Prediction Model for the Risk of HIV Infection among MSM in China: Validation and Stability
Abstract
:1. Introduction
2. Methods
2.1. Study Population and Eligibility Criteria
2.2. Participants and Procedure
2.3. Measurement
2.3.1. Socio-Demographic Variables
2.3.2. Psychosocial Variables
Involuntary Subordination
Sexual Compulsivity
Social Support
2.3.3. Behavioral Variables
Multiple Sexual Partners
Unprotected Anal Intercourse
Alcohol Use before Sex
Drug use before Sex
Voluntary HIV Counseling and Testing (VCT)
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Participants
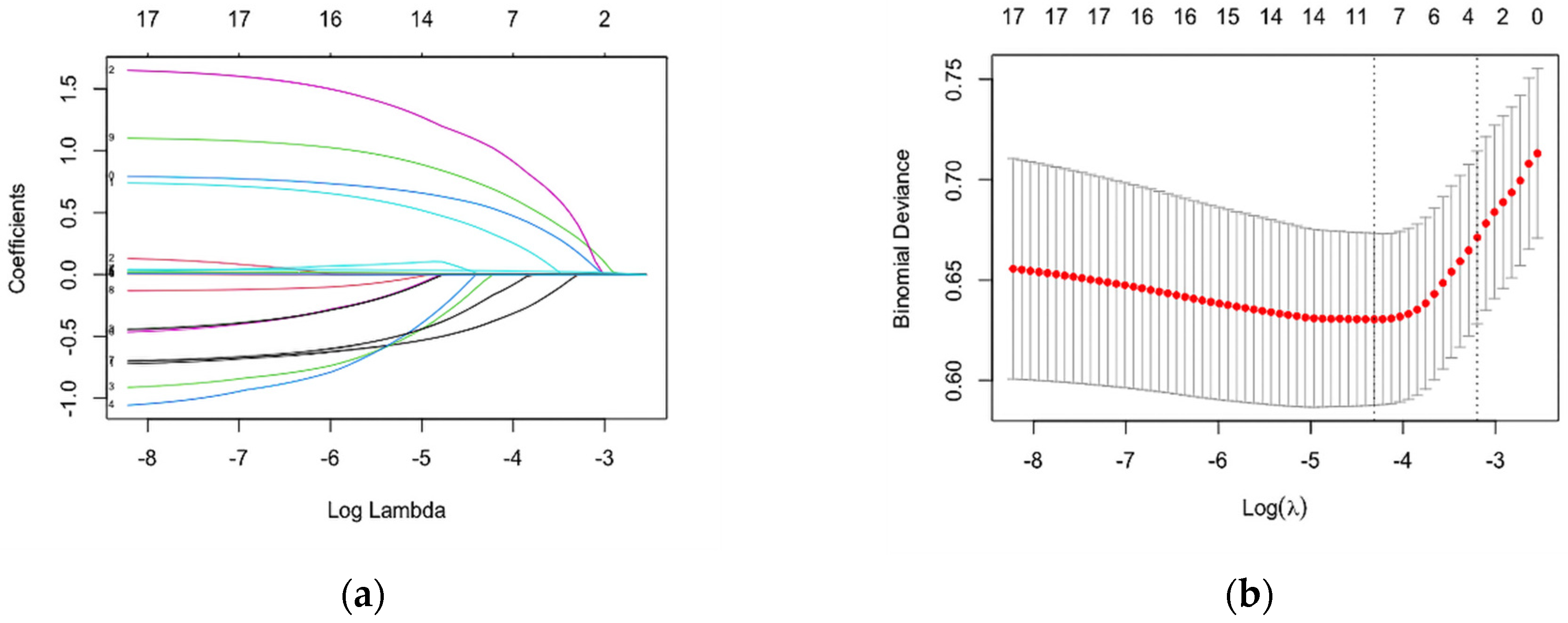
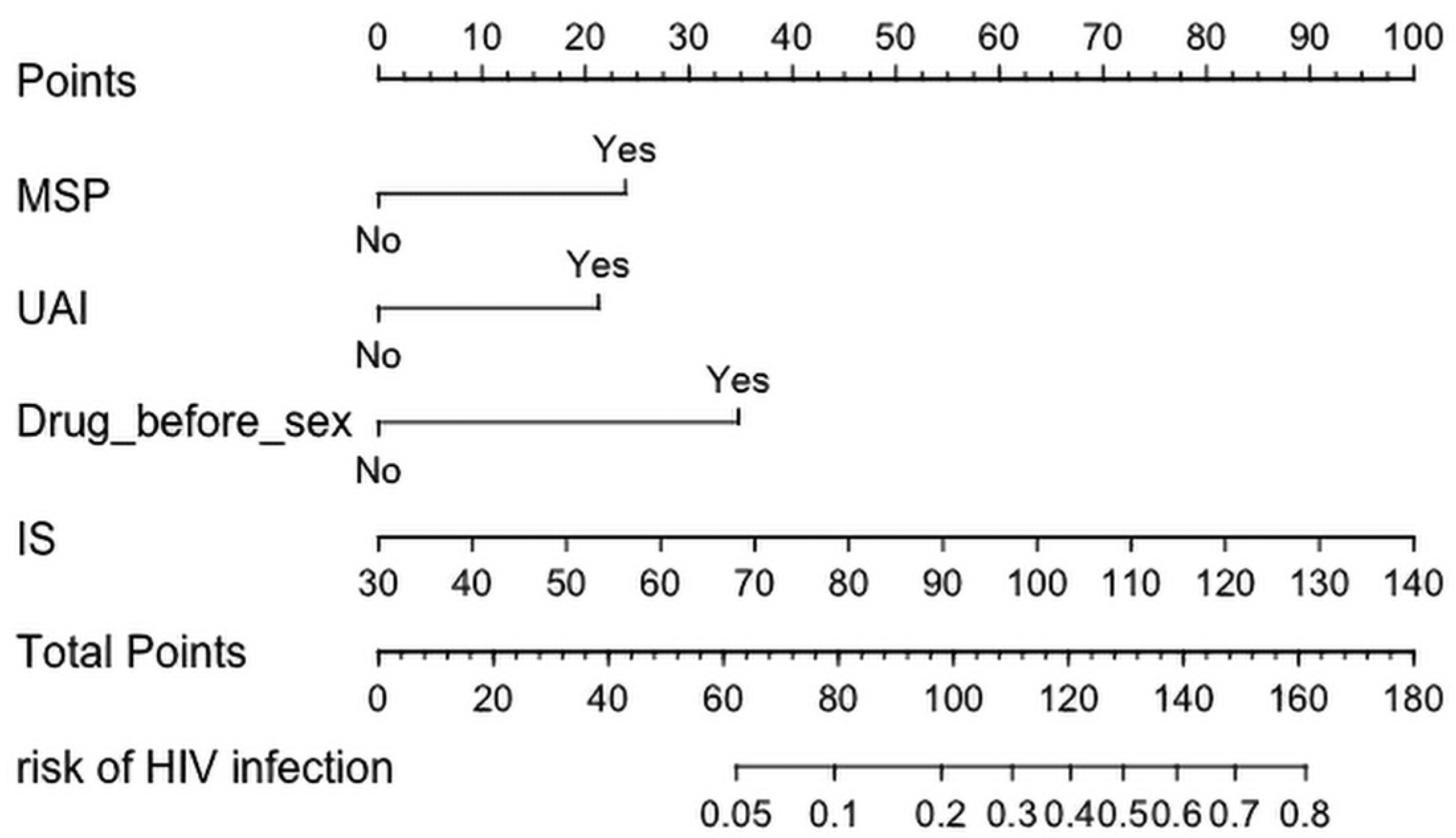
3.2. Construction and Development of a Risk Assessment Tool for HIV Infection among MSM
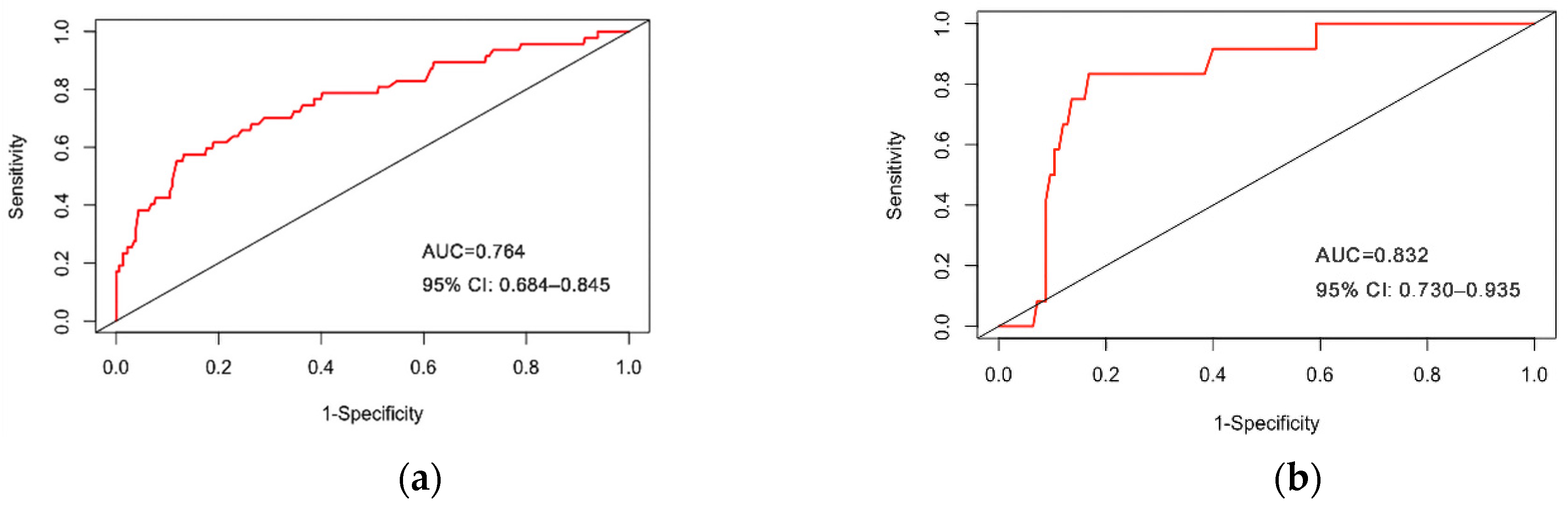
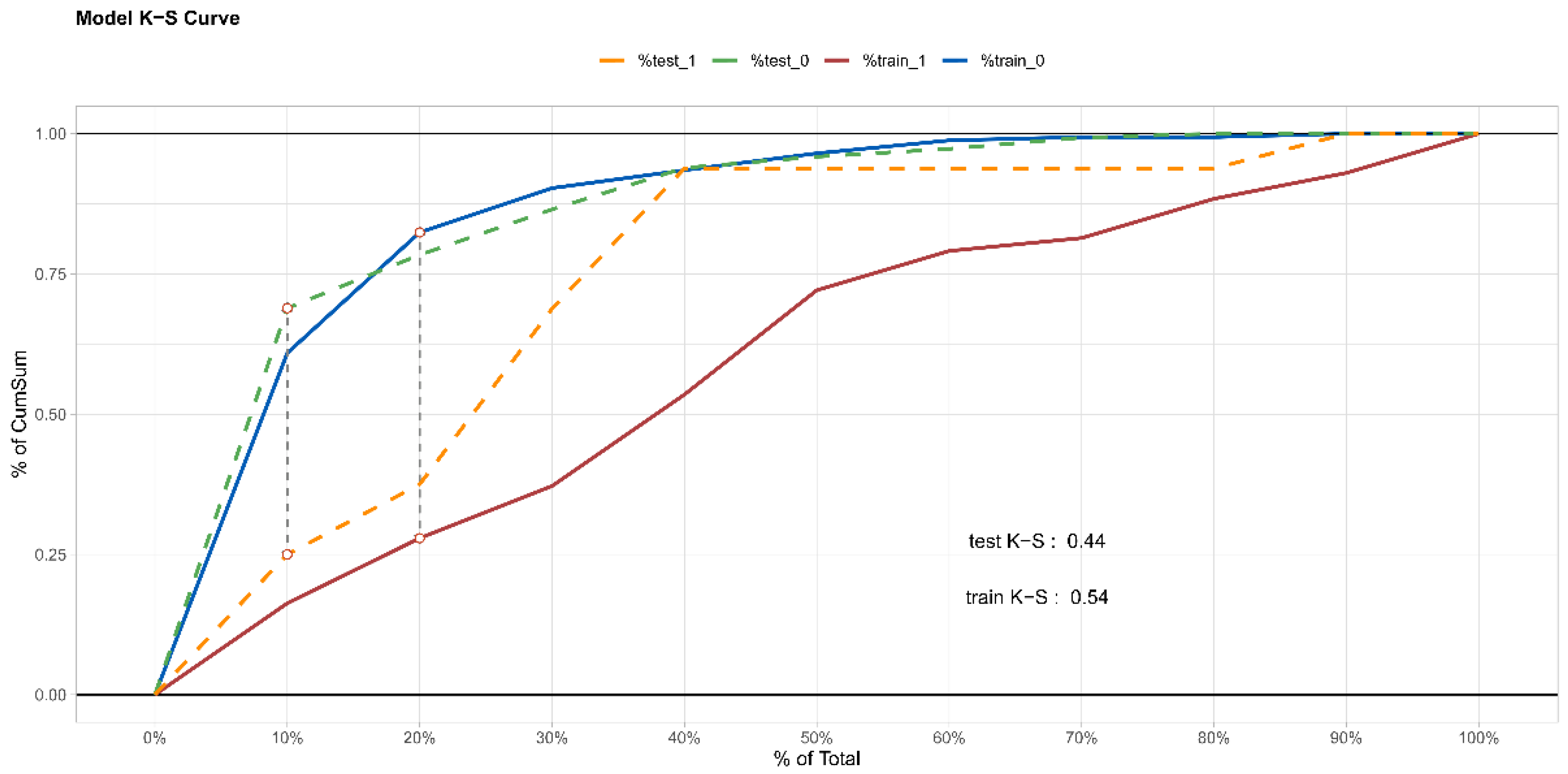
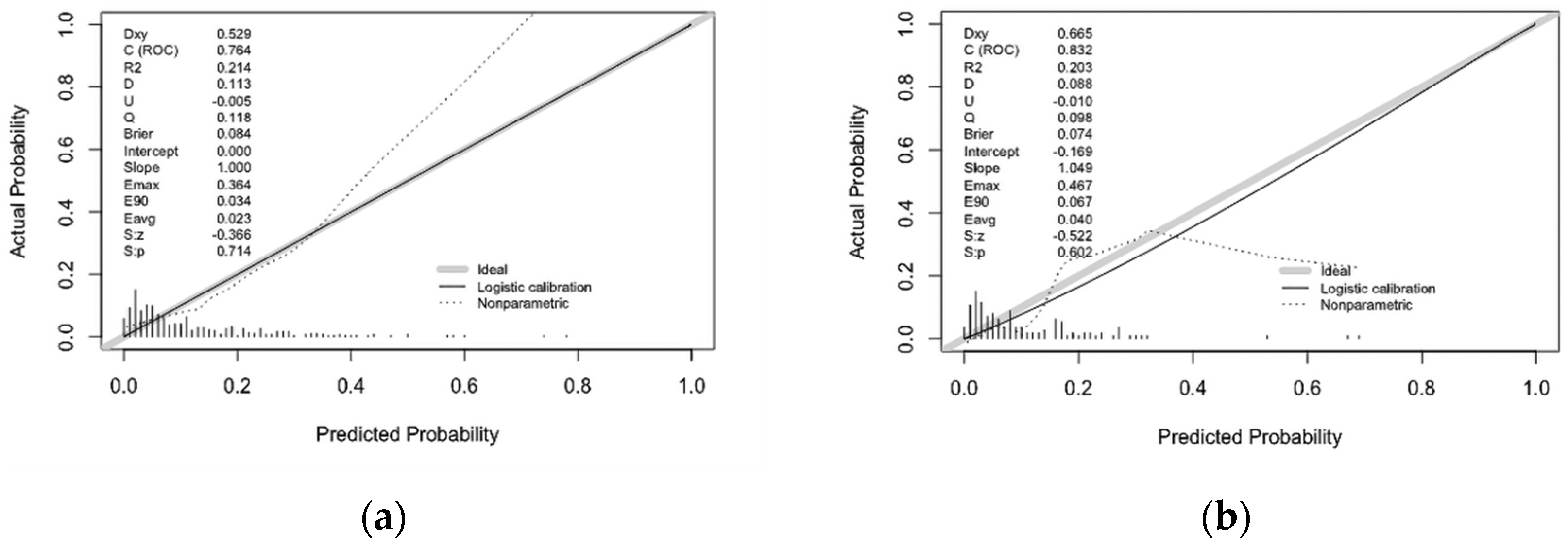
3.3. Internal Validation of Prediction Model
4. Discussion
4.1. Association of UAI and MSP with HIV Infection among MSM
4.2. Association of Drug Use before Sex with HIV Infection among MSM
4.3. Association of IS with HIV Infection among MSM
4.4. Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- 2020 Global AIDS Update—Seizing the Moment—Tackling Entrenched Inequalities to End Epidemics. Available online: https://www.unaids.org/en/resources/documents/2020/global-aids-report (accessed on 6 July 2020).
- Poteat, T.; Ackerman, B.; Diouf, D.; Ceesay, N.; Mothopeng, T.; Odette, K.Z.; Kouanda, S.; Ouedraogo, H.G.; Simplice, A.; Kouame, A.; et al. HIV prevalence and behavioral and psychosocial factors among transgender women and cisgender men who have sex with men in 8 African countries: A cross-sectional analysis. PLoS Med. 2017, 14, e1002422. [Google Scholar] [CrossRef] [Green Version]
- Rutledge, S.E.; Jemmott, J.B., 3rd; O’Leary, A.; Icard, L.D. What’s In an Identity Label? Correlates of Sociodemographics, Psychosocial Characteristics, and Sexual Behavior Among African American Men Who Have Sex With Men. Arch. Sex. Behav. 2018, 47, 157–167. [Google Scholar] [CrossRef]
- Frye, V.; Nandi, V.; Egan, J.; Cerda, M.; Greene, E.; Van Tieu, H.; Ompad, D.C.; Hoover, D.R.; Lucy, D.; Baez, E.; et al. Sexual orientation- and race-based discrimination and sexual HIV risk behavior among urban MSM. AIDS Behav. 2015, 19, 257–269. [Google Scholar] [CrossRef]
- Reilly, K.H.; Neaigus, A.; Jenness, S.M.; Wendel, T.; Marshall, D.M.; Hagan, H. Experiences of Discrimination and HIV Risk Among Men Who Have Sex With Men in New York City. Am. J. Mens. Health 2016, 10, 505–514. [Google Scholar] [CrossRef] [Green Version]
- Hirshfield, S.; Lewis, K.E.; Silver, M.; Gordon, R.J. Interpersonal Stigma, Mental Health, and Sexual Compulsivity Among an Online U.S. Sample of Men Who Have Sex with Men Living with HIV. AIDS Behav. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Mo, P.K.H.; Chen, X.; Lam, E.H.K.; Li, J.; Kahler, C.W.; Lau, J.T.F. The Moderating Role of Social Support on the Relationship Between Anxiety, Stigma, and Intention to Use Illicit Drugs Among HIV-Positive Men Who Have Sex with Men. AIDS Behav. 2020, 24, 55–64. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, S.; Wang, Z.; Wang, Y.; Jiang, X.; Xu, G.; Cai, Y. The association between involuntary subordination and common mental disorders among men who have sex with men (MSM) in Shanghai, China. BMC Psychiatry 2019, 19, 369. [Google Scholar] [CrossRef] [Green Version]
- Sturman, E.D. Involuntary subordination and its relation to personality, mood, and submissive behavior. Psychol. Assess. 2011, 23, 262–276. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, X.; Zhang, Z.; Luo, M.; Shen, Q.; Dong, Y.; Wang, Y.; Cai, Y. Co-Occurring Psychosocial Problems and Multiple Sexual Partners among Men Who Have Sex with Men in Shanghai, China: A Syndemic Approach. J. Sex Res. 2018, 55, 892–901. [Google Scholar] [CrossRef]
- Brown, M.J.; Serovich, J.M.; Kimberly, J.A. Outcome Expectancy and Sexual Compulsivity Among Men Who Have Sex with Men Living with HIV. AIDS Behav. 2016, 20, 1667–1674. [Google Scholar] [CrossRef] [Green Version]
- Jerome, R.C.; Woods, W.J.; Moskowitz, J.T.; Carrico, A.W. The Psychological Context of Sexual Compulsivity Among Men Who Have Sex with Men. AIDS Behav. 2016, 20, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Parsons, J.T.; Grov, C.; Golub, S.A. Sexual compulsivity, co-occurring psychosocial health problems, and HIV risk among gay and bisexual men: Further evidence of a syndemic. Am. J. Public Health 2012, 102, 156–162. [Google Scholar] [CrossRef]
- Qiao, S.; Li, X.; Stanton, B. Social support and HIV-related risk behaviors: A systematic review of the global literature. AIDS Behav. 2014, 18, 419–441. [Google Scholar] [CrossRef] [Green Version]
- Saleh, L.D.; van den Berg, J.J.; Chambers, C.S.; Operario, D. Social support, psychological vulnerability, and HIV risk among African American men who have sex with men. Psychol. Health 2016, 31, 549–564. [Google Scholar] [CrossRef]
- Hermanstyne, K.A.; Green, H.D., Jr.; Cook, R.; Tieu, H.V.; Dyer, T.V.; Hucks-Ortiz, C.; Wilton, L.; Latkin, C.; Shoptaw, S. Social Network Support and Decreased Risk of Seroconversion in Black MSM: Results of the BROTHERS (HPTN 061) Study. J. Acquir. Immune Defic. Syndr. 2018, 78, 163–168. [Google Scholar] [CrossRef]
- Shuper, P.A.; MacLachlan, D.J.; Joharchi, N.; Guimond, T.H.; Maxwell, J.; Adam, B.D. HIV Risk and Protective Factors in the Context of Alcohol and Substance Use During Pride. AIDS Behav. 2018, 22, 2797–2806. [Google Scholar] [CrossRef]
- Cohen, S.; Wills, T.A. Stress, social support, and the buffering hypothesis. Psychol. Bull. 1985, 98, 310–357. [Google Scholar] [CrossRef]
- Wohl, A.R.; Galvan, F.H.; Myers, H.F.; Garland, W.; George, S.; Witt, M.; Cadden, J.; Operskalski, E.; Jordan, W.; Carpio, F. Social support, stress and social network characteristics among HIV-positive Latino and African American women and men who have sex with men. AIDS Behav. 2010, 14, 1149–1158. [Google Scholar] [CrossRef]
- Menza, T.W.; Hughes, J.P.; Celum, C.L.; Golden, M.R. Prediction of HIV acquisition among men who have sex with men. Sex. Transm. Dis. 2009, 36, 547–555. [Google Scholar] [CrossRef] [Green Version]
- Haukoos, J.S.; Lyons, M.S.; Lindsell, C.J.; Hopkins, E.; Bender, B.; Rothman, R.E.; Hsieh, Y.H.; Maclaren, L.A.; Thrun, M.W.; Sasson, C.; et al. Derivation and validation of the Denver Human Immunodeficiency Virus (HIV) risk score for targeted HIV screening. Am. J. Epidemiol. 2012, 175, 838–846. [Google Scholar] [CrossRef] [Green Version]
- Hoenigl, M.; Weibel, N.; Mehta, S.R.; Anderson, C.M.; Jenks, J.; Green, N.; Gianella, S.; Smith, D.M.; Little, S.J. Development and validation of the San Diego Early Test Score to predict acute and early HIV infection risk in men who have sex with men. Clin. Infect. Dis. 2015, 61, 468–475. [Google Scholar] [CrossRef] [Green Version]
- Hu, P.; Zhong, F.; Cheng, W.B.; Xu, H.F.; Ling, L. Study on the infectious risk model of AIDS among men who have sex with men in Guangzhou. Zhonghua Liu Xing Bing Xue Za Zhi 2012, 33, 667–671. [Google Scholar]
- Yin, L.; Zhao, Y.; Peratikos, M.B.; Song, L.; Zhang, X.; Xin, R.; Sun, Z.; Xu, Y.; Zhang, L.; Hu, Y.; et al. Risk Prediction Score for HIV Infection: Development and Internal Validation with Cross-Sectional Data from Men Who Have Sex with Men in China. AIDS Behav. 2018, 22, 2267–2276. [Google Scholar] [CrossRef]
- Kalichman, S.C.; Rompa, D. Sexual sensation seeking and Sexual Compulsivity Scales: Reliability, validity, and predicting HIV risk behavior. J. Pers. Assess. 1995, 65, 586–601. [Google Scholar] [CrossRef]
- Cella, D.F.; Cherin, E.A. Quality of life during and after cancer treatment. Compr. Ther. 1988, 14, 69–75. [Google Scholar]
- Dahlem, N.W.; Zimet, G.D.; Walker, R.R. The Multidimensional Scale of Perceived Social Support: A confirmation study. J. Clin. Psychol. 1991, 47, 756–761. [Google Scholar] [CrossRef]
- Mo, R.; Shi, R.; Hu, Y.; Hu, F. Nomogram-Based Prediction of the Risk of Diabetic Retinopathy: A Retrospective Study. J. Diabetes Res. 2020, 2020, 7261047. [Google Scholar] [CrossRef]
- Wu, B.; Niu, Z.; Hu, F. Study on Risk Factors of Peripheral Neuropathy in Type 2 Diabetes Mellitus and Establishment of Prediction Model. Diabetes Metab. J. 2021, 45, 526–538. [Google Scholar] [CrossRef]
- Krakower, D.S.; Gruber, S.; Hsu, K.; Menchaca, J.T.; Maro, J.C.; Kruskal, B.A.; Wilson, I.B.; Mayer, K.H.; Klompas, M. Development and validation of an automated HIV prediction algorithm to identify candidates for pre-exposure prophylaxis: A modelling study. Lancet HIV 2019, 6, e696–e704. [Google Scholar] [CrossRef]
- Marcus, J.L.; Hurley, L.B.; Krakower, D.S.; Alexeeff, S.; Silverberg, M.J.; Volk, J.E. Use of electronic health record data and machine learning to identify candidates for HIV pre-exposure prophylaxis: A modelling study. Lancet HIV 2019, 6, e688–e695. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhou, B.; Meng, S.; Jiang, J.; Huang, S.; Lu, X.; Wu, N.; Xie, Z.; Deng, J.; Chen, X.; et al. Development and external-validation of a nomogram for predicting the survival of hospitalised HIV/AIDS patients based on a large study cohort in western China. Epidemiol. Infect. 2020, 148, e84. [Google Scholar] [CrossRef] [Green Version]
- Aghaizu, A.; Wayal, S.; Nardone, A.; Parsons, V.; Copas, A.; Mercey, D.; Hart, G.; Gilson, R.; Johnson, A.M. Sexual behaviours, HIV testing, and the proportion of men at risk of transmitting and acquiring HIV in London, UK, 2000–2013: A serial cross-sectional study. Lancet HIV 2016, 3, e431–e440. [Google Scholar] [CrossRef] [Green Version]
- Zhong, F.; Liang, B.; Xu, H.; Cheng, W.; Fan, L.; Han, Z.; Liang, C.; Gao, K.; Mai, H.; Qin, F.; et al. Increasing HIV and decreasing syphilis prevalence in a context of persistently high unprotected anal intercourse, six consecutive annual surveys among men who have sex with men in Guangzhou, China, 2008 to 2013. PLoS ONE 2014, 9, e103136. [Google Scholar] [CrossRef] [Green Version]
- Baggaley, R.F.; White, R.G.; Boily, M.C. HIV transmission risk through anal intercourse: Systematic review, meta-analysis and implications for HIV prevention. Int. J. Epidemiol. 2010, 39, 1048–1063. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.Z.; Xu, J.J.; Qian, H.Z.; You, B.X.; Zhang, J.; Zhang, J.M.; Hu, Q.H.; Chu, Z.X.; Liu, S.Y.; Jiang, Y.J.; et al. High prevalence of HIV infection and unprotected anal intercourse among older men who have sex with men in China: A systematic review and meta-analysis. BMC Infect. Dis. 2014, 14, 531. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Huang, Z.; Dong, Z.; Zhang, S.; Han, J.; Jin, M. Prevalence of high-risky behaviors in transmission of HIV among high school and college student MSM in China: A meta-analysis. BMC Public Health 2015, 15, 1272. [Google Scholar] [CrossRef] [Green Version]
- Chapin-Bardales, J.; Rosenberg, E.S.; Sullivan, P.S.; Jenness, S.M.; Paz-Bailey, G. Trends in Number and Composition of Sex Partners Among Men Who Have Sex With Men in the United States, National HIV Behavioral Surveillance, 2008–2014. J. Acquir. Immune Defic. Syndr. 2019, 81, 257–265. [Google Scholar] [CrossRef]
- Castillo, R.; Konda, K.A.; Leon, S.R.; Silva-Santisteban, A.; Salazar, X.; Klausner, J.D.; Coates, T.J.; Cáceres, C.F. HIV and Sexually Transmitted Infection Incidence and Associated Risk Factors Among High-Risk MSM and Male-to-Female Transgender Women in Lima, Peru. J. Acquir. Immune Defic. Syndr. 2015, 69, 567–575. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, F.; Ding, F.; Lin, X.; Wang, X.; Liu, N.; Liu, X.; Wang, W.; Zhang, H. Unprotected sexual behaviors and related factors of HIV-positive MSM with multiple sexual partners. Zhonghua Liu Xing Bing Xue Za Zhi 2016, 37, 517–521. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Y.L.; Chen, H.L.; Xia, J. Nitrite inhalants use, sexual behaviors and HIV/syphilis infection among men who have sex with men in Chongqing, China. Infect. Dis. Poverty 2020, 9, 127. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Van Khuu, N.; Nguyen, P.D.; Tran, H.P.; Phan, H.T.T.; Phan, L.T.; Detels, R. Sociodemographic Factors, Sexual Behaviors, and Alcohol and Recreational Drug Use Associated with HIV Among Men Who Have Sex with Men in Southern Vietnam. AIDS Behav. 2016, 20, 2357–2371. [Google Scholar] [CrossRef]
- Carey, J.W.; Mejia, R.; Bingham, T.; Ciesielski, C.; Gelaude, D.; Herbst, J.H.; Sinunu, M.; Sey, E.; Prachand, N.; Jenkins, R.A.; et al. Drug use, high-risk sex behaviors, and increased risk for recent HIV infection among men who have sex with men in Chicago and Los Angeles. AIDS Behav. 2009, 13, 1084–1096. [Google Scholar] [CrossRef]
- Xu, J.J.; Zhang, C.; Hu, Q.H.; Chu, Z.X.; Zhang, J.; Li, Y.Z.; Lu, L.; Wang, Z.; Fu, J.H.; Chen, X.; et al. Recreational drug use and risks of HIV and sexually transmitted infections among Chinese men who have sex with men: Mediation through multiple sexual partnerships. BMC Infect. Dis. 2014, 14, 642. [Google Scholar] [CrossRef] [Green Version]
- Mao, X.; Leuba, S.I.; Hu, Q.; Yan, H.; Wang, Z.; Lu, L.; Zhuang, M.; Chen, X.; Fu, J.; Geng, W.; et al. Use of multiple recreational drugs is associated with new HIV infections among men who have sex with men in China: A multicenter cross-sectional survey. BMC Public Health 2021, 21, 354. [Google Scholar] [CrossRef]
- Rosińska, M.; Gios, L.; Nöstlinger, C.; Vanden Berghe, W.; Marcus, U.; Schink, S.; Sherriff, N.; Jones, A.M.; Folch, C.; Dias, S.; et al. Prevalence of drug use during sex amongst MSM in Europe: Results from a multi-site bio-behavioural survey. Int. J. Drug Policy 2018, 55, 231–241. [Google Scholar] [CrossRef]
- Guerras, J.M.; Hoyos Miller, J.; Agustí, C.; Chanos, S.; Pichon, F.; Kuske, M.; Cigan, B.; Fuertes, R.; Stefanescu, R.; Ooms, L.; et al. Association of Sexualized Drug Use Patterns with HIV/STI Transmission Risk in an Internet Sample of Men Who Have Sex with Men from Seven European Countries. Arch. Sex. Behav. 2021, 50, 461–477. [Google Scholar] [CrossRef]
- Park, J.W.; Dobs, A.S.; Ho, K.S.; Palella, F.J.; Seaberg, E.C.; Weiss, R.E.; Detels, R. Characteristics and Longitudinal Patterns of Erectile Dysfunction Drug Use Among Men Who Have Sex with Men in the U.S. Arch. Sex. Behav. 2021, 50, 2887–2896. [Google Scholar] [CrossRef] [PubMed]
- Santos, G.M.; Coffin, P.O.; Das, M.; Matheson, T.; DeMicco, E.; Raiford, J.L.; Vittinghoff, E.; Dilley, J.W.; Colfax, G.; Herbst, J.H. Dose-response associations between number and frequency of substance use and high-risk sexual behaviors among HIV-negative substance-using men who have sex with men (SUMSM) in San Francisco. J. Acquir. Immune Defic. Syndr. 2013, 63, 540–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerras, J.M.; Hoyos, J.; García de Olalla, P.; de la Fuente, L.; Herrero, L.; Palma, D.; Del Romero, J.; García-Pérez, J.N.; Belza, M.J.; The Methysos Project, G. Comparison of Polydrug Use Prevalences and Typologies between Men Who Have Sex with Men and General Population Men, in Madrid and Barcelona. Int. J. Environ. Res. Public Health 2021, 18, 11609. [Google Scholar] [CrossRef]
- Sewell, J.; Miltz, A.; Lampe, F.C.; Cambiano, V.; Speakman, A.; Phillips, A.N.; Stuart, D.; Gilson, R.; Asboe, D.; Nwokolo, N.; et al. Poly drug use, chemsex drug use, and associations with sexual risk behaviour in HIV-negative men who have sex with men attending sexual health clinics. Int. J. Drug Policy 2017, 43, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Wang, H.; She, R.; Zhang, S.; Tsamlag, L.; Shen, Q.; Shi, Y.; Wang, Z.; Lau, J.T.F.; Wang, Y.; et al. Feelings of Entrapment and Defeat Mediate the Association Between Self-Esteem and Depression Among Transgender Women Sex Workers in China. Front. Psychol. 2019, 10, 2241. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Shi, Y.; Zhang, S.; Tsamlag, L.; Wang, H.; Chang, R.; Peng, Z.; Wang, Y.; Shang, M.; Cai, Y. How involuntary subordination and social support influence the association between self-esteem and depression: A moderated mediation model. BMC Psychiatry 2019, 19, 390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, P.; Allan, S.; Trent, D.R. Involuntary subordination or dependency as key dimensions of depressive vulnerability? J. Clin. Psychol. 1995, 51, 740–752. [Google Scholar] [CrossRef]

| Characteristic | Total Populations (n = 547) | Training Set (n = 410, 75%) | Testing Set (n = 137, 25%) | p-Value |
|---|---|---|---|---|
| Age (years old) | 28.0 (25.0, 33.0) | 28.0 (25.0, 33.0) | 27.0 (24.5, 34.0) | 0.933 |
| Employment status | 0.991 | |||
| Employed | 447 (81.7) | 335 (81.7) | 112 (81.8) | |
| Unemployed | 100 (18.3) | 75 (18.3) | 25 (18.2) | |
| Highest education level | 0.858 | |||
| Senior high school or less | 157 (28.7) | 119 (29.0) | 38 (27.7) | |
| College degree or above | 390 (71.3) | 291 (71.0) | 99 (72.3) | |
| Current marital status | 0.195 | |||
| Single | 434 (79.3) | 329 (80.2) | 105 (76.6) | |
| Married 1 | 82 (15.0) | 62 (15.1) | 20 (14.6) | |
| Divorced or widowed | 31 (5.7) | 19 (4.7) | 12 (8.8) | |
| Income(CNY) | 0.332 | |||
| <3000 2 | 133 (24.3) | 99(24.1) | 34(24.8) | |
| 3000–6000 | 211 (38.6) | 152(37.1) | 59(43.1) | |
| >6000 | 203 (37.1) | 159(38.8) | 44(32.1) | |
| Residence status | 0.079 | |||
| Local | 389 (71.1) | 127 (31.0) | 31 (22.6) | |
| Non-local | 158 (28.9) | 283 (69.0) | 106 (77.4) | |
| Sexual orientation | 0.714 | |||
| Non-homosexual | 157 (28.7) | 116 (28.3) | 41 (29.9) | |
| Gay/homosexual | 390 (71.3) | 294 (71.7) | 96 (70.1) | |
| Have had a VCT | 0.822 | |||
| No | 251 (45.9) | 187 (45.6) | 64 (46.7) | |
| Yes | 296 (54.1) | 223 (54.4) | 73 (53.3) | |
| Alcohol use before having sex | 0.415 | |||
| No | 278 (50.8) | 213 (52.0) | 65 (47.4) | |
| Yes | 269 (49.2) | 197 (48.0) | 72 (52.6) | |
| Drug use before having sex | 1.000 | |||
| No | 530 (96.9) | 397 (96.8) | 133 (97.1) | |
| Yes | 17 (3.1) | 13 (3.2) | 4 (2.9) | |
| MSP | 0.502 | |||
| No | 250 (45.7) | 184 (44.9) | 66 (48.2) | |
| Yes | 297 (54.3) | 226 (55.1) | 71 (51.8) | |
| UAI | 0.188 | |||
| No | 249 (45.5) | 180 (43.9) | 69 (50.4) | |
| Yes | 298 (54.5) | 230 (56.1) | 68 (49.6) | |
| Involuntary subordination | 80.52 ± 18.06 | 80.31 ± 18.23 | 81.12 ± 17.57 | 0.649 |
| Social support | 61.0 (51.0, 70.0) | 62.00 (51.0, 70.0) | 59.00 (50.5, 69.0) | 0.448 |
| Sexual compulsivity | 23.0 (19.0, 26.0) | 23.00(19.0, 26.0) | 23.00(20.0, 26.0) | 0.906 |
| Intercept and Variables | Estimate | Prediction Model | ||
|---|---|---|---|---|
| Wald Values | Odds Ratio (95% CI) | p-Value | ||
| Intercept | −6.862 | 51.835 | 0.000 (0.000, 0.006) | <0.001 |
| UAI | 0.931 | 5.698 | 2.536 (1.219, 5.703) | 0.017 |
| MSP | 1.043 | 6.720 | 2.837 (1.338, 6.591) | 0.010 |
| Drug use before sex | 1.522 | 5.323 | 4.579 (1.184, 16.437) | 0.021 |
| Involuntary subordination | 0.040 | 17.161 | 1.041 (1.022, 1.061) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Liu, S.; Xia, D.; Xu, C.; Yu, X.; Chen, H.; Wang, R.; Liu, Y.; Dong, J.; Hu, F.; et al. Prediction Model for the Risk of HIV Infection among MSM in China: Validation and Stability. Int. J. Environ. Res. Public Health 2022, 19, 1010. https://doi.org/10.3390/ijerph19021010
Dong Y, Liu S, Xia D, Xu C, Yu X, Chen H, Wang R, Liu Y, Dong J, Hu F, et al. Prediction Model for the Risk of HIV Infection among MSM in China: Validation and Stability. International Journal of Environmental Research and Public Health. 2022; 19(2):1010. https://doi.org/10.3390/ijerph19021010
Chicago/Turabian StyleDong, Yinqiao, Shangbin Liu, Danni Xia, Chen Xu, Xiaoyue Yu, Hui Chen, Rongxi Wang, Yujie Liu, Jingwen Dong, Fan Hu, and et al. 2022. "Prediction Model for the Risk of HIV Infection among MSM in China: Validation and Stability" International Journal of Environmental Research and Public Health 19, no. 2: 1010. https://doi.org/10.3390/ijerph19021010
APA StyleDong, Y., Liu, S., Xia, D., Xu, C., Yu, X., Chen, H., Wang, R., Liu, Y., Dong, J., Hu, F., Cai, Y., & Wang, Y. (2022). Prediction Model for the Risk of HIV Infection among MSM in China: Validation and Stability. International Journal of Environmental Research and Public Health, 19(2), 1010. https://doi.org/10.3390/ijerph19021010







