Association of Japanese and Mediterranean Dietary Patterns with Muscle Weakness in Japanese Community-Dwelling Middle-Aged and Older Adults: Post Hoc Cross-Sectional Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Dietary Survey
2.3. Japanese Dietary Pattern
2.4. Mediterranean Dietary Pattern
2.5. Muscle Weakness
2.6. Other Variables
2.7. Statistical Analysis
2.8. Ethical Considerations
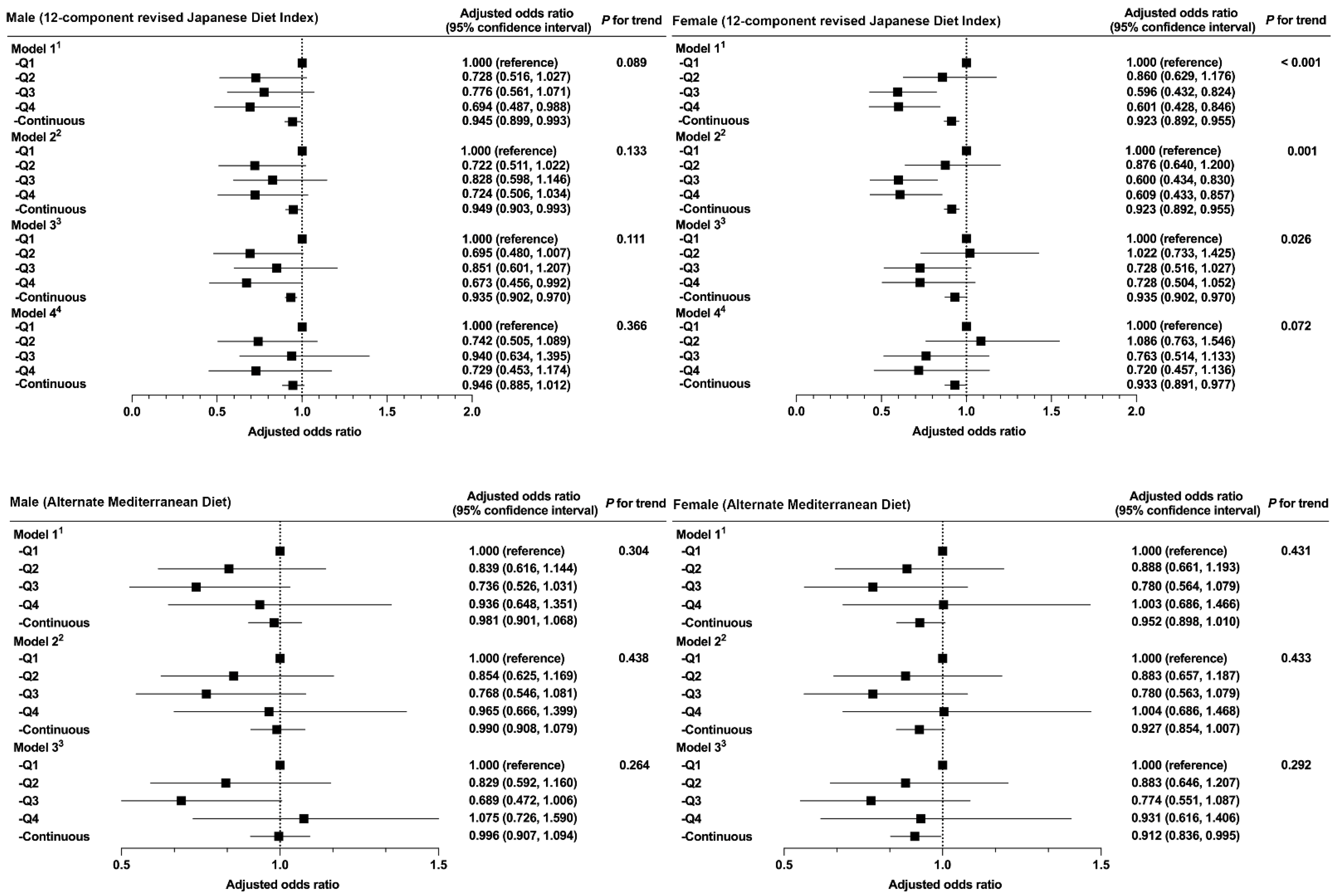
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tada, N.; Maruyama, C.; Koba, S.; Tanaka, H.; Birou, S.; Teramoto, T.; Sasaki, J. Japanese dietary lifestyle and cardiovascular disease. J. Atheroscler. Thromb. 2011, 18, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean diet, its components, and cardiovascular disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef]
- Zhang, S.; Tomata, Y.; Sugawara, Y.; Tsuduki, T.; Tsuji, I. The Japanese dietary pattern is associated with longer disability-free survival time in the general elderly population in the Ohsaki Cohort 2006 Study. J. Nutr. 2019, 149, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Tomata, Y.; Watanabe, T.; Sugawara, Y.; Chou, W.T.; Kakizaki, M.; Tsuji, I. Dietary patterns and incident functional disability in elderly Japanese: The Ohsaki Cohort 2006 Study. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Mitrou, P.N.; Kipnis, V.; Thiébaut, A.C.; Reedy, J.; Subar, A.F.; Wirfalt, E.; Flood, A.; Mouw, T.; Hollenbeck, A.R.; Leitzmann, M.F.; et al. Mediterranean dietary pattern and prediction of all-cause mortality in a US population: Results from the NIH-AARP Diet and Health Study. Arch. Intern. Med. 2007, 167, 2461–2468. [Google Scholar] [CrossRef] [PubMed]
- Abe, S.; Zhang, S.; Tomata, Y.; Tsuduki, T.; Sugawara, Y.; Tsuji, I. Japanese diet and survival time: The Ohsaki Cohort 1994 Study. Clin. Nutr. 2020, 39, 298–303. [Google Scholar] [CrossRef]
- Tomata, Y.; Zhang, S.; Kaiho, Y.; Tanji, F.; Sugawara, Y.; Tsuji, I. Nutritional characteristics of the Japanese diet: A cross-sectional study of the correlation between Japanese diet index and nutrient intake among community-based elderly Japanese. Nutrition 2019, 57, 115–121. [Google Scholar] [CrossRef]
- Zhang, S.; Otsuka, R.; Tomata, Y.; Shimokata, H.; Tange, C.; Tomida, M.; Nishita, Y.; Matsuyama, S.; Tsuji, I. A cross-sectional study of the associations between the traditional Japanese diet and nutrient intakes: The NILS-LSA project. Nutr. J. 2019, 18, 43. [Google Scholar] [CrossRef]
- Liu, H.X.; Ding, G.; Yu, W.J.; Liu, T.F.; Yan, A.Y.; Chen, H.Y.; Zhang, A.H. Association between frailty and incident risk of disability in community-dwelling elder people: Evidence from a meta-analysis. Public Health 2019, 175, 90–100. [Google Scholar] [CrossRef]
- Nakamura, K.; Yoshida, D.; Honda, T.; Hata, J.; Shibata, M.; Hirakawa, Y.; Furuta, Y.; Kishimoto, H.; Ohara, T.; Kitazono, T.; et al. Prevalence and mortality of sarcopenia in a community-dwelling older Japanese population: The Hisayama Study. J. Epidemiol. 2021, 31, 320–327. [Google Scholar] [CrossRef]
- Satake, S.; Arai, H. The revised Japanese version of the Cardiovascular Health Study criteria (revised J-CHS criteria). Geriatr. Gerontol. Int. 2020, 20, 992–993. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.C.; Manini, T.M. Sarcopenia =/= dynapenia. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 829–834. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307 e2. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, S.; Travison, T.G.; Manini, T.M.; Patel, S.; Pencina, K.M.; Fielding, R.A.; Magaziner, J.M.; Newman, A.B.; Kiel, D.P.; Cooper, C.; et al. Sarcopenia Definition: The Position Statements of the Sarcopenia Definition and Outcomes Consortium. J. Am. Geriatr. Soc. 2020, 68, 1410–1418. [Google Scholar] [CrossRef]
- Wind, A.E.; Takken, T.; Helders, P.J.; Engelbert, R.H. Is grip strength a predictor for total muscle strength in healthy children, adolescents, and young adults? Eur. J. Pediatr. 2010, 169, 281–287. [Google Scholar] [CrossRef]
- Suga, H.; Hashimoto, H. Age threshold for recommending higher protein intake to prevent age-related muscle weakness: A cross-sectional study in Japan. PLoS ONE 2018, 13, e0208169. [Google Scholar] [CrossRef]
- Sahni, S.; Dufour, A.B.; Fielding, R.A.; Newman, A.B.; Kiel, D.P.; Hannan, M.T.; Jacques, P.F. Total carotenoid intake is associated with reduced loss of grip strength and gait speed over time in adults: The Framingham Offspring Study. Am. J. Clin. Nutr. 2021, 113, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Pahor, M.; Bartali, B.; Cherubini, A.; Penninx, B.W.; Williams, G.R.; Atkinson, H.; Martin, A.; Guralnik, J.M.; Ferrucci, L. Antioxidants and physical performance in elderly persons: The Invecchiare in Chianti (InCHIANTI) Study. Am. J. Clin. Nutr. 2004, 79, 289–294. [Google Scholar] [CrossRef]
- Barrea, L.; Muscogiuri, G.; Di Somma, C.; Tramontano, G.; De Luca, V.; Illario, M.; Colao, A.; Savastano, S. Association between Mediterranean diet and hand grip strength in older adult women. Clin. Nutr. 2019, 38, 721–729. [Google Scholar] [CrossRef]
- Yoshida, D.; Ninomiya, T.; Doi, Y.; Hata, J.; Fukuhara, M.; Ikeda, F.; Mukai, N.; Kiyohara, Y. Prevalence and causes of functional disability in an elderly general population of Japanese: The Hisayama Study. J. Epidemiol. 2012, 22, 222–229. [Google Scholar] [CrossRef]
- Tomata, Y.; Sugiyama, K.; Kaiho, Y.; Honkura, K.; Watanabe, T.; Zhang, S.; Sugawara, Y.; Tsuji, I. Dietary patterns and incident dementia in elderly Japanese: The Ohsaki Cohort 2006 Study. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 1322–1328. [Google Scholar] [CrossRef]
- Jung, H.; Tanaka, S.; Iwamoto, Y.; Kawano, T.; Yamasaki, M.; Tanaka, R. Reductions in muscle strength and range of motion cause locomotion disability via locomotion-related functional limitation in Japanese older adults: A cross-sectional study. J. Aging Res. 2021, 2021, 6627767. [Google Scholar] [CrossRef]
- Sum, G.; Ishida, M.; Koh, G.C.; Singh, A.; Oldenburg, B.; Lee, J.T. Implications of multimorbidity on healthcare utilisation and work productivity by socioeconomic groups: Cross-sectional analyses of Australia and Japan. PLoS ONE 2020, 15, e0232281. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Comparison of relative validity of food group intakes estimated by comprehensive and brief-type self-administered diet history questionnaires against 16 d dietary records in Japanese adults. Public Health Nutr. 2011, 14, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Honda, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Both comprehensive and brief self-administered diet history questionnaires satisfactorily rank nutrient intakes in Japanese adults. J. Epidemiol. 2012, 22, 151–159. [Google Scholar] [CrossRef]
- Saji, N.; Tsuduki, T.; Murotani, K.; Hisada, T.; Sugimoto, T.; Kimura, A.; Niida, S.; Toba, K.; Sakurai, T. Relationship between the Japanese-style diet, gut microbiota, and dementia: A cross-sectional study. Nutrition 2021, 94, 111524. [Google Scholar] [CrossRef]
- Suzuki, N.; Goto, Y.; Ota, H.; Kito, K.; Mano, F.; Joo, E.; Ikeda, K.; Inagaki, N.; Nakayama, T. Characteristics of the Japanese diet described in epidemiologic publications: A qualitative systematic review. J. Nutr. Sci. Vitaminol. 2018, 64, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Abe, S.K.; Saito, E.; Sawada, N.; Tsugane, S.; Ito, H.; Lin, Y.; Tamakoshi, A.; Sado, J.; Kitamura, Y.; Sugawara, Y.; et al. Coffee consumption and mortality in Japanese men and women: A pooled analysis of eight population-based cohort studies in Japan (Japan Cohort Consortium). Prev. Med. 2019, 123, 270–277. [Google Scholar] [CrossRef]
- Chen, G.D.; Dong, X.W.; Zhu, Y.Y.; Tian, H.Y.; He, J.; Chen, Y.M. Adherence to the Mediterranean diet is associated with a higher BMD in middle-aged and elderly Chinese. Sci. Rep. 2016, 6, 25662. [Google Scholar] [CrossRef]
- Fung, T.T.; Meyer, H.E.; Willett, W.C.; Feskanich, D. Association between diet quality scores and risk of hip fracture in postmenopausal women and men aged 50 years and older. J. Acad. Nutr. Diet. 2018, 118, 2269–2279.e4. [Google Scholar] [CrossRef]
- Fung, T.T.; Rexrode, K.M.; Mantzoros, C.S.; Manson, J.E.; Willett, W.C.; Hu, F.B. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation 2009, 119, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Koyano, W.; Shibata, H.; Nakazato, K.; Haga, H.; Suyama, Y. Measurement of competence: Reliability and validity of the TMIG index of competence. Arch. Gerontol. Geriatr. 1991, 13, 103–116. [Google Scholar] [CrossRef]
- Doyev, R.; Axelrod, R.; Keinan-Boker, L.; Shimony, T.; Goldsmith, R.; Nitsan, L.; Dichtiar, R.; Sinai, T. Energy intake is highly associated with handgrip strength in community-dwelling elderly adults. J. Nutr. 2021, 151, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Alhussain, M.H.; MM, A.L. Association between fish consumption and muscle mass and function in middle-age and older adults. Front. Nutr. 2021, 8, 746880. [Google Scholar] [CrossRef]
- Ganapathy, A.; Nieves, J.W. Nutrition and sarcopenia-what do we know? Nutrients 2020, 12, 1755. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, F.; Hashimoto, Y.; Kaji, A.; Sakai, R.; Kawate, Y.; Okamura, T.; Kitagawa, N.; Okada, H.; Nakanishi, N.; Majima, S.; et al. Habitual miso (fermented soybean paste) consumption is associated with a low prevalence of sarcopenia in patients with type 2 diabetes: A cross-sectional study. Nutrients 2020, 13, 72. [Google Scholar] [CrossRef]
- Tak, Y.J.; Lee, J.G.; Yi, Y.H.; Kim, Y.J.; Lee, S.; Cho, B.M.; Cho, Y.H. Association of handgrip strength with dietary intake in the Korean population: Findings based on the seventh Korea National Health and Nutrition Examination Survey (KNHANES VII-1), 2016. Nutrients 2018, 10, 1180. [Google Scholar] [CrossRef]
- Bartali, B.; Frongillo, E.A.; Stipanuk, M.H.; Bandinelli, S.; Salvini, S.; Palli, D.; Morais, J.A.; Volpato, S.; Guralnik, J.M.; Ferrucci, L. Protein intake and muscle strength in older persons: Does inflammation matter? J. Am. Geriatr. Soc. 2012, 60, 480–484. [Google Scholar] [CrossRef]
- Frampton, J.; Murphy, K.G.; Frost, G.; Chambers, E.S. Higher dietary fibre intake is associated with increased skeletal muscle mass and strength in adults aged 40 years and older. J. Cachexia Sarcopenia Muscle 2021, 12, 2134–2144. [Google Scholar] [CrossRef]
- Balboa-Castillo, T.; Struijk, E.A.; Lopez-Garcia, E.; Banegas, J.R.; Rodríguez-Artalejo, F.; Guallar-Castillon, P. Low vitamin intake is associated with risk of frailty in older adults. Age Ageing 2018, 47, 872–879. [Google Scholar] [CrossRef]
- Bekfani, T.; Pellicori, P.; Morris, D.; Ebner, N.; Valentova, M.; Sandek, A.; Doehner, W.; Cleland, J.G.; Lainscak, M.; Schulze, P.C.; et al. Iron deficiency in patients with heart failure with preserved ejection fraction and its association with reduced exercise capacity, muscle strength and quality of life. Clin. Res. Cardiol. 2019, 108, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Barbagallo, M.; Lauretani, F.; Bandinelli, S.; Bos, A.; Corsi, A.M.; Simonsick, E.M.; Ferrucci, L. Magnesium and muscle performance in older persons: The InCHIANTI Study. Am. J. Clin. Nutr. 2006, 84, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Writing Group for the European Working Group on Sarcopenia in Older, P.; the Extended Group for, E., Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Hamer, M.; Molloy, G.J. Association of C-reactive protein and muscle strength in the English Longitudinal Study of Ageing. Age 2009, 31, 171–177. [Google Scholar] [CrossRef]
- Ma, Y.; Griffith, J.A.; Chasan-Taber, L.; Olendzki, B.C.; Jackson, E.; Stanek, E.J., III; Li, W.; Pagoto, S.L.; Hafner, A.R.; Ockene, I.S. Association between dietary fiber and serum C-reactive protein. Am. J. Clin. Nutr. 2006, 83, 760–766. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Rahmat, A.; Patimah, I.; Khaza’ai, H.; Abed, Y. Effect of vitamin C on inflammation and metabolic markers in hypertensive and/or diabetic obese adults: A randomized controlled trial. Drug Des. Dev. Ther. 2015, 9, 3405–3412. [Google Scholar] [CrossRef]
- Maier, J.A.; Castiglioni, S.; Locatelli, L.; Zocchi, M.; Mazur, A. Magnesium and inflammation: Advances and perspectives. Semin Cell Dev. Biol. 2021, 115, 37–44. [Google Scholar] [CrossRef]
- Murakami, K.; Livingstone, M.B.E.; Sasaki, S. Thirteen-year trends in dietary patterns among Japanese adults in the National Health and Nutrition Survey 2003(-)2015: Continuous westernization of the Japanese diet. Nutrients 2018, 10, 994. [Google Scholar] [CrossRef]
- Nunes, J.P.; Grgic, J.; Cunha, P.M.; Ribeiro, A.S.; Schoenfeld, B.J.; de Salles, B.F.; Cyrino, E.S. What influence does resistance exercise order have on muscular strength gains and muscle hypertrophy? A systematic review and meta-analysis. Eur. J. Sport Sci. 2021, 21, 149–157. [Google Scholar] [CrossRef]


| Characteristics | rJDI12 Group | aMed Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | p | Q1 | Q2 | Q3 | Q4 | p | |
| Range of scores | 0–4 | 5–6 | 7–8 | 9–12 | 0–3 | 4 | 5 | 6–8 | ||
| Participants, n | 1581 | 1506 | 1674 | 1270 | 2706 | 1438 | 1203 | 684 | ||
| Age, years | 61.3 (6.9) | 62.3 (7.0) | 63.4 (7.0) | 64.5 (6.6) | <0.001 | 61.7 (7.0) | 63.4 (7.0) | 63.7 (6.8) | 64.8 (6.6) | <0.001 |
| Female sex, n (%) | 835 (52.8) | 801 (53.2) | 892 (53.3) | 705 (55.5) | 0.488 | 1555 (57.5) | 744 (51.7) | 620 (51.5) | 314 (45.9) | <0.001 |
| BMI, kg/m2 | 23.2 (3.3) | 23.1 (3.1) | 23.2 (3.0) | 23.0 (2.9) | 0.288 | 23.0 (3.2) | 23.2 (3.1) | 23.2 (3.1) | 23.3 (2.9) | 0.157 |
| Cerebrovascular diseases, n (%) | 0.040 | 0.003 | ||||||||
| -Yes | 49 (3.1) | 40 (2.7) | 40 (2.4) | 42 (3.3) | 65 (2.4) | 42 (2.9) | 41 (3.4) | 23 (3.4) | ||
| -No | 962 (60.8) | 929 (61.7) | 784 (65.2) | 458 (67.0) | 1651 (61.0) | 913 (63.5) | 784 (65.2) | 458 (67.0) | ||
| -Missing | 570 (36.1) | 537 (35.7) | 552 (33.0) | 395 (31.1) | 990 (36.6) | 483 (33.6) | 378 (31.4) | 203 (29.7) | ||
| Coronary heart diseases, n (%) | 0.039 | <0.001 | ||||||||
| -Yes | 147 (9.3) | 126 (8.4) | 169 (10.1) | 137 (10.8) | 212 (7.8) | 147 (10.2) | 134 (11.1) | 86 (12.6) | ||
| -No | 864 (54.6) | 843 (56.0) | 953 (56.9) | 738 (58.1) | 1504 (55.6) | 808 (56.2) | 691 (57.4) | 395 (57.7) | ||
| -Missing | 570 (36.1) | 537 (35.7) | 552 (33.0) | 395 (10.8) | 990 (36.6) | 483 (33.6) | 378 (31.4) | 203 (29.7) | ||
| Diabetes, n (%) | 0.033 | <0.001 | ||||||||
| -Yes | 143 (9.0) | 162 (10.8) | 160 (9.6) | 125 (9.8) | 221 (8.2) | 163 (11.3) | 127 (10.6) | 79 (11.5) | ||
| -No | 869 (55.0) | 807 (53.6) | 962 (57.5) | 750 (59.1) | 1495 (55.2) | 793 (55.1) | 698 (58.0) | 402 (58.8) | ||
| -Missing | 569 (36.0) | 537 (35.7) | 552 (33.0) | 395 (31.1) | 990 (36.6) | 482 (33.5) | 378 (31.4) | 203 (29.7) | ||
| Cancer, n (%) | 0.095 | 0.001 | ||||||||
| -Yes | 65 (4.1) | 68 (4.5) | 80 (4.8) | 60 (4.7) | 122 (4.5) | 53 (3.7) | 66 (5.5) | 32 (4.7) | ||
| -No | 946 (59.8) | 901 (59.8) | 1042 (62.2) | 815 64.2) | 1594 (58.9) | 902 (62.7) | 759 (63.1) | 449 (65.6) | ||
| -Missing | 570 (36.1) | 537 (35.7) | 552 (33.0) | 395 (31.1) | 990 (36.6) | 483 (33.6) | 378 (31.4) | 203 (29.7) | ||
| Smoking status, n (%) | <0.001 | 0.001 | ||||||||
| -Current | 398 (25.2) | 341 (22.6) | 273 (16.3) | 186 (14.6) | 597 (22.1) | 282 (19.6) | 217 (18.0) | 102 (14.9) | ||
| -Former | 376 (23.8) | 343 (22.8) | 439 (26.2) | 316 (24.9) | 620 (22.9) | 360 (25.0) | 299 (24.9) | 195 (28.5) | ||
| -Never | 763 (48.3) | 784 (52.1) | 922 (55.1) | 738 (58.1) | 1422 (52.5) | 756 (52.6) | 662 (55.0) | 367 (53.7) | ||
| -Missing | 44 (2.8) | 38 (2.5) | 40 (2.4) | 30 (2.4) | 67 (2.5) | 40 (2.8) | 25 (2.1) | 20 (2.9) | ||
| Alcohol consumption, n (%) | 0.108 | <0.001 | ||||||||
| -Every day | 346 (21.9) | 334 (22.2) | 384 (22.9) | 232 (18.3) | 558 (20.6) | 331 (23.0) | 238 (19.8) | 169 (24.7) | ||
| -Sometimes | 538 (34.0) | 513 (34.1) | 564 (33.7) | 476 (37.5) | 891 (32.9) | 489 (34.0) | 449 (37.3) | 262 (38.3) | ||
| -Never | 645 (40.8) | 609 (40.4) | 666 (39.8) | 508 (40.0) | 1166 (43.1) | 569 (39.6) | 469 (39.0) | 224 (32.7) | ||
| -Missing | 52 (3.3) | 50 (3.3) | 60 (3.6) | 54 (4.3) | 91 (3.4) | 49 (3.4) | 47 (3.9) | 29 (4.2) | ||
| Time spent walking, n (%) | <0.001 | 0.120 | ||||||||
| -≥1 h/d | 571 (36.1) | 618 (41.0) | 753 (45.0) | 593 (46.7) | 1079 (39.9) | 620 (43.1) | 521 (43.3) | 315 (46.1) | ||
| -0.5–1 h/d | 568 (35.9) | 558 (37.1) | 601 (35.9) | 443 (34.9) | 1006 (37.2) | 505 (35.1) | 439 (36.5) | 220 (32.2) | ||
| -<0.5 h/d | 397 (25.1) | 306 (20.3) | 290 (17.3) | 212 (16.7) | 564 (20.8) | 287 (20.0) | 219 (18.2) | 135 (19.7) | ||
| -Missing | 45 (2.8) | 24 (1.6) | 30 (1.8) | 22 (1.7) | 57 (2.1) | 26 (1.8) | 24 (2.0) | 14 (2.0) | ||
| TMIG-IC score, points | 5 (5–5) | 5 (5–5) | 5 (5–5) | 5 (5–5) | 0.036 | 5 (5–5) | 5 (5–5) | 5 (5–5) | 5 (5–5) | 0.099 |
| Energy, kcal/d | 1441 (449) | 1778 (474) | 2035 (536) | 2409 (633) | <0.001 | 1847 (629) | 1901 (644) | 1939 (600) | 1986 (605) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimizu, A.; Okada, K.; Tomata, Y.; Uno, C.; Kawase, F.; Momosaki, R. Association of Japanese and Mediterranean Dietary Patterns with Muscle Weakness in Japanese Community-Dwelling Middle-Aged and Older Adults: Post Hoc Cross-Sectional Analysis. Int. J. Environ. Res. Public Health 2022, 19, 12636. https://doi.org/10.3390/ijerph191912636
Shimizu A, Okada K, Tomata Y, Uno C, Kawase F, Momosaki R. Association of Japanese and Mediterranean Dietary Patterns with Muscle Weakness in Japanese Community-Dwelling Middle-Aged and Older Adults: Post Hoc Cross-Sectional Analysis. International Journal of Environmental Research and Public Health. 2022; 19(19):12636. https://doi.org/10.3390/ijerph191912636
Chicago/Turabian StyleShimizu, Akio, Kiwako Okada, Yasutake Tomata, Chiharu Uno, Fumiya Kawase, and Ryo Momosaki. 2022. "Association of Japanese and Mediterranean Dietary Patterns with Muscle Weakness in Japanese Community-Dwelling Middle-Aged and Older Adults: Post Hoc Cross-Sectional Analysis" International Journal of Environmental Research and Public Health 19, no. 19: 12636. https://doi.org/10.3390/ijerph191912636
APA StyleShimizu, A., Okada, K., Tomata, Y., Uno, C., Kawase, F., & Momosaki, R. (2022). Association of Japanese and Mediterranean Dietary Patterns with Muscle Weakness in Japanese Community-Dwelling Middle-Aged and Older Adults: Post Hoc Cross-Sectional Analysis. International Journal of Environmental Research and Public Health, 19(19), 12636. https://doi.org/10.3390/ijerph191912636







