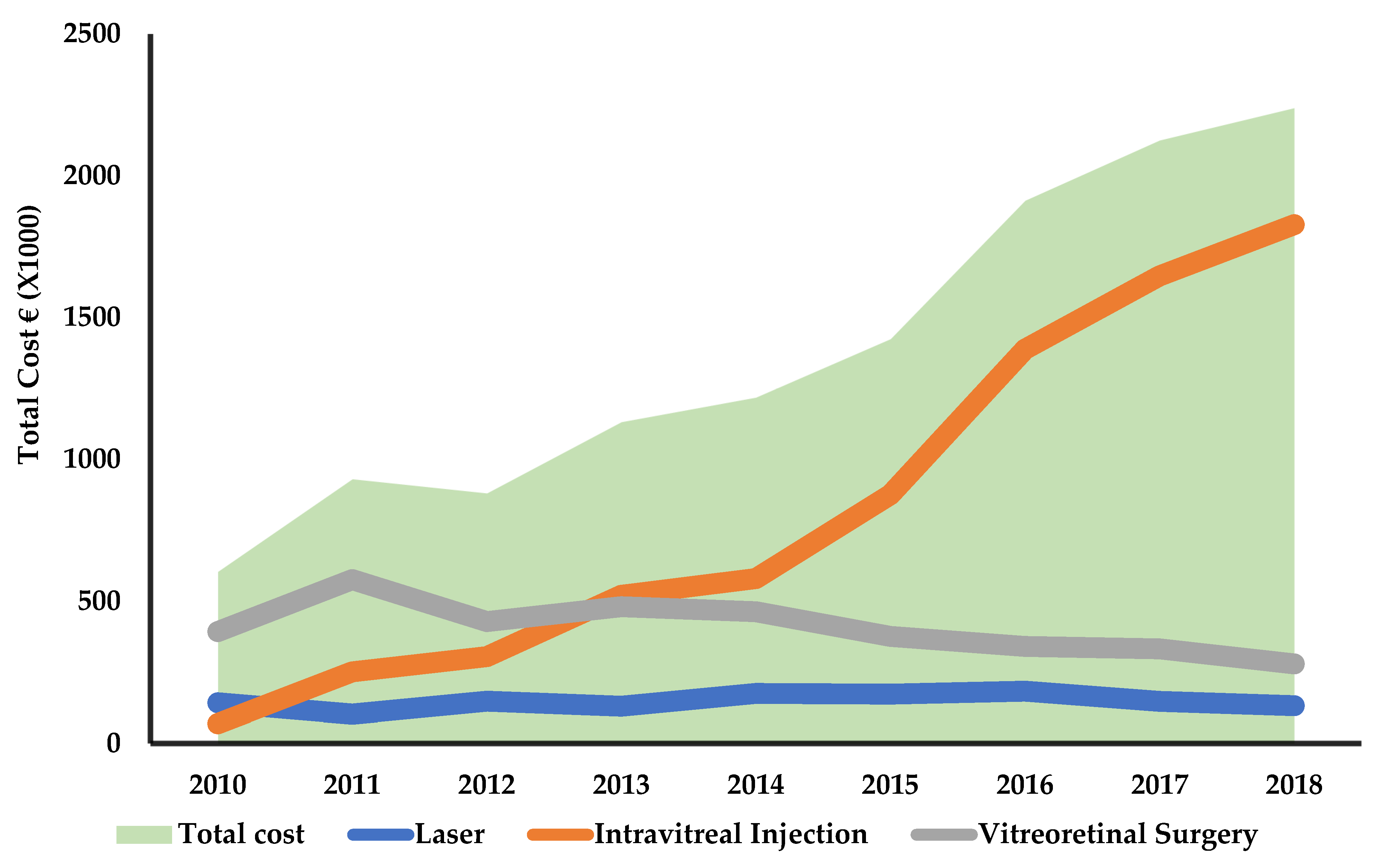
3. Results
3.1. Hospital Utilization
3.2. Treatment Costs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- World Healh Organisation (WHO). Global Report on Diabetes; World Healh Organisation: Geneva, Switzerland, 2016; Volume 978.
- European Society of Cardiology (ESC). Global Statistics on Diabetes; ESC Press Office: Sophia, France; Available online: https://www.escardio.org/Education/Diabetes-and-CVD/Recommended-Reading/global-statistics-on-diabetes (accessed on 9 March 2020).
- International Diabetes Federation (IDF). Diabetic Mocular Edema. Available online: https://idf.org/54-our-activities/562-diabetic-macular-edema-dme.html (accessed on 9 March 2020).
- Scanlon, P.H.; Wilkinson, C.P.; Aldington, S.J.; Matthews, D.R. A Practical Manual of Diabetic Retinopathy Management; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Neubauer, A.S.; Ulbig, M.W. Laser Treatment in Diabetic Retinopathy. Ophthalmologica 2007, 221, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Gardner, T.W.; Chew, E.Y. Future Opportunities in Diabetic Retinopathy Research. Curr. Opin. Endocrinol. Diabetes Obesity. 2016, 23, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Ollendorf, D.A.; Colby, J.A.; Pearson, S.D. Comparative Effectiveness of Anti-Vegf Agents for Diabetic Macular Edema. Int. J. Technol. Assess. Health Care 2013, 29, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Heier, J.S.; Bressler, N.M.; Avery, R.L.; Bakri, S.J.; Boyer, D.S.; Brown, D.M.; Dugel, P.U.; Freund, K.B.; Glassman, A.R.; Kim, J.E.; et al. Comparison of Aflibercept, Bevacizumab, and Ranibizumab for Treatment of Diabetic Macular Edema: Extrapolation of Data to Clinical Practice. JAMA Ophthalmol. 2016, 134, 95–99. [Google Scholar] [CrossRef]
- Wells, J.A.; Glassman, A.R.; Ayala, A.R.; Jampol, L.M.; Aiello, L.P.; Antoszyk, A.N.; Arnold-Bush, B.; Baker, C.W.; Bressler, N.M.; Browning, D.J.; et al. Aflibercept, Bevacizumab, or Ranibizumab for Diabetic Macular Edema: Two-Year Results from a Comparative Effectiveness Randomized Clinical Trial. Ophthalmology. Ophthalmology 2016, 123, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Matonti, F.; Pommier, S.; Meyer, F.; Hajjar, C.; Merite, P.Y.; Parrat, E.; Rouhette, H.; Rebollo, O.; Guigou, S. Long-Term Efficacy and Safety of Intravitreal Dexamethasone Implant for the Treatment of Diabetic Macular Edema. Eur. J. Ophthalmol. 2016. [Google Scholar] [CrossRef]
- Boyer, D.S.; Hopkins, J.J.; Sorof, J.; Ehrlich, J.S. Anti-Vascular Endothelial Growth Factor Therapy for Diabetic Macular Edema. Ther. Adv. Endocrinol. Metabolism. 2013, 4, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Berrocal, M.H.; Acaba-Berrocal, L. Early Pars Plana Vitrectomy for Proliferative Diabetic Retinopathy: Update and Review of Current Literature. Curr. Opin. Ophthalmol. 2021, 32, 203–208. [Google Scholar] [CrossRef]
- Berrocal, M.H.; Chenworth, M.L.; Acaba, L.A. Management of Giant Retinal Tear Detachments. J. Ophthalmic Vis. Res. 2017, 12, 93–97. [Google Scholar] [CrossRef]
- Jackson, T.L.; Nicod, E.; Angelis, A.; Grimaccia, F.; Prevost, A.T.; Simpson, A.R.H.; Kanavos, P. Pars Plana Vitrectomy for Vitreomacular Traction Syndrome: A Systematic Review and Metaanalysis of Safety and Efficacy. Retina 2013, 33, 2012–2017. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Garcia-Arumi, J.; Bandello, F.; Berg, K.; Chakravarthy, U.; Gerendas, B.S.; Jonas, J.; Larsen, M.; Tadayoni, R.; Loewenstein, A. Guidelines for the Management of Diabetic Macular Edema by the European Society of Retina Specialists (EURETINA). Ophthalmologica 2017, 237, 185–222. [Google Scholar] [CrossRef] [PubMed]
- Wirostko, B.; Beusterien, K.; Grinspan, J.; Ciulla, T.; Gonder, J.; Barsdorf, A.; Pleil, A. Patient Preferences in the Treatment of Diabetic Retinopathy. Patient Prefer. Adherence 2011, 5, 229–237. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mason, L.; Crosson, J.N.; Mason, J.O.; Mcgwin, G. Patient Preferences with Regard to Laser versus Intravitreal Injections in the Treatment of Diabetic Macular Edema. J. Ophthalmol. 2017, 2017, 7398470. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, B.P.; Schachat, A.P. A Review of Clinical Trials of Anti-VEGF Agents for Diabetic Retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2010, 248, 915–930. [Google Scholar] [CrossRef] [PubMed]
- Diabetic Retinopathy Clinical Research Network. A Phase II Randomized Clinical Trial of Intravitreal Bevacizumab for Diabetic Macular Edema. Ophthalmology 2007, 10, 1860–1867. [Google Scholar]
- Singer, M.A.; Bell, D.J.; Woods, P.; Pollard, J.; Boord, T.; Herro, A.; Porbandarwalla, S. Effect of Combination Therapy with Bevacizumab and Dexamethasone Intravitreal Implant in Patients with Retinal Vein Occlusion. Retina 2012, 32, 1289–1294. [Google Scholar] [CrossRef]
- Nazari, H.; Modarres, M.; Parvaresh, M.M.; Ghasemi Falavarjani, K. Intravitreal Bevacizumab in Combination with Laser Therapy for the Treatment of Severe Retinopathy of Prematurity (ROP) Associated with Vitreous or Retinal Hemorrhage. Graefe’s Arch. Clin. Exp. Ophthalmol. 2010, 248, 1713–1718. [Google Scholar] [CrossRef]
- Diabetic Retinopathy Clinical Research Network, (DRCR); Wells, J.A.; Glassman, A.R.; Ayala, A.R.; Jampol, L.M.; Aiello, L.P.; Antoszyk, A.N.; Arnold-Bush, B.; Baker, C.W.; Bressler, N.M.; et al. Aflibercept, Bevacizumab, or Ranibizumab for Diabetic Macular Edema. N. Engl. J. Med. 2015, 372, 1193–1203. [Google Scholar] [CrossRef]
- Norwegian Medicines Agency. Guidelines for the Submission of Documentation for Single Technology Assessment (STA) of Pharmaceuticals. Available online: https://legemiddelverket.no/english/public-funding-and-pricing/documentation-for-sta/guidelines-for-the-submission-of-documentation-for-single-technology-assessment-sta-of-pharmaceuticals (accessed on 22 January 2021).
- NoMA. Unit Costs. Available online: https://legemiddelverket.no/Documents/Offentligfinansieringogpris/Dokumentasjontilmetodevurdering/Enhetskostnadsdatabase_kostnader_0040618.pdf (accessed on 9 March 2020).
- Helsedirektoratet. DRG-Systemet. Available online: https://www.helsedirektoratet.no/tema/finansiering/innsatsstyrt-finansiering-og-drg-systemet/drg-systemet#drglogikkdefinisjonstabeller (accessed on 21 September 2022).
- Lode, H.E.; Gjølberg, T.T.; Foss, S.; Sivertsen, M.S.; Brustugun, J.; Andersson, Y.; Jørstad, Ø.K.; Moe, M.C.; Andersen, J.T. A New Method for Pharmaceutical Compounding and Storage of Anti-VEGF Biologics for Intravitreal Use in Silicone Oil-Free Prefilled Plastic Syringes. Sci. Rep. 2019, 9, 18021. [Google Scholar] [CrossRef]
- Helsedirektoratet. Regelverk for Innsatsstyrt Finansiering 2021 (ISF-Regelverket). Available online: https://www.helsedirektoratet.no/tema/finansiering/innsatsstyrt-finansiering-og-drg-systemet/innsatsstyrt-finansieringisf#:~:text=kodeverkene%20fra%202022-,Regelverk%202021,behandling%20av%20rusmiddelavhengige%20(TSB) (accessed on 30 September 2022).
- StataCorp. Stata Statistical Software: Release 16; StataCorp LLC: College Station, TX, USA, 2016. [Google Scholar]
- Gross, J.G.; Glassman, A.R.; Jampol, L.M.; Inusah, S.; Aiello, L.P.; Antoszyk, A.N.; Baker, C.W.; Berger, B.B.; Bressler, N.M.; Browning, D.; et al. Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. JAMA J. Am. Med. Assoc. 2015, 314, 2137–2146. [Google Scholar] [CrossRef]
- Glassman, A.R.; Beaulieu, W.T.; Maguire, M.G.; Antoszyk, A.N.; Chow, C.C.; Elman, M.J.; Jampol, L.M.; Salehi-Had, H.; Sun, J.K. Visual Acuity, Vitreous Hemorrhage, and Other Ocular Outcomes after Vitrectomy vs Aflibercept for Vitreous Hemorrhage Due to Diabetic Retinopathy: A Secondary Analysis of a Randomized Clinical Trial. JAMA Ophthalmol. 2021, 139, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Tóth, G.; Limburg, H.; Szabó, D.; Sándor, G.L.; Nagy, Z.Z.; Németh, J. Rapid Assessment of Avoidable Blindness-Based Healthcare Costs of Diabetic Retinopathy in Hungary and Its Projection for the Year 2045. Br. J. Ophthalmol. 2021, 105, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Kim, Y.Y.; Jo, Y.J.; Oh, J.; Lee, J.E.; Lee, J.E.; Park, D.H.; Kang, S.W.; Lee, W.K.; Kim, H.K.; et al. Healthcare Utilization and Treatment Patterns in Diabetic Macular Edema in Korea: A Retrospective Chart Review. J. Korean Med. Sci. 2019, 34, e118. [Google Scholar] [CrossRef] [PubMed]
- Spooner, K.L.; Guinan, G.; Koller, S.; Hong, T.; Chang, A.A. Burden Of Treatment Among Patients Undergoing Intravitreal Injections For Diabetic Macular Oedema In Australia. Diabetes Metab. Syndr. Obes. 2019, 12, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.M.; Wykoff, C.C.; Boyer, D.; Heier, J.S.; Clark, W.L.; Emanuelli, A.; Higgins, P.M.; Singer, M.; Weinreich, D.M.; Yancopoulos, G.D.; et al. Evaluation of Intravitreal Aflibercept for the Treatment of Severe Nonproliferative Diabetic Retinopathy: Results from the PANORAMA Randomized Clinical Trial. JAMA Ophthalmol. 2021, 139, 946–955. [Google Scholar] [CrossRef]
- Haas, M.; Hall, J. The Economic Evaluation of Health Care; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Happich, M.; Reitberger, U.; Breitscheidel, L.; Ulbig, M.; Watkins, J. The Economic Burden of Diabetic Retinopathy in Germany in 2002. Graefe’s Arch. Clin. Exp. Ophthalmol. 2008, 246, 151–159. [Google Scholar] [CrossRef]
- Shea, A.M.; Curtis, L.H.; Hammill, B.G.; Kowalski, J.W.; Ravelo, A.; Lee, P.P.; Sloan, F.A.; Schulman, K.A. Resource Use and Costs Associated With Diabetic Macular Edema in Elderly Persons. Arch. Ophthalmol. 2008, 126, 1748–1754. [Google Scholar] [CrossRef]
- Sun, J.K. Intravitreal Anti-VEGF Therapy with Prompt or Deferred Laser Compared with Steroid with Prompt Laser and Prompt Laser Alone for Treatment of Diabetic Macular Edema. Curr. Diab. Rep. 2011, 11, 227–229. [Google Scholar] [CrossRef]
- Kiss, S.; Liu, Y.; Brown, J.; Holekamp, N.M.; Almony, A.; Campbell, J.; Kowalski, J.W. Clinical Utilization of Anti-Vascular Endothelial Growth-Factor Agents and Patient Monitoring in Retinal Vein Occlusion and Diabetic Macular Edema. Clin. Ophthalmol. 2014, 8, 1611–1621. [Google Scholar] [CrossRef]
- Mensah-Debrah, A.; Amissah Arthur, K.N.; Kumah, D.B.; Akuffo, K.O.; Osei Duah, I.; Bascaran, C. Situational Analysis of Diabetic Retinopathy Treatment Services in Ghana. BMC Health Serv. Res. 2021, 21, 584. [Google Scholar] [CrossRef]
- Teo, Z.L.; Tham, Y.C.; Yu, M.; Cheng, C.Y.; Wong, T.Y.; Sabanayagam, C. Do We Have Enough Ophthalmologists to Manage Vision-Threatening Diabetic Retinopathy? A Global Perspective. Eye 2020, 34, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Bonora, E.; Cataudella, S.; Marchesini, G.; Miccoli, R.; Vaccaro, O.; Fadini, G.P.; Martini, N.; Rossi, E. Clinical Burden of Diabetes in Italy in 2018: A Look at a Systemic Disease from the ARNO Diabetes Observatory. BMJ Open Diab. Res. Care 2020, 8, e001191. [Google Scholar] [CrossRef] [PubMed]
- Caro, J.J.; Ward, A.J.; O’Brien, J.A. Lifetime Costs of Complications Resulting from Type 2 Diabetes in the U.S. Diabetes Care 2002, 25, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Köster, I.; Von Ferber, L.; Ihle, P.; Schubert, I.; Hauner, H. The Cost Burden of Diabetes Mellitus: The Evidence from Germany—CoDiM Study. Diabetologia 2006, 49, 1498–1504. [Google Scholar] [CrossRef] [PubMed]
- Seuring, T.; Archangelidi, O.; Suhrcke, M. The Economic Costs of Type 2 Diabetes: A Global Systematic Review. Pharmacoeconomics 2015, 33, 811–831. [Google Scholar] [CrossRef]
- Gillies, M.C.; Lim, L.L.; Campain, A.; Quin, G.J.; Salem, W.; Li, J.; Goodwin, S.; Aroney, C.; McAllister, I.L.; Fraser-Bell, S. A Randomized Clinical Trial of Intravitreal Bevacizumab versus Intravitreal Dexamethasone for Diabetic Macular Edema: The BEVORDEX Study. Ophthalmology 2014, 121, 2473–2481. [Google Scholar] [CrossRef]
- Iovino, C.; Mastropasqua, R.; Lupidi, M.; Bacherini, D.; Pellegrini, M.; Bernabei, F.; Borrelli, E.; Sacconi, R.; Carnevali, A.; D’aloisio, R.; et al. Intravitreal Dexamethasone Implant as a Sustained Release Drug Delivery Device for the Treatment of Ocular Diseases: A Comprehensive Review of the Literature. Pharmaceutics 2020, 12, 703. [Google Scholar] [CrossRef]
- Muchira, J.; Stuart-shor, E.; Kariuki, J.; Mukuna, A.; Ndigirigi, I.; Gakage, L.; Mutuma, V.; Karani, A. International Journal of Africa Nursing Sciences Distribution and Characteristics of Risk Factors for Cardiovascular—Metabolic Disease in a Rural Kenyan Community. Int. J. Afr. Nurs. Sci. 2015, 3, 76–81. [Google Scholar] [CrossRef][Green Version]
- Hertzberg, S.N.; Moe, M.C.; Jørstad, Ø.K.; Petrovski, B.É.; Burger, E.; Petrovski, G. Healthcare Expenditure of Intravitreal Anti-vascular Endothelial Growth Factor Inhibitors Compared with Dexamethasone Implant for Diabetic Macular Oedema. Acta Ophthalmol. 2022. [Google Scholar] [CrossRef]
- Berg, K.; Roald, A.B.; Navaratnam, J.; Bragadóttir, R. An 8-Year Follow-up of Anti-Vascular Endothelial Growth Factor Treatment with a Treat-and-Extend Modality for Neovascular Age-Related Macular Degeneration. Acta Ophthalmol. 2017, 95, 796–802. [Google Scholar] [CrossRef]
- Okoye, O.; Okonkwo, O.; Oderinlo, O.; Hassan, K.; Ijasan, A. Bilateral Concomitant Intravitreal Anti-Vascular Endothelial Growth Factor Injection: Experience in a Nigerian Tertiary Private Eye Care Facility. Niger. J. Clin. Pract. 2016, 19, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Giocanti-Auregan, A.; Tadayoni, R.; Grenet, T.; Fajnkuchen, F.; Nghiem-Buffet, S.; Delahaye-Mazza, C.; Quentel, G.; Cohen, S.Y. Estimation of the Need for Bilateral Intravitreal Anti-VEGF Injections in Clinical Practice. BMC Ophthalmol. 2016, 16, 142. [Google Scholar] [CrossRef]
- Eichenbaum, D.A.; Duerr, E.; Patel, H.R.; Pollack, S.M. Monthly Versus Treat-and-Extend Ranibizumab for Diabetic Macular Edema: A Prospective, Randomized Trial. Ophthalmic Surg. Lasers Imaging Retin. 2018, 49, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.F.; Rosenberg, D.; Deonarain, D.M.; Phillips, M.R.; Thabane, L.; Kaiser, P.; Garg, S.; Sivaprasad, S.; Wykoff, C.C.; Chaudhary, V.; et al. Treat-and-Extend Versus Alternate Dosing Strategies with Anti-Vascular Endothelial Growth Factor Agents to Treat Center Involving Diabetic Macular Edema: A Sys- Tematic Review and Meta-Analysis of 2346 Eyes. Surv. Ophthalmol. 2022, 67, 1346–1363. [Google Scholar] [CrossRef]
- Wykoff, C.C.; Abreu, F.; Adamis, A.P.; Basu, K.; Eichenbaum, D.A.; Haskova, Z.; Lin, H.; Loewenstein, A.; Mohan, S.; Pearce, I.A.; et al. Efficacy, Durability, and Safety of Intravitreal Faricimab with Extended Dosing up to Every 16 Weeks in Patients with Diabetic Macular Oedema (YOSEMITE and RHINE): Two Randomised, Double-Masked, Phase 3 Trials. Lancet 2022, 399, 741–755. [Google Scholar] [CrossRef]
- Chia, M.A.; Keane, P.A. Beyond Anti-VEGF: Can Faricimab Reduce Treatment Burden for Retinal Disease? Lancet 2022, 399, 697–699. [Google Scholar] [CrossRef]
- Memon, S.; Ahsan, S.; Alvi, R.; Fawwad, A.; Basit, A.; Shera, S.; Sheikh, S.A.; Fahim, M.F. Retinal Screening Acceptance, Laser Treatment Uptake and Follow-up Response in Diabetics Requiring Laser Therapy in an Urban Diabetes Care Centre. J. Coll. Physicians Surg. Pak. 2015, 25, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Kashim, R.M.; Newton, P.; Ojo, O. Diabetic Retinopathy Screening: A Systematic Review on Patients’ Non-Attendance. Int. J. Environ. Res. Public Health 2018, 15, 157. [Google Scholar] [CrossRef]
- Kilstad, H.N.; Sjølie, A.K.; Gøransson, L.; Hapnes, R.; Henschien, H.J.; Alsbirk, K.E.; Fossen, K.; Bertelsen, G.; Holstad, G.; Bergrem, H. Prevalence of Diabetic Retinopathy in Norway: Report from a Screening Study. Acta Ophthalmol. 2012, 90, 609–612. [Google Scholar] [CrossRef]
- Cai, C.X.; Li, Y.; Zeger, S.L.; McCarthy, M.L. Social Determinants of Health Impacting Adherence to Diabetic Retinopathy Examinations. BMJ Open Diabetes Res. Care 2021, 9, e002374. [Google Scholar] [CrossRef]
- Kao, C.C.; Hsieh, H.M.; Lee, D.Y.; Hsieh, K.P.; Sheu, S.J. Importance of Medication Adherence in Treatment Needed Diabetic Retinopathy. Sci. Rep. 2021, 11, 19100. [Google Scholar] [CrossRef] [PubMed]


| Item | Unit Cost in EUR (Excluding VAT) | Source |
|---|---|---|
| DR treatment per treatment per patient | ||
| Laser | 164.25 | 36R (The Norwegian Directorate of Health (DofH), 2021) [27] |
| Intravitreal injection | 137.45 | NoMA Unit cost database |
| Vitreoretinal Surgery | 8504.59 | 36E (DofH, 2021) [27] |
| Visit to specialist cost | 70.22 | NoMA Unit cost database |
| Intraocular pressure measurement cost | 70.25 | NoMA Unit cost database |
| Intravitreal injection drugs per injection per patient | ||
| Bevacizumab | 38.24 | NoMA Drug cost database |
| Ranibizumab | 338.89 | NoMA Drug cost database |
| Aflibercept | 326.75 | NoMA Drug cost database |
| Dexamethasone implant | 1075.08 | NoMA Drug cost database |
| Patient time and transport | ||
| Transport cost per journey | 58.17 | NoMA Unit cost database |
| Patient time—cost per hour | 23.61 | NoMA Unit cost database |
| 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | Average 2005–2018 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Laser | 1.09 (0.14) | 1.81 (0.17) | 1.87 (0.10) | 1.88 (0.10) | 1.65 (0.10) | 1.72 (0.13) | 1.04 (0.08) | 1.24 (0.09) | 1.04 (0.10) | 1.38 (0.11) | 1.07 (0.08) | 0.95 (0.07) | 0.64 (0.05) | 0.54 (0.05) | 2.2 (0.04) |
| Intravitreal injections | 0.08 (0.03) | 0.02 (0.01) | 0.10 (0.02) | 0.16 (0.03) | 0.35 (0.06) | 0.70 (0.10) | 1.95 (0.18) | 2.05 (0.15) | 2.74 (0.20) | 2.78 (0.17) | 3.20 (0.16) | 4.33 (0.17) | 4.36 (0.15) | 4.73 (0.14) | 4.7 (0.07) |
| Vitreoretinal surgery | 0.54 (0.06) | 0.40 (0.05) | 0.20 (0.03) | 0.22 (0.03) | 0.20 (0.03) | 0.22 (0.03) | 0.27 (0.04) | 0.16 (0.02) | 0.18 (0.03) | 0.17 (0.03) | 0.11 (0.02) | 0.08 (0.01) | 0.07 (0.01) | 0.05 (0.01) | 1.2 (0.02) |
| Treated patients | 91 | 128 | 260 | 265 | 217 | 208 | 249 | 300 | 315 | 318 | 405 | 487 | 580 | 611 |
| Year | Treatment | Observed Average (EUR) | Bootstrapped Standard Error | 95% Confidence Interval | Total Cost per Patient |
|---|---|---|---|---|---|
| 2010 | Laser | 899 | 59 | 785–1014 | 2906 |
| Intravitreal injection | 1116 | 115 | 892–1341 | ||
| Vitreoretinal Surgery | 10,348 | 555 | 9261–11,436 | ||
| 2011 | Laser | 750 | 43 | 666–833 | 3737 |
| Intravitreal injection | 1977 | 141 | 1701–2253 | ||
| Vitreoretinal surgery | 10,882 | 733 | 9445–12,318 | ||
| 2012 | Laser | 844 | 50 | 747–941 | 2935 |
| Intravitreal injection | 1752 | 94 | 1567–1937 | ||
| Vitreoretinal surgery | 9308 | 325 | 8672–9945 | ||
| 2013 | Laser | 862 | 57 | 751–973 | 3592 |
| Intravitreal injection | 2604 | 146 | 2316–2891 | ||
| Vitreoretinal surgery | 10,226 | 554 | 9140–11,312 | ||
| 2014 | Laser | 1043 | 61 | 924–1161 | 3831 |
| Intravitreal injection | 2774 | 131 | 2517–3032 | ||
| Vitreoretinal surgery | 12,188 | 1168 | 9898–14,478 | ||
| 2015 | Laser | 971 | 52 | 868–1073 | 3518 |
| Intravitreal injection | 2960 | 115 | 2735–3184 | ||
| Vitreoretinal surgery | 10,438 | 678 | 9109–11,766 | ||
| 2016 | Laser | 1073 | 52 | 972–1174 | 3928 |
| Intravitreal injection | 3645 | 115 | 3419–3871 | ||
| Vitreoretinal surgery | 9737 | 607 | 8547–10,927 | ||
| 2017 | Laser | 880 | 45 | 792–967 | 3664 |
| Intravitreal injection | 3437 | 103 | 3236–3638 | ||
| Vitreoretinal surgery | 10,377 | 611 | 9180–11,573 | ||
| 2018 | Laser | 836 | 41 | 755–917 | 3665 |
| Intravitreal injection | 3442 | 89 | 3268–3616 | ||
| Vitreoretinal surgery | 10,357 | 670 | 9043–11,670 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hertzberg, S.N.W.; Jørstad, Ø.K.; Petrovski, B.É.; Bragadottir, R.; Steffensen, L.A.; Moe, M.C.; Burger, E.A.; Petrovski, G. Transition from Laser to Intravitreal Injections for Diabetic Retinopathy: Hospital Utilization and Costs from an Extended Healthcare Perspective. Int. J. Environ. Res. Public Health 2022, 19, 12603. https://doi.org/10.3390/ijerph191912603
Hertzberg SNW, Jørstad ØK, Petrovski BÉ, Bragadottir R, Steffensen LA, Moe MC, Burger EA, Petrovski G. Transition from Laser to Intravitreal Injections for Diabetic Retinopathy: Hospital Utilization and Costs from an Extended Healthcare Perspective. International Journal of Environmental Research and Public Health. 2022; 19(19):12603. https://doi.org/10.3390/ijerph191912603
Chicago/Turabian StyleHertzberg, Silvia Nanjala Walekhwa, Øystein K. Jørstad, Beáta Éva Petrovski, Ragnheidur Bragadottir, Leif Arthur Steffensen, Morten Carstens Moe, Emily A. Burger, and Goran Petrovski. 2022. "Transition from Laser to Intravitreal Injections for Diabetic Retinopathy: Hospital Utilization and Costs from an Extended Healthcare Perspective" International Journal of Environmental Research and Public Health 19, no. 19: 12603. https://doi.org/10.3390/ijerph191912603
APA StyleHertzberg, S. N. W., Jørstad, Ø. K., Petrovski, B. É., Bragadottir, R., Steffensen, L. A., Moe, M. C., Burger, E. A., & Petrovski, G. (2022). Transition from Laser to Intravitreal Injections for Diabetic Retinopathy: Hospital Utilization and Costs from an Extended Healthcare Perspective. International Journal of Environmental Research and Public Health, 19(19), 12603. https://doi.org/10.3390/ijerph191912603









