Brain Fog and Quality of Life at Work in Non-Hospitalized Patients after COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Data Collection
2.2. Assessment of the Brain Fog Symptoms and the Quality of Life
2.3. Psychometric Analysis
2.4. Statistics and Bioethics
3. Results
3.1. Psychometric Properties of the BF-COVID Questionnaire
3.2. The Quality of Life at Work before and after COVID-19
3.3. Association between the Quality of Life at Work and the Number of Brain Fog Symptoms
3.4. Association between the Quality of Life at Work and Severity of Brain Fog Symptoms
3.5. Impairment of the Quality of Life at Work before COVID-19
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kumari, M.; Lu, R.-M.; Li, M.-C.; Huang, J.-L.; Hsu, F.-F.; Ko, S.-H.; Ke, F.-Y.; Su, S.-C.; Liang, K.-H.; Yuan, J.P.-Y.; et al. A Critical Overview of Current Progress for COVID-19: Development of Vaccines, Antiviral Drugs, and Therapeutic Antibodies. J. Biomed. Sci. 2022, 29, 68. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, S.; Bhopal, R.; Nguyen Tien, H. Review of Infective Dose, Routes of Transmission and Outcome of COVID-19 Caused by the SARS-COV-2: Comparison with Other Respiratory Viruses. Epidemiol. Infect. 2021, 149, e96. [Google Scholar] [CrossRef] [PubMed]
- Priyanka; Choudhary, O.P.; Singh, I. Protective Immunity against COVID-19: Unravelling the Evidences for Humoral vs. Cellular Components. Travel Med. Infect. Dis. 2021, 39, 101911. [Google Scholar] [CrossRef]
- Vitiello, A.; Ferrara, F.; Troiano, V.; La Porta, R. COVID-19 Vaccines and Decreased Transmission of SARS-CoV-2. Inflammopharmacology 2021, 29, 1357–1360. [Google Scholar] [CrossRef]
- Jennings, G.; Monaghan, A.; Xue, F.; Mockler, D.; Romero-Ortuño, R. A Systematic Review of Persistent Symptoms and Residual Abnormal Functioning Following Acute COVID-19: Ongoing Symptomatic Phase vs. Post-COVID-19 Syndrome. J. Clin. Med. 2021, 10, 5913. [Google Scholar] [CrossRef]
- Korompoki, E.; Gavriatopoulou, M.; Hicklen, R.S.; Ntanasis-Stathopoulos, I.; Kastritis, E.; Fotiou, D.; Stamatelopoulos, K.; Terpos, E.; Kotanidou, A.; Hagberg, C.A.; et al. Epidemiology and Organ Specific Sequelae of Post-Acute COVID19: A Narrative Review. J. Infect. 2021, 83, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Keijsers, K.; Broeders, M.; Baptista Lopes, V.; Klinkert, A.; van Baar, J.; Nahar-van Venrooij, L.; Kerckhoffs, A. Memory Impairment and Concentration Problems in COVID-19 Survivors 8 Weeks after Non-ICU Hospitalization: A Retrospective Cohort Study. J. Med. Virol. 2022; online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, R.; Ghosh, A.K.; Nyman, M.A.; Croghan, I.T.; Grach, S.L.; Anstine, C.V.; Salonen, B.R.; Hurt, R.T. PROMIS Scales for Assessment of Persistent Post-COVID Symptoms: A Cross Sectional Study. J. Prim. Care Community Health 2021, 12, 21501327211030412. [Google Scholar] [CrossRef] [PubMed]
- Menges, D.; Ballouz, T.; Anagnostopoulos, A.; Aschmann, H.E.; Domenghino, A.; Fehr, J.S.; Puhan, M.A. Burden of Post-COVID-19 Syndrome and Implications for Healthcare Service Planning: A Population-Based Cohort Study. PLoS ONE 2021, 16, e0254523. [Google Scholar] [CrossRef]
- Lindert, J.; Sancassiani, F.; Massa, E.; Nardi, A.E. An Overview of the International Literature on Health-Related Quality of Life, Mental Health and Psychosocial Issues in People with Cancer. Clin. Pract. Epidemiol. Ment. Health 2022, 17, 253–256. [Google Scholar] [CrossRef]
- Zeng, N.; Zhao, Y.-M.; Yan, W.; Li, C.; Lu, Q.-D.; Liu, L.; Ni, S.-Y.; Mei, H.; Yuan, K.; Shi, L.; et al. A Systematic Review and Meta-Analysis of Long Term Physical and Mental Sequelae of COVID-19 Pandemic: Call for Research Priority and Action. Mol. Psychiatry 2022. [Google Scholar] [CrossRef] [PubMed]
- Sirayder, U.; Inal-Ince, D.; Kepenek-Varol, B.; Acik, C. Long-Term Characteristics of Severe COVID-19: Respiratory Function, Functional Capacity, and Quality of Life. Int. J. Environ. Res. Public Health 2022, 19, 6304. [Google Scholar] [CrossRef]
- Piras, I.; Piazza, M.F.; Piccolo, C.; Azara, A.; Piana, A.; Finco, G.; Galletta, M. Experiences, Emotions, and Health Consequences among COVID-19 Survivors after Intensive Care Unit Hospitalization. Int. J. Environ. Res. Public Health 2022, 19, 6263. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Pérez, O.; Merino, E.; Leon-Ramirez, J.-M.; Andres, M.; Ramos, J.M.; Arenas-Jiménez, J.; Asensio, S.; Sanchez, R.; Ruiz-Torregrosa, P.; Galan, I.; et al. Post-Acute COVID-19 Syndrome. Incidence and Risk Factors: A Mediterranean Cohort Study. J. Infect. 2021, 82, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Daher, A.; Balfanz, P.; Cornelissen, C.; Müller, A.; Bergs, I.; Marx, N.; Müller-Wieland, D.; Hartmann, B.; Dreher, M.; Müller, T. Follow up of Patients with Severe Coronavirus Disease 2019 (COVID-19): Pulmonary and Extrapulmonary Disease Sequelae. Respir. Med. 2020, 174, 106197. [Google Scholar] [CrossRef] [PubMed]
- Fradelos, E.C.; Boutlas, S.; Tsimitrea, E.; Sistou, A.; Tourlakopoulos, K.; Papathanasiou, I.V.; Gourgoulianis, K.I. Perceived Symptoms, Mental Health and Quality of Life after Hospitalization in COVID-19 Patients. J. Pers. Med. 2022, 12, 728. [Google Scholar] [CrossRef]
- Franco, J.V.A.; Garegnani, L.I.; Oltra, G.V.; Metzendorf, M.-I.; Trivisonno, L.F.; Sgarbossa, N.; Ducks, D.; Heldt, K.; Mumm, R.; Barnes, B.; et al. Long-Term Health Symptoms and Sequelae Following SARS-CoV-2 Infection: An Evidence Map. Int. J. Environ. Res. Public Health 2022, 19, 9915. [Google Scholar] [CrossRef]
- Wose Kinge, C.; Hanekom, S.; Lupton-Smith, A.; Akpan, F.; Mothibi, E.; Maotoe, T.; Lebatie, F.; Majuba, P.; Sanne, I.; Chasela, C. Persistent Symptoms among Frontline Health Workers Post-Acute COVID-19 Infection. Int. J. Environ. Res. Public Health 2022, 19, 5933. [Google Scholar] [CrossRef]
- O’ Mahony, L.; Buwalda, T.; Blair, M.; Forde, B.; Lunjani, N.; Ambikan, A.; Neogi, U.; Barrett, P.; Geary, E.; O’Connor, N.; et al. Impact of Long COVID on Health and Quality of Life. HRB Open Res. 2022, 5, 31. [Google Scholar] [CrossRef]
- Aiyegbusi, O.L.; Hughes, S.E.; Turner, G.; Rivera, S.C.; McMullan, C.; Chandan, J.S.; Haroon, S.; Price, G.; Davies, E.H.; Nirantharakumar, K.; et al. Symptoms, Complications and Management of Long COVID: A Review. J. R. Soc. Med. 2021, 114, 428–442. [Google Scholar] [CrossRef]
- Halpin, S.J.; McIvor, C.; Whyatt, G.; Adams, A.; Harvey, O.; McLean, L.; Walshaw, C.; Kemp, S.; Corrado, J.; Singh, R.; et al. Postdischarge Symptoms and Rehabilitation Needs in Survivors of COVID-19 Infection: A Cross-Sectional Evaluation. J. Med. Virol. 2021, 93, 1013–1022. [Google Scholar] [CrossRef]
- Rogers, J.P.; Chesney, E.; Oliver, D.; Pollak, T.A.; McGuire, P.; Fusar-Poli, P.; Zandi, M.S.; Lewis, G.; David, A.S. Psychiatric and Neuropsychiatric Presentations Associated with Severe Coronavirus Infections: A Systematic Review and Meta-Analysis with Comparison to the COVID-19 Pandemic. Lancet Psychiatry 2020, 7, 611–627. [Google Scholar] [CrossRef]
- Huang, L.; Li, X.; Gu, X.; Zhang, H.; Ren, L.; Guo, L.; Liu, M.; Wang, Y.; Cui, D.; Wang, Y.; et al. Health Outcomes in People 2 Years after Surviving Hospitalisation with COVID-19: A Longitudinal Cohort Study. Lancet Respir. Med. 2022, 10, 863–876. [Google Scholar] [CrossRef]
- Shanbehzadeh, S.; Tavahomi, M.; Zanjari, N.; Ebrahimi-Takamjani, I.; Amiri-arimi, S. Physical and Mental Health Complications Post-COVID-19: Scoping Review. J. Psychosom. Res. 2021, 147, 110525. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Boon, G.J.A.M.; Barco, S.; Endres, M.; Miranda Geelhoed, J.J.; Knauss, S.; Rezek, S.A.; Spruit, M.A.; Vehreschild, J.; Siegerink, B. The Post-COVID-19 Functional Status Scale: A Tool to Measure Functional Status over Time after COVID-19. Eur. Respir. J. 2020, 56, 10–12. [Google Scholar] [CrossRef]
- Drabik, L.; Derbisz, J.; Chatys-Bogacka, Z.; Mazurkiewicz, I.; Sawczynska, K.; Kesek, T.; Czepiel, J.; Wrona, P.; Szaleniec, J.; Wojcik-Bugajska, M.; et al. Neurological Prognostic Factors in Hospitalized Patients with COVID-19. Brain Sci. 2022, 12, 193. [Google Scholar] [CrossRef]
- Campiglio, L.; Priori, A. Neurological Symptoms in Acute COVID-19 Infected Patients: A Survey among Italian Physicians. PLoS ONE 2020, 15, e0238159. [Google Scholar] [CrossRef]
- Hüfner, K.; Tymoszuk, P.; Ausserhofer, D.; Sahanic, S.; Pizzini, A.; Rass, V.; Galffy, M.; Böhm, A.; Kurz, K.; Sonnweber, T.; et al. Who Is at Risk of Poor Mental Health Following Coronavirus Disease-19 Outpatient Management? Front. Med. 2022, 9, 792881. [Google Scholar] [CrossRef]
- Shah, W.; Hillman, T.; Playford, E.D.; Hishmeh, L. Managing the Long Term Effects of Covid-19: Summary of NICE, SIGN, and RCGP Rapid Guideline. BMJ 2021, 372, 10–13. [Google Scholar] [CrossRef]
- Park, D.-I. Development and Validation of a Knowledge, Attitudes and Practices Questionnaire on COVID-19 (KAP COVID-19). Int. J. Environ. Res. Public Health 2021, 18, 7493. [Google Scholar] [CrossRef]
- Acal, C.; Aguilera, A.M.; Escabias, M. New Modeling Approaches Based on Varimax Rotation of Functional Principal Components. Mathematics 2020, 8, 2085. [Google Scholar] [CrossRef]
- Rojas-Valverde, D.; Pino-Ortega, J.; Gómez-Carmona, C.D.; Rico-González, M. A Systematic Review of Methods and Criteria Standard Proposal for the Use of Principal Component Analysis in Team’s Sports Science. Int. J. Environ. Res. Public Health 2020, 17, 8712. [Google Scholar] [CrossRef] [PubMed]
- Carden, S.; Camper, T.; Holtzman, N. Cronbach’s Alpha under Insufficient Effort Responding: An Analytic Approach. Stats 2018, 2, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A Review of Methods to Deal with It and a Simulation Study Evaluating Their Performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Sydor, W.; Wizner, B.; Strach, M.; Bociąga-Jasik, M.; Mydel, K.; Olszanecka, A.; Sanak, M.; Małecki, M.; Wójkowska-Mach, J.; Chrzan, R.; et al. CRACoV-HHS: An Interdisciplinary Project for Multi-Specialist Hospital and Non-Hospital Care for Patients with SARS-CoV-2 Infection as Well Hospital Staff Assessment for Infection Exposure. Folia Med. Cracov. 2021, 61, 5–44. [Google Scholar] [CrossRef]
- Graham, E.L.; Clark, J.R.; Orban, Z.S.; Lim, P.H.; Szymanski, A.L.; Taylor, C.; DiBiase, R.M.; Jia, D.T.; Balabanov, R.; Ho, S.U.; et al. Persistent Neurologic Symptoms and Cognitive Dysfunction in Non-Hospitalized Covid-19 “Long Haulers”. Ann. Clin. Transl. Neurol. 2021, 8, 1073–1085. [Google Scholar] [CrossRef]
- Ali, S.T.; Kang, A.K.; Patel, T.R.; Clark, J.R.; Perez-Giraldo, G.S.; Orban, Z.S.; Lim, P.H.; Jimenez, M.; Graham, E.L.; Batra, A.; et al. Evolution of Neurologic Symptoms in Non-Hospitalized COVID-19 “Long Haulers”. Ann. Clin. Transl. Neurol. 2022, 9, 950–961. [Google Scholar] [CrossRef] [PubMed]
- Weerahandi, H.; Hochman, K.A.; Simon, E.; Blaum, C.; Chodosh, J.; Duan, E.; Garry, K.; Kahan, T.; Karmen-Tuohy, S.L.; Karpel, H.C.; et al. Post-Discharge Health Status and Symptoms in Patients with Severe COVID-19. J. Gen. Intern. Med. 2021, 36, 738–745. [Google Scholar] [CrossRef]
- Garrigues, E.; Janvier, P.; Kherabi, Y.; Le Bot, A.; Hamon, A.; Gouze, H.; Doucet, L.; Berkani, S.; Oliosi, E.; Mallart, E.; et al. Post-Discharge Persistent Symptoms and Health-Related Quality of Life after Hospitalization for COVID-19. J. Infect. 2020, 81, e4–e6. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-Month Consequences of COVID-19 in Patients Discharged from Hospital: A Cohort Study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Haller, J.; Kocalevent, R.D.; Nienhaus, A.; Peters, C.; Bergelt, C.; Koch-Gromus, U. Persistent Fatigue Symptoms Following COVID-19 Infection in Healthcare Workers: Risk Factors and Impact on Quality of Life. Bundesgesundheitsblatt Gesundh. Gesundh. 2022, 65, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Chopra, V.; Flanders, S.A.; O’Malley, M.; Malani, A.N.; Prescott, H.C. Sixty-Day Outcomes among Patients Hospitalized with COVID-19. Ann. Intern. Med. 2021, 174, 576–578. [Google Scholar] [CrossRef] [PubMed]
- Roed, C.; Sørensen, H.T.; Rothman, K.J.; Skinhøj, P.; Obel, N. Employment and Disability Pension after Central Nervous System Infections in Adults. Am. J. Epidemiol. 2015, 181, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Raman, B.; Cassar, M.P.; Tunnicliffe, E.M.; Filippini, N.; Griffanti, L.; Alfaro-Almagro, F.; Okell, T.; Sheerin, F.; Xie, C.; Mahmod, M.; et al. Medium-Term Effects of SARS-CoV-2 Infection on Multiple Vital Organs, Exercise Capacity, Cognition, Quality of Life and Mental Health, Post-Hospital Discharge. EClinicalMedicine 2021, 31, 100683. [Google Scholar] [CrossRef] [PubMed]
- Hugon, J.; Queneau, M.; Sanchez Ortiz, M.; Msika, E.F.; Farid, K.; Paquet, C. Cognitive Decline and Brainstem Hypometabolism in Long COVID: A Case Series. Brain Behav. 2022, 12, e2513. [Google Scholar] [CrossRef]
- Borodovitsyna, O.; Flamini, M.; Chandler, D. Noradrenergic Modulation of Cognition in Health and Disease. Neural Plast. 2017, 2017, 6031478. [Google Scholar] [CrossRef] [Green Version]
- Hugon, J.; Msika, E.F.; Queneau, M.; Farid, K.; Paquet, C. Long COVID: Cognitive Complaints (Brain Fog) and Dysfunction of the Cingulate Cortex. J. Neurol. 2022, 269, 44–46. [Google Scholar] [CrossRef]
- Malik, P.; Patel, K.; Pinto, C.; Jaiswal, R.; Tirupathi, R.; Pillai, S.; Patel, U. Post-Acute COVID-19 Syndrome (PCS) and Health-Related Quality of Life (HRQoL)—A Systematic Review and Meta-Analysis. J. Med. Virol. 2022, 94, 253–262. [Google Scholar] [CrossRef]
- Hellemons, M.E.; Huijts, S.; Bek, L.M.; Berentschot, J.C.; Nakshbandi, G.; Schurink, C.A.M.; Vlake, J.H.; van Genderen, M.E.; van Bommel, J.; Gommers, D.; et al. Persistent Health Problems beyond Pulmonary Recovery up to 6 Months after Hospitalization for COVID-19: A Longitudinal Study of Respiratory, Physical, and Psychological Outcomes. Ann. Am. Thorac. Soc. 2022, 19, 551–561. [Google Scholar] [CrossRef]
- Peluso, M.J.; Daniel Kelly, J.; Lu, S.; Goldberg, S.A.; Davidson, M.C.; Mathur, S.; Durstenfeld, M.S.; Spinelli, M.A.; Hoh, R.; Tai, V.; et al. Persistence, Magnitude, and Patterns of Postacute Symptoms and Quality of Life Following Onset of SARS-CoV-2 Infection: Cohort Description and Approaches for Measurement. Open Forum Infect. Dis. 2022, 9, ofab640. [Google Scholar] [CrossRef]
- Seeble, J.; Waterboer, T.; Hippchen, T.; Simon, J.; Kirchner, M.; Lim, A.; Muller, B.; Merle, U. Persistent Symptoms in Adult Patients 1 Year after Coronavirus Disease 2019 (COVID-19): A Prospective Cohort Study. Clin. Infect. Dis. 2022, 74, 1191–1198. [Google Scholar] [CrossRef]
- Tabacof, L.; Tosto-Mancuso, J.; Wood, J.; Cortes, M.; Kontorovich, A.; McCarthy, D.; Rizk, D.; Rozanski, G.; Breyman, E.; Nasr, L.; et al. Post-Acute COVID-19 Syndrome Negatively Impacts Physical Function, Cognitive Function, Health-Related Quality of Life, and Participation. Am. J. Phys. Med. Rehabil. 2022, 101, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xu, X.; Zhang, L.; Zheng, D.; Liu, Y.; Feng, B.; Hu, J.; Lin, Q.; Xi, X.; Wang, Q.; et al. Post-Traumatic Stress Disorder Symptoms and Quality of Life of COVID-19 Survivors at 6-Month Follow-Up: A Cross-Sectional Observational Study. Front. Psychiatry 2022, 12, 782478. [Google Scholar] [CrossRef] [PubMed]
- O’Kelly, B.; Vidal, L.; Avramovic, G.; Broughan, J.; Connolly, S.P.; Cotter, A.G.; Cullen, W.; Glaspy, S.; McHugh, T.; Woo, J.; et al. Assessing the Impact of COVID-19 at 1-Year Using the SF-12 Questionnaire: Data from the Anticipate Longitudinal Cohort Study. Int. J. Infect. Dis. 2022, 118, 236–243. [Google Scholar] [CrossRef]
- Fisher, K.A.; Olson, S.M.; Tenforde, M.W.; Self, W.H.; Wu, M.; Lindsell, C.J.; Shapiro, N.I.; Files, D.C.; Gibbs, K.W.; Erickson, H.L.; et al. Symptoms and Recovery among Adult Outpatients with and without COVID-19 at 11 Healthcare Facilities—July 2020, United States. Influenza Other Respi. Viruses 2021, 15, 345–351. [Google Scholar] [CrossRef]
- Van Den Borst, B.; Peters, J.B.; Brink, M.; Schoon, Y.; Bleeker-Rovers, C.P.; Schers, H.; Van Hees, H.W.H.; Van Helvoort, H.; Van Den Boogaard, M.; Van Der Hoeven, H.; et al. Comprehensive Health Assessment 3 Months after Recovery from Acute Coronavirus Disease 2019 (COVID-19). Clin. Infect. Dis. 2021, 73, E1089–E1098. [Google Scholar] [CrossRef]
- O’keefe, J.B.; Minton, H.C.; Morrow, M.; Johnson, C.; Moore, M.A.; O’keefe, G.A.D.; Benameur, K.; Higdon, J.; Fairley, J.K. Postacute Sequelae of SARS-CoV-2 Infection and Impact on Quality of Life 1-6 Months after Illness and Association with Initial Symptom Severity. Open Forum Infect. Dis. 2021, 8, ofab352. [Google Scholar] [CrossRef]
- Rass, V.; Ianosi, B.A.; Zamarian, L.; Beer, R.; Sahanic, S.; Lindner, A.; Kofler, M.; Schiefecker, A.J.; Mahlknecht, P.; Heim, B.; et al. Factors Associated with Impaired Quality of Life Three Months after Being Diagnosed with COVID-19. Qual. Life Res. 2022, 31, 1401–1414. [Google Scholar] [CrossRef]
- Taboada, M.; Moreno, E.; Cariñena, A.; Rey, T.; Pita-Romero, R.; Leal, S.; Sanduende, Y.; Rodríguez, A.; Nieto, C.; Vilas, E.; et al. Quality of Life, Functional Status, and Persistent Symptoms after Intensive Care of COVID-19 Patients. Br. J. Anaesth. 2021, 126, e110–e113. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603. [Google Scholar] [CrossRef]
- Arnold, D.T.; Hamilton, F.W.; Milne, A.; Morley, A.J.; Viner, J.; Attwood, M.; Noel, A.; Gunning, S.; Hatrick, J.; Hamilton, S.; et al. Patient Outcomes after Hospitalisation with COVID-19 and Implications for Follow-up: Results from a Prospective UK Cohort. Thorax 2021, 76, 399–401. [Google Scholar] [CrossRef]
- Bungenberg, J.; Humkamp, K.; Hohenfeld, C.; Rust, M.I.; Ermis, U.; Dreher, M.; Hartmann, N.K.; Marx, G.; Binkofski, F.; Finke, C.; et al. Long COVID-19: Objectifying Most Self-reported Neurological Symptoms. Ann. Clin. Transl. Neurol. 2022, 9, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.; Zhao, Y.; Cai, H.; Sha, S.; Zhang, Q.; Lei, S.; Lok, G.K.I.; Chow, I.H.I.; Cheung, T.; Su, Z.; et al. Network Analysis of Depression, Anxiety, Insomnia and Quality of Life among Macau Residents during the COVID-19 Pandemic. J. Affect. Disord. 2022, 311, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Vagheggini, G.; Marzetti, F.; Miniati, M.; Bernardeschi, L.; Miccoli, M.; Boni Brivio, G.; Meini, S.; Panait, E.; Cini, E.; Gemignani, A. Pulmonary Function and Psychological Burden Three Months after COVID-19: Proposal of a Comprehensive Multidimensional Assessment Protocol. Healthcare 2022, 10, 612. [Google Scholar] [CrossRef]
- Braude, P.; McCarthy, K.; Strawbridge, R.; Short, R.; Verduri, A.; Vilches-Moraga, A.; Hewitt, J.; Carter, B. Frailty Is Associated with Poor Mental Health 1 Year after Hospitalisation with COVID-19. J. Affect. Disord. 2022, 310, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Vaira, L.A.; Gessa, C.; Deiana, G.; Salzano, G.; Maglitto, F.; Lechien, J.R.; Saussez, S.; Piombino, P.; Biglio, A.; Biglioli, F.; et al. The Effects of Persistent Olfactory and Gustatory Dysfunctions on Quality of Life in Long-COVID-19 Patients. Life 2022, 12, 141. [Google Scholar] [CrossRef]
- Pavli, A.; Theodoridou, M.; Maltezou, H.C. Post-COVID Syndrome: Incidence, Clinical Spectrum, and Challenges for Primary Healthcare Professionals. Arch. Med. Res. 2021, 52, 575–581. [Google Scholar] [CrossRef]
- O’Sullivan, O. Long-Term Sequelae Following Previous Coronavirus Epidemics. Clin. Med. J. R. Coll. Physicians London 2021, 21, E68–E70. [Google Scholar] [CrossRef]
- Castro, J.P.; Kierkegaard, M.; Zeitelhofer, M. A Call to Use the Multicomponent Exercise Tai Chi to Improve Recovery From COVID-19 and Long COVID. Front. Public Health 2022, 10, 827645. [Google Scholar] [CrossRef]
- Al-Jabr, H.; Windle, K.; Thompson, D.R.; Jenkins, Z.M.; Castle, D.J.; Ski, C.F. Long COVID Optimal Health Program (LC-OHP) to Enhance Psychological and Physical Health: Protocol for a Feasibility Randomized Controlled Trial. JMIR Res. Protoc. 2022, 11, e36673. [Google Scholar] [CrossRef]
- Harenwall, S.; Heywood-Everett, S.; Henderson, R.; Godsell, S.; Jordan, S.; Moore, A.; Philpot, U.; Shepherd, K.; Smith, J.; Bland, A.R. Post-Covid-19 Syndrome: Improvements in Health-Related Quality of Life Following Psychology-Led Interdisciplinary Virtual Rehabilitation. J. Prim. Care Community Health 2021, 12, 21501319211067674. [Google Scholar] [CrossRef]
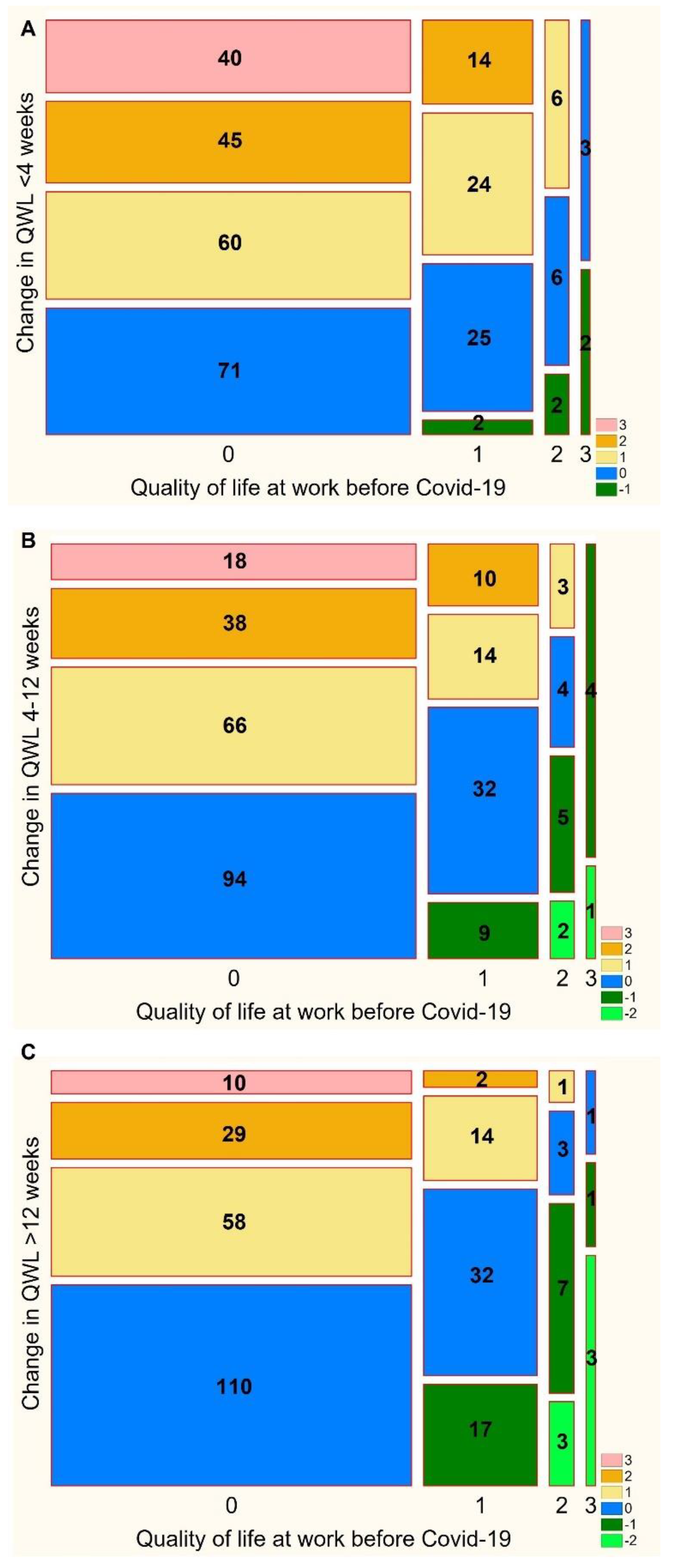

| Quality of Life at Work | Before COVID-19 (n = 300) | 0–4 Weeks (n = 300) | 4–8 Weeks (n = 300) | >12 Weeks (n = 291) | p-Value |
|---|---|---|---|---|---|
| Normal | 216 (72.00) | 73 (24.33) | 105 (35.00) | 130 (44.67) | <0.001 |
| Mild impairment | 65 (21.67) | 87 (29.00) | 104 (34.67) | 100 (34.64) | |
| Moderate impairment | 14 (4.67) | 77 (25.67) | 60 (20.00) | 47 (16.15) | |
| Severe impairment | 5 (1.67) | 63 (21.00) | 31 (10.33) | 14 (4.81) |
| Quality of Life at Work | Number of Symptoms of Brain Fog (median, IQR) | |||
|---|---|---|---|---|
| Before COVID-19 | 0–4 Weeks | 4–12 Weeks | >12 Weeks | |
| Normal | 0 (0–1) | 1 (0–3) | 1 (0–2) | 0 (0–1) |
| Mild impairment | 1 (0–3) * | 3 (1–4) * | 3 (1–5) * | 2 (1–4) * |
| Moderate impairment | 1 (0–3) | 4 (2–6) *# | 4 (2–6) * | 5 (2–6) *# |
| Severe impairment | 0 (0–1) | 5 (3–6) *# | 5 (2–6) *# | 5 (4–6) *# |
| Before COVID-19 | Deterioration in QoL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0–4 Weeks | 4–12 Weeks | >12 Weeks | ||||||||
| No | Yes | p-Value | No | Yes | p-Value | No | Yes | p-Value | ||
| 1. Writing, reading, and counting, n (%) | ||||||||||
| 0 | 294 (97.02) | 94 (84.68) | 124 (65.61) | <0.001 | 132 (87.42) | 112 (75.17) | 0.013 | 164 (92.66) | 85 (7.56) | <0.001 |
| 1 | 5 (1.65) | 10 (9.01) | 42 (22.22) | 15 (9.93) | 20 (13.42) | 11 (6.21) | 21 (18.42) | |||
| 2 | 3 (0.99) | 4 (3.60) | 21 (11.11) | 3 (1.99) | 15 (10.07) | 1 (0.56) | 6 (5.26) | |||
| 3 | 0 (0.00) | 3 (2.70) | 2 (1.06) | 1 (0.66) | 2 (1.34) | 1 (0.56) | 2 (1.75) | |||
| 2. Answering the questions in an understandable or unambiguous manner, n (%) | ||||||||||
| 0 | 286 (94.38) | 81 (72.97) | 95 (50.26) | 0.001 | 116 (76.82) | 73 (48.99) | <0.001 | 152 (85.88) | 61 (53.51) | <0.001 |
| 1 | 15 (4.95) | 20 (18.02) | 54 (28.57) | 32 (21.19) | 48 (32.21) | 21 (11.86) | 35 (30.70) | |||
| 2 | 1 (0.33) | 9 (8.11) | 36 (19.05) | 2 (1.32) | 24 (16.11) | 4 (2.26) | 16 (14.04) | |||
| 3 | 1 (0.33) | 1 (0.90) | 2 (2.12) | 1 (0.66) | 4 (2.68) | 0 (0.00) | 2 (1.75) | |||
| 3. Thoughts communicating during a conversation in a way that others can understand, n (%) | ||||||||||
| 0 | 263 (86.80) | 65 (58.56) | 79 (41.80) | 0.009 | 105 (69.54) | 63 (42.28) | <0.001 | 145 (81.92) | 54 (47.37) | <0.001 |
| 1 | 37 (12.21) | 32 (28.83) | 57 (30.16) | 41 (27.15) | 55 (36.91) | 27 (15.25) | 41 (35.96) | |||
| 2 | 2 (0.66) | 12 (10.81) | 43 (22.75) | 3 (1.99) | 24 (16.11) | 3 (1.69) | 14 (12.28) | |||
| 3 | 1 (0.33) | 2 (1.80) | 10 (5.29) | 2 (1.32) | 7 (4.70) | 2 (1.13) | 5 (4.39) | |||
| 4. Performing several independent tasks simultaneously, n (%) | ||||||||||
| 0 | 257 (84.82) | 71 (63.96) | 62 (30.80) | <0.001 | 99 (65.56) | 51 (34.23) | <0.001 | 137 (77.40) | 49 (42.98) | <0.001 |
| 1 | 42 (13.86) | 20 (18.02) | 58 (30.69) | 43 (28.48) | 55 (36.91) | 32 (18.08) | 38 (33.33) | |||
| 2 | 3 (0.99) | 18 (16.22) | 43 (22.75) | 7 (4.64) | 34 (22.82) | 6 (3.39) | 22 (19.30) | |||
| 3 | 1 (0.33) | 2 (1.80) | 26 (13.76) | 2 (1.32) | 9 (6.04) | 2 (1.13) | 5 (4.39) | |||
| 5. Recalling new information, n (%) | ||||||||||
| 0 | 241 (79.54) | 56 (50.45) | 55 (29.10) | <0.001 | 88 (58.28) | 44 (29.53) | <0.001 | 130 (73.45) | 36 (31.58) | <0.001 |
| 1 | 50 (16.50) | 31 (27.93) | 50 (26.46) | 48 (31.79) | 59 (39.60) | 34 (19.21) | 46 (40.35) | |||
| 2 | 12 (3.96) | 18 (16.22) | 55 (29.10) | 10 (6.62) | 32 (21.48) | 7 (3.95) | 20 (17.54) | |||
| 3 | 0 (0.00) | 6 (5.41) | 29 (15.34) | 5 (3.31) | 14 (9.40) | 7 (3.95) | 12 (10.53) | |||
| 6. Remembering information from the past; for example, recognizing people or remembering events, n (%) | ||||||||||
| 0 | 266 (87.79) | 89 (80.18) | 125 (66.14) | 0.025 | 126 (83.44) | 105 (70.47) | 0.003 | 157 (88.70) | 81 (70.05) | <0.001 |
| 1 | 37 (12.21) | 20 (18.02) | 47 (24.87) | 24 (15.89) | 30 (20.13) | 19 (10.73) | 26 (22.81) | |||
| 2 | 0 (0.00) | 2 (1.80) | 14 (7.41) | 1 (0.66) | 12 (8.05) | 0 (0.00) | 6 (5.26) | |||
| 3 | 0 (0.00) | 0 (0.00) | 3 (1.59) | 0 (0.00) | 2 (1.34) | 1 (0.56) | 1 (0.88) | |||
| 7. Determining the current date and naming the days of the week, n (%) | ||||||||||
| 0 | 282 (93.07) | 92 (82.88) | 149 (78.84) | 0.734 | 133 (88.08) | 118 (79.19) | 0.060 | 164 (92.66) | 92 (80.70) | 0.016 |
| 1 | 19 (6.27) | 13 (11.71) | 25 (13.23) | 14 (9.27) | 21 (14.09) | 10 (5.65) | 18 (15.79) | |||
| 2 | 1 (0.33) | 4 (3.60) | 12 (6.35) | 4 (2.65) | 5 (3.36) | 3 (1.69) | 3 (2.63) | |||
| 3 | 1 (0.33) | 2 (1.80) | 3 (1.59) | 0 (0.00) | 5 (3.36) | 0 (0.00) | 1 (0.88) | |||
| 8. Finding the right way in a familiar place, n (%) | ||||||||||
| 0 | 295 (97.36) | 103 (92.79) | 160 (84.66) | 0.008 | 142 (94.04) | 127 (85.23) | 0.014 | 170 (96.05) | 100 (87.72) | 0.012 |
| 1 | 5 (1.65) | 2 (1.80) | 24 (12.70) | 6 (3.97) | 19 (12.75) | 4 92.26) | 13 (11.40) | |||
| 2 | 3 (0.99) | 4 (3.60) | 4 (2.12) | 3 (1.99) | 1 (0.67) | 2 (1.13) | 1 (0.88) | |||
| 3 | 0 (0.00) | 2 (1.80) | 1 (0.53) | 0 (0.00) | 2 (1.34) | 1 (0.56) | 0 (0.00) | |||
| Univariable Analysis | Multivariable Analysis Model A | Multivariable Analysis Model B | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| <4 weeks after COVID-19 | ||||||
| Age (per year) | 1.00 (0.98–1.02) | 0.921 | - | - | - | - |
| Female sex | 1.30 (0.74–2.30) | 0.367 | - | - | - | - |
| Writing, reading, and counting (pp) | 1.70 (1.15–2.50) | 0.008 | - | - | - | - |
| Answering the questions (pp) | 1.89 (1.34–2.66) | <0.001 | - | - | - | - |
| Thoughts communication (pp) | 1.65 (1.23–2.21) | <0.001 | x | x | x | x |
| Performing several tasks (pp) | 2.00 (1.52 –2.64) | <0.001 | 1.96 (1.48–2.58) | <0.001 | x | x |
| Recalling new information (pp) | 1.73 (1.35–2.22) | <0.001 | x | x | 1.65 (1.28–2.13) | <0.001 |
| Remembering information from the past (pp) | 1.96 (1.25–3.07) | 0.003 | 1.88 (1.18–3.00) | 0.008 | 1.75 (1.10–2.80) | 0.019 |
| AIC | 365.20 | 375.27 | ||||
| AUC (95% CI) | 0.694 (0.632–0.756) | 0.662 (0.600–0.724) | ||||
| The Hosmer–Lemeshow test p-value | 0.586 | 0.791 | ||||
| 4–12 weeks after COVID-19 | ||||||
| Age (per year) | 1.02 (1.00–1.04) | 0.041 | 0.46 (0.25–0.85) | 0.014 | - | - |
| Female sex | 0.71 (0.40–1.25) | 0.231 | - | - | - | - |
| Writing, reading, and counting (pp) | 1.88 (1.22–2.86) | 0.003 | - | - | - | - |
| Answering the questions (pp) | 2.83 (1.92–4.20) | <0.001 | 1.99 (1.27–3.14) | 0.003 | 2.63 (1.78–3.87) | <0.001 |
| Thoughts communication (pp) | 2.49 (1.75–3.55) | <0.001 | x | x | x | x |
| Performing several tasks (pp) | 2.63 (1.89–3.66) | <0.001 | 2.05 (1.40–3.01) | <0.001 | - | - |
| Recalling new information (pp) | 2.15 (1.61–2.89) | <0.001 | x | x | 1.41 (0.94–2.12) | 0.097 |
| Remembering information from the past (pp) | 2.15 (1.37–3.38) | <0.001 | - | - | 1.87 (1.65–3.00) | 0.010 |
| Current date (pp) | 1.69 (1.09–2.63) | 0.020 | - | - | - | - |
| Finding the right way (pp) | 1.87 (1.01–3.450) | 0.047 | - | - | - | - |
| AIC | 370.38 | 380.88 | ||||
| AUC (95% CI) | 0.727 (0.669–0.785) | 0.689 (0.627–0.751) | ||||
| The Hosmer–Lemeshow test p-value | 0.243 | 0.963 | ||||
| >12 weeks after COVID-19 | ||||||
| Age (per year) | 1.03 (1.01–1.06) | 0.026 | 1.03 (1.01–1.05) | 0.025 | 1.03 (1.01–1.06) | 0.016 |
| Female sex | 1.07 (0.59–1.93) | 0.828 | - | - | - | - |
| Writing, reading, and counting (pp) | 2.86 (1.63–5.03) | <0.001 | - | - | - | - |
| Answering the questions (pp) | 3.65 (2.32–5.74) | <0.001 | 2.00 (1.47–2.36) | 0.001 | 2.42 (1.39–4.20) | 0.002 |
| Thoughts communication (pp) | 3.12 (2.08–4.70) | <0.001 | x | x | x | x |
| Performing several tasks (pp) | 2.85 (1.98–4.09) | <0.001 | 1.75 (1.15–2.66) | 0.009 | - | - |
| Recalling new information (pp) | 2.64 (1.92–3.62) | <0.001 | x | x | 1.70 (1.15–2.43) | 0.008 |
| Remembering information from the past (pp) | 2.66 (1.57–4.52) | <0.001 | 2.21 (1.24–3.92) | 0.007 | 2.01 (1.12–3.64) | 0.021 |
| Current date (pp) | 2.20 (1.23–3.94) | 0.008 | - | - | - | - |
| AIC | 334.86 | 334.58 | ||||
| AUC (95% CI) | 0.764 (0.707–0.822) | 0.767 (0.709–0.825) | ||||
| The Hosmer–Lemeshow test p-value | 0.944 | 0.288 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatys-Bogacka, Z.; Mazurkiewicz, I.; Slowik, J.; Bociaga-Jasik, M.; Dzieza-Grudnik, A.; Slowik, A.; Wnuk, M.; Drabik, L. Brain Fog and Quality of Life at Work in Non-Hospitalized Patients after COVID-19. Int. J. Environ. Res. Public Health 2022, 19, 12816. https://doi.org/10.3390/ijerph191912816
Chatys-Bogacka Z, Mazurkiewicz I, Slowik J, Bociaga-Jasik M, Dzieza-Grudnik A, Slowik A, Wnuk M, Drabik L. Brain Fog and Quality of Life at Work in Non-Hospitalized Patients after COVID-19. International Journal of Environmental Research and Public Health. 2022; 19(19):12816. https://doi.org/10.3390/ijerph191912816
Chicago/Turabian StyleChatys-Bogacka, Zaneta, Iwona Mazurkiewicz, Joanna Slowik, Monika Bociaga-Jasik, Anna Dzieza-Grudnik, Agnieszka Slowik, Marcin Wnuk, and Leszek Drabik. 2022. "Brain Fog and Quality of Life at Work in Non-Hospitalized Patients after COVID-19" International Journal of Environmental Research and Public Health 19, no. 19: 12816. https://doi.org/10.3390/ijerph191912816
APA StyleChatys-Bogacka, Z., Mazurkiewicz, I., Slowik, J., Bociaga-Jasik, M., Dzieza-Grudnik, A., Slowik, A., Wnuk, M., & Drabik, L. (2022). Brain Fog and Quality of Life at Work in Non-Hospitalized Patients after COVID-19. International Journal of Environmental Research and Public Health, 19(19), 12816. https://doi.org/10.3390/ijerph191912816







