Transfer of Metal(loid)s from Soil to Leaves and Trunk Xylem Sap of Medicinal Plants and Possible Health Risk Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Schematic Drawing of the Study
2.2. Sample Collection
2.3. Sample Preparation and Digestion
2.4. Sample Analysis
2.5. Transfer Factor
2.6. Estimated Daily Intake
2.7. Target Hazard Quotient
2.8. Statistical Analysis
3. Results
3.1. Concentration of the Meta(loid)s in Soil, Trunk Xylem Sap and Leaves of the D. alata
- For distances D1, D2 and D3 from highway (Table 1), the results obtained for the concentration of Al, As, Cd, Co, Fe, Mg, Mo, Ni, P and Se are considerably higher than those reported by Brazil/Conama, and other countries as China and USA;
- The obtained Cr, Mn and Pb levels in three sampling sites of soil at D1, D2 and D3, are below than those reported Brazil/Conama, China and USA;
- The obtained results for Cu and Zn levels in three sampling sites of soil at D1, D2 and D3, are below than those reported by Brazil/Conama; however, these values are highest than the values set by China and USA.
3.2. Transfer Factor
- For distances of 5 m from highway edge: P (1.99) > Mg (0.14) > Al (0.05) > Mn (0.04) > Fe (0.03) > Se (0.02) > Cd (0.02) > Cr (0.01). The TF value for P is greater than 1.
- For distances of 20 m from highway edge: P (2.21) > Mg (0.14) > Mn (0.12) > Al (0.07) > Fe (0.03) > Cd (0.02) > Se (0.02) > Cr (0.01). For P, TF > 1.
- For distances of 35 m from highway edge: P (2.64) > Mg (0.15) > Mn (0.13) > Al (0.07) > Fe (0.04) > Cd (0.03) > Se (0.02) > Cr (0.02). Only for Al, the value of the TF > 1. For this distance, with excess of Al, all TF values < 1.
3.3. Health Risk Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steffan, J.J.; Brevik, E.C.; Burgess, L.C.; Cerdà, A. The effect of soil on human health: An overview. Eur. J. Soil Sci. 2018, 69, 159–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pepper, I.L. The soil health-human health Nexus. Crit. Rev. Environ. Sci. Tech. 2013, 43, 2617–2652. [Google Scholar] [CrossRef]
- Brevik, E.C.; Burgess, L. Soil: Influence on human health. In Encyclopedia of Environmental Management; Jorgensen, S.V., Ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 1–13. [Google Scholar]
- Gupta, D.; Fatima, A.; Singh, P.; Correspondence, P.; Singh, S.; Prasad, S. Repercussion of soil pollution on plants. Regul. Rivers Res. Manag. 2019, 6, 89–98. [Google Scholar]
- FAO; UNEP. Global Assessment of Soil Pollution—Summary for policy makers; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Polluting Our Soils Is Polluting Our Future. 2020. Available online: http://www.fao.org/fao-stories/article/en/c/1126974/ (accessed on 5 June 2021).
- Dey, S.; Mehta, N.S. Automobile pollution control using catalysis. Resour. Environ. Sustain. 2020, 2, 100006. [Google Scholar] [CrossRef]
- European Environment Agency (EEA). Heavy Metal Emissions. Available online: https://www.eea.europa.eu/data-and-maps/indicators/eea32-heavy-metal-hm-emissions-1/assessment-10 (accessed on 30 September 2021).
- EC, IED 2010/75/EU, 2010. Available online: https://www.eea.europa.eu/data-and-maps/indicators/eea32-heavy-metal-hm-emissions-1/assessment-8/#_ftnref4 (accessed on 30 September 2021).
- Machate, D.J.; Melo, E.S.P.; Arakaki, D.G.; Guimaraes, R.C.A.; Hiane, P.A.; Bogo, D.; Pott, A.; Nascimento, V.A. High concentration of heavy metal and metalloid levels in edible Campomanesia adamantium pulp from anthropic areas. Int. J. Environ. Res. Public Health 2021, 18, 5503. [Google Scholar] [CrossRef]
- Tschinkel, P.F.S.; Melo, E.S.P.; Pereira, H.S.; Silva, K.R.N.; Arakaki, D.G.; Lima, N.V.; Fernandes, M.R.; Leite, L.C.S.; Melo Eliane, S.P.; Melnikov, P.; et al. The hazardous level of heavy metals in different medicinal plants and their decoctions in water: A public health problem in Brazi. Bio. Med. Res. Int. 2020, 2020. [Google Scholar] [CrossRef] [Green Version]
- Mirecki, N.; Rukie, A.; Šunić, L.; Milenkovic, L.; Ilic, Z.S. Transfer factor as indicator of heavy metals content in plants. Fresenius Environ. Bull. 2015, 24, 4212–4219. [Google Scholar]
- Zheljazkova, V.D.J.; Jeliazkovaa, E.A.; Kovachevab, N.; Dzhurmanski, A. Environmental and experimental botany metal uptake by medicinal plant species grown in soils contaminated by a smelter. Environ. Exp. Bot. 2008, 64, 207–216. [Google Scholar] [CrossRef]
- Mota, E.M.S.; Novaes, C.R.D.B.; Silva, L.B.; Chaves, L.J. Structure of the phenotypic variability of fruit and seeds of D. alata vogel (Fabaceae). Rev. Bras. Frutic. 2020, 42, 6. [Google Scholar] [CrossRef]
- Sano, S.M.; Ribeiro, J.F.; Brito, M.A. Baru: Biologia e uso. Planaltina, DF: Embrapa Cerrados. 2004. Available online: https://www.infoteca.cnptia.embrapa.br/infoteca/handle/doc/566595 (accessed on 26 December 2021).
- Sanches, R.M. Estudo Fitoquímico e Propriedades Biológicas da D. alata Vogel (baru). Dissertation of Master, Iniversity of the Ilha Solteira, Ilha Solteira, Brazil, 2014. Available online: http://hdl.handle.net/11449/123176 (accessed on 5 June 2021).
- Nazato, V.S.; Rubem-Mauro, L.; Vieira, N.A.; Rocha-Junior, D.; Silva, M.G.; Lopes, P.S.; Dal-Belo, C.A.; Cogo, J.C.; dos Santos, M.G.; da Cruz-Höfling, M.A.; et al. In vitro antiophidian properties of D. alata Vogel bark extracts. Molecules 2010, 15, 5956–5970. [Google Scholar] [CrossRef] [Green Version]
- Takemoto, E.; Okada, I.A.; Garbelotti, M.L.; Tavares, M. Aued-Pimentel, S. Chemical composition of seeds and oil of baru (D. alata Vog.) native from Pirenópolis, State of Goiás. Brazil. Rev. Inst. Adolfo Lutz 2001, 60, 113–117. [Google Scholar]
- Boni, T.S.; Maltoni, K.L.; Mizobata, K.K.G.S. Dipteryx alata Seedlings Nutritional Status in a Recovery Area in the Brazilian Savannah. Floresta Ambiente 2020, 27, e20180125. [Google Scholar] [CrossRef]
- Marceli Borges Fiovarante, Elaboração, Aceitabilidade de Bebida Fermentada a Base de Extrato Hidrosoluvel da Amendoa do Baru (D. alata Vog), 2015, Universidade Federal de Mato Grosso do Sul, Campo Grande/Ms. Brazil. 2015. Available online: https://repositorio.ufms.br/bitstream/123456789/2641/1/MARCELI%20BORGES%20FIORAVANTE.pdf (accessed on 5 June 2021).
- Greco, A.S. Desenvolvimento de Método Analítico para Determinação de Selênio em Castanhas do Cerrado por Espectrometria de Absorção Atômica com Geração de Hidreto. Anderson dos Santos Greco.—Dourados, MS: UFGD. 2016. Available online: https://files.ufgd.edu.br/arquivos/arquivos/78/MESTRADO-QUIMICA/Disserta%C3%A7%C3%A3o%20%20Anderson%20dos%20Santos%20Greco.pdf (accessed on 5 June 2021).
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of sources, chemistry, risks and best available strategies for remediation. Int. Sch. Res. Not. 2011, 2011. [Google Scholar] [CrossRef] [Green Version]
- Zwolak, A.; Sarzyńska, M.; Szpyrka, E.; Stawarczyk, K. Sources of soil pollution by heavy metals and their accumulation in vegetables: A review. Water Air Soil Pollut. 2019, 230, 164. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, A.C., Jr.; Nacke, H.; Schwantes, D.; Coelho, D.F. Heavy Metal Contamination in Brazilian Agricultural Soils Due to Application of Fertilizers. 2014. Available online: https://www.intechopen.com/chapters/46144 (accessed on 22 June 2021).
- Falahi-Ardakani, A. Contamination of environment with heavy metals emitted from automotives. Ecotoxicol. Environ. Saf. 1984, 8, 152–161. [Google Scholar] [CrossRef]
- Hulskotte, J.; Roskam, G.; Denier van der Gon, H.A. Elemental composition of current automotive braking materials and derived air emission factors. Atmos. Environ. 2014, 99, 436–445. [Google Scholar] [CrossRef]
- Souza, I.D.; Melo, E.S.P.; Nascimento Valdir, A.; Pereira, H.S.; Silva, K.R.N.; Espindola, P.R.; Tschinkel, P.F.S.; Ramos, E.M.; Reis, F.J.M.; Ramos, I.B.; et al. Potential health risks of macro- and microelements in commercial medicinal plants used to treatment of diabetes. Bio. Med. Res. Int. 2021, 2021. Available online: https://www.hindawi.com/journals/bmri/2021/6678931/ (accessed on 26 December 2021). [CrossRef]
- Vachová, P.; Vach, M.; Najnarová, E. Using expansive grasses for monitoring heavy metal pollution in the vicinity of roads. Environ. Pollut. 2017, 229, 94–101. [Google Scholar] [CrossRef]
- Miclean, M.; Cadar, O.; Levei, E.A.; Roman, R.; Ozunu, A.; Levei, L. Metal (Pb, Cu, Cd, and Zn) Transfer along food chain and health risk assessment through raw milk consumption from free-range cows. Int. J. Environ. Res. Public Health 2019, 16, 4064. [Google Scholar] [CrossRef] [Green Version]
- United States Environmental Protection Agency (USEPA). Method 3051A “Microwave Assisted Acid Digestion of Sediments, Sludge and Oils” Revision 1, January 1998. Available online: https://www.epa.gov/sites/default/files/2015-12/documents/3051a.pdf (accessed on 10 August 2021).
- Long, G.L.; Winefordner, J.D. Limit of detection: A closer look at the IUPAC definition. Anal. Chem. 1983, 55, 712a–724a. [Google Scholar]
- Jolly, Y.N.; Islam, A.; Akbar, S. Transfer of metals from soil to vegetables and possible health risk assessment. SpringerPlus 2013, 2, 385. [Google Scholar] [CrossRef] [Green Version]
- Blaylock, M.J.; Salt, D.E.; Dushenkov, S.; Zakharova, O.; Gussman, C.; Kapulnik, Y.; Ensley, B.D.; Raskin, I. Enhanced accumulation of Pb in Indian Mustard by soil applied chelating agents. Environ. Sci. Technol. 1997, 31, 860–865. [Google Scholar] [CrossRef]
- Brooks, R.R.; Lee, J.; Reeves, R.D.; Jaffre, T. Detection of nickeliferous rocks by analysis of herbarium specimens of indicator plants. J. Geochem. Explor. 1977, 7, 49–58. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Food and Nutrition Board; Committee to Review the Dietary Reference Intakes for Sodium and Potassium. Dietary Reference Intakes for Sodium and Potassium; Oria, M., Harrison, M., Stallings, V.A., Eds.; National Academies Press (US): Washington, DC, USA, 2019; Available online: https://www.ncbi.nlm.nih.gov/books/NBK538102/ (accessed on 10 August 2021).
- Sipter, E.; Rozsa, E.; Gruiz, K.; Tatrai, E.; Morvai, V. Site-specific risk assessment in contaminated vegetable gardens. Chemosphere 2008, 71, 1301–1307. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (USEPA). Regional Screening Levels (RSLs)—Generic Tables. Available online: https://semspub.epa.gov/work/HQ/400750.pdf (accessed on 22 June 2021).
- Chemical Update Worksheet. CAS #: 7439-95-4, Revision Date: 21 September 2015. Available online: https://www.michigan.gov/documents/deq/deq-rrd-chem-MagnesiumDatasheet_527861_7.pdf (accessed on 7 July 2021).
- Gerba, C.P. Risk Assessment, Chapter 14, pag 221. Available online: https://web.iitd.ac.in/~arunku/files/CEL899_Y13/Gerba%20Risk%20Assessment.pdf (accessed on 10 September 2021).
- Kamunda, C.; Mathuthu, M.; Madhuku, M. Health risk assessment of heavy metals in soils from witwatersrand gold mining Basin, South Africa. Int. J. Environ. Res. Public Health 2016, 13, 663. [Google Scholar] [CrossRef]
- Dray, S.; Dufour, A.B.; Chessel, D. The ade4 package-II: Two-table and K-table methods. R News 2007, 7, 47–52. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austia, 2021. Available online: http://www.R-project.org. (accessed on 26 December 2021).
- Ministério do Meio Ambiente, Conselho Nacional do Meio Ambiente. Resolution No 420, de 28 de Dezembro de 2009. Brazil. Available online: http://hab.eng.br/wp-content/uploads/2017/09/resolucao-conama-420-2009-gerenciamento-de-acs.pdf (accessed on 22 June 2021).
- Chen, J.; Wei, F.; Zheng, C.; Wu, Y.; Adriano, D.C. Background concentrations of elements in soils of China. Water Air Soil Pollut. 1991, 57, 699–712. [Google Scholar] [CrossRef]
- Monitoring and Sampling Manual: Environmental Protection (Water) Policy. Brisbane: Department of Environment and Science Government. Available online: https://environment.des.qld.gov.au/__data/assets/pdf_file/0031/89914/monitoring-sampling-manual-2018.pdf (accessed on 21 November 2021).
- Swaileh, K.M.; Rabay’a, N.; Ezzughayyar, A.; Rabbo, A.A. Concentrations of heavy metals in roadside soils, plants, and landsnails from the West Bank, Palestine. Journal of environmental science and health. Part A. Toxic Hazard. Subst. Environ. Eng. 2001, 36, 765–778. [Google Scholar] [CrossRef]
- Viard, B.; Pihan, F.; Promeyrat, S.; Pihan, J.-C. Integrated assessment of heavy metal (Pb, Zn, Cd) highway pollution: Bioaccumulation in soil, Graminaceae and land snails. Chemosphere 2004, 55, 1349–1359. [Google Scholar] [CrossRef]
- Kuklová, M.; Hniličková, H.; Hnilička, F.; Pivková, I.; Kukla, J. Impact of expressway on physiology of plants and accumulation of risk elements in forest ecosystems. Plant Soil Environ. 2019, 65, 46–53. [Google Scholar] [CrossRef]
- Maiorana, S.; Teoldi, F.; Silvani, S.; Mancini, A.; Sanguineti, A.; Mariani, F.; Cella, C.; Lopez, A.; Potenza, M.A.C.; Lodi, M.; et al. Phytotoxicity of wear debris from traditional and innovative brake pads. Environ. Int. 2018, 123, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.A.; Arrizabalaga, I.; Bustamante, J.; Goienaga, N.; Arana, G.; Madariaga, J.M. Diagnosing the Impact of Traffic on Roadside Soils Through Chemometric Analysis on the Concentrations of More Than 60 Metals Measured by ICP/MS. In Highway and Urban Environment; Rauch, S., Morrison, G., Monzón, A., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 336–393. [Google Scholar]
- Mapani, B.; Křibek, B. Environmental and Health impacts of mining in Africa. In Proceedings of the Annual Workshop IGCP/SIDA Project No. 594, Windhoek, Namibia, 5–6 July 2012; pp. 31–32, Czech Geological Survey. ISBN 978-80-7075-781-9. Available online: http://www.geology.cz/igcp594/windhoek/proceedings-of-the-workshop.pdf (accessed on 26 December 2021).
- Page, V.; Feller, U. Heavy Metals in Crop Plants: Transport and redistribution processes on the whole plant level. Agronomy 2015, 5, 447–463. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, S.; Davie, W.J. Xylem Sap pH Increase: A drought signal received at the apoplastic face of the guard cell that lnvolves the suppression of saturable abscisic acid uptake by the epidermal symplast. Plant. Physiol. 1997, 113, 559–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dambrine, E.; Martin, F.; Carisey, N.; Granier, A.; Hallgren, J.E.; Bishop, K. Xylem sap composition: A tool for investigating mineral uptake and cycling in adult spruce. Plant. Soil 1995, 168–169, 233–241. [Google Scholar] [CrossRef]
- Alam, S.; Kamei, S.; Kawai, S. Effect of iron deficiency on the chemical composition of the xylem xap of barley. Soil Sci. Plant Nutr. 2001, 47, 643–649. [Google Scholar] [CrossRef]
- Leita, L.; Mondini, C.; de Nobili, M.; Simoni, A.; Sequi, P. Heavy metal content in xylem sap (Vitis Vinifera) from mining and smelting areas. Environ. Monit Assess. 1998, 50, 189–200. [Google Scholar] [CrossRef]
- Ferguson, A.R. Xylem sap from Actinidia chinensis: Apparent differences in the sap composition arising from the method of collection. Ann. Bot. 1980, 46, 791–801. [Google Scholar] [CrossRef]
- Álvarez-Fernández, A.; Díaz-Benito, P.; Abadía, A.; Lopez-Millan, A.-F.; Abadía, J. Metal species involved in long distance metal transport in plants. Front. Plant. Sci. 2014, 5, 105. [Google Scholar] [CrossRef] [Green Version]
- Azab, E.; Hegazy, A.K. Monitoring the Efficiency of Rhazya stricta L. Plants in Phytoremediation of Heavy Metal-Contaminated Soil. Plants 2020, 9, 1057. [Google Scholar] [CrossRef]
- Takagi, D.; Miyagi, A.; Tazoe, Y.; Suganami, M.; Kawai-Yamada, M.; Ueda, A.; Suzuki, Y.; Noguchi, K.; Hirotsu, N.; Makino, A. Phosphorus toxicity disrupts Rubisco activation and reactive oxygen species defence systems by phytic acid accumulation in leaves. Plant. Cell Environ. 2020, 43, 2033–2053. [Google Scholar] [CrossRef]
- Robles, H. (Ed.) Phosphorus. In Philip Wexler, Encyclopedia of Toxicology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 920–921. ISBN 9780123864550. [Google Scholar] [CrossRef]
- Menezes-Filho, J.A.; Paes, C.R.; Pontes, A.M.C.; Moreira, J.C.; Sarcinelli, P.N.; Mergler, D. High levels of hair manganese in children living in the vicinity of a ferromanganese alloy production plant. Neurotoxicology 2009, 30, 1207–1213. [Google Scholar] [CrossRef] [Green Version]
- Ajib, F.A.; Childress, J.M. Magnesium Toxicity. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021; Available online: https://www.ncbi.nlm.nih.gov/books/NBK554593/ (accessed on 10 September 2021). [PubMed]
- Ngole, V.M.; Ekosse, G.I.E. Copper, nickel and zinc contamination in soils within the precincts of mining and landfilling environments. Int. J. Environ. Sci. Technol. 2012, 9, 485–494. [Google Scholar] [CrossRef] [Green Version]
- Gebre, D.G.; Debelie, H.D. Heavy metal pollution of soil around solid waste dumping sites and its impact on adjacent community: The case of Shashemane open landfill. Ethiopia 2018, 5, 169–178. [Google Scholar]
- Kanmani, S.; Gandhimathi, R. Assessment of heavy metal contamination in soil due to leachate migration from an open dumping site. Appl. Water Sci. 2013, 3, 193–205. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Cao, Q.; Zheng, Y.M.; Huang, Y.Z.; Zhu, Y.G. Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ. Pollut. 2008, 152, 686–692. [Google Scholar] [CrossRef]
- Awad, M.; Liu, Z.; Skalicky, M.; Dessoky, E.S.; Brestic, M.; Mbarki, S.; Rastogi, A.; EL Sabagh, A. Fractionation of Heavy Metals in Multi-Contaminated Soil Treated with Biochar Using the Sequential Extraction Procedure. Biomolecules 2021, 11, 448. [Google Scholar] [CrossRef]
- Basta, N.T.; Ryan, J.A.; Chaney, R.L. Trace element chemistry in residual-treated soil: Key concepts and metal bioavailability. J. Environ. Qual. 2005, 34, 49–63. [Google Scholar] [CrossRef] [Green Version]
- United States Environmental Protection Agency (USEPA). Aluminum: Material-Specific. Data. 2018. Available online: https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/aluminum-material-specific-data#AluminumOverview (accessed on 3 September 2021).
- Osman, M.A.; Yang, F.; Massey, I.Y. Exposure routes and health effects of heavy metals on children. Biometals 2019, 32, 563–573. [Google Scholar] [CrossRef]
- Lajayer, B.A.; Moghadam, N.K.; Maghsoodi, M.R.; Kariman, K. Phytoextraction of heavy metals from contaminated soil, water and atmosphere using ornamental plants: Mechanisms and efficiency improvement strategies. Environ. Sci. Pollut. Res. 2019, 26, 8468–8484. [Google Scholar] [CrossRef]
- Awad, M.; El-Desoky, M.A.; Ghallab, A.; Kubes, J.; Abdel-Mawly, S.E.; Danish, S.; Ratnasekera, D.; Sohidul Islam, M.; Skalicky, M.; Brestic, M.; et al. Ornamental Plant Efficiency for Heavy Metals Phytoextraction from Contaminated Soils Amended with Organic Materials. Molecules 2021, 26, 3360. [Google Scholar] [CrossRef]
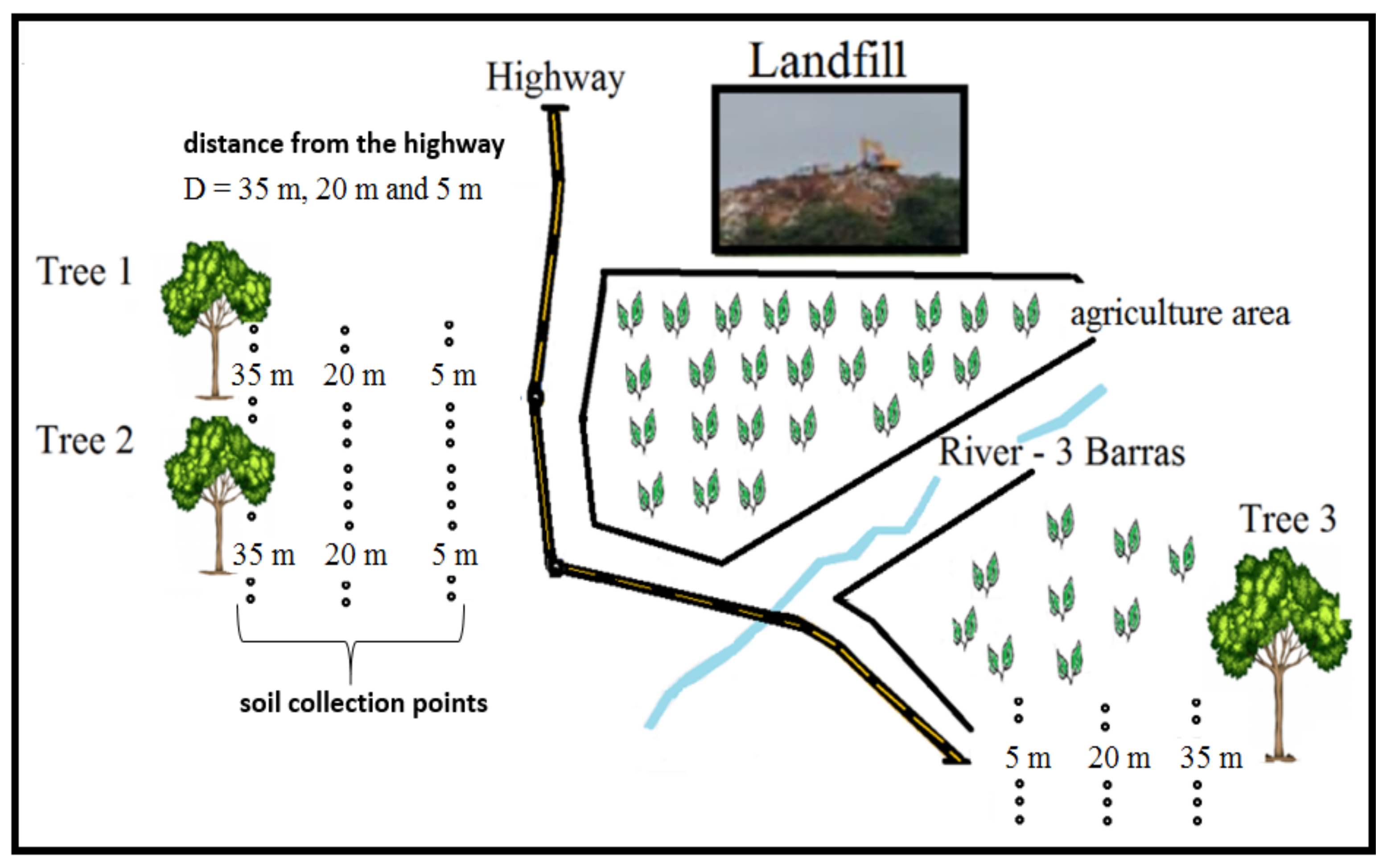

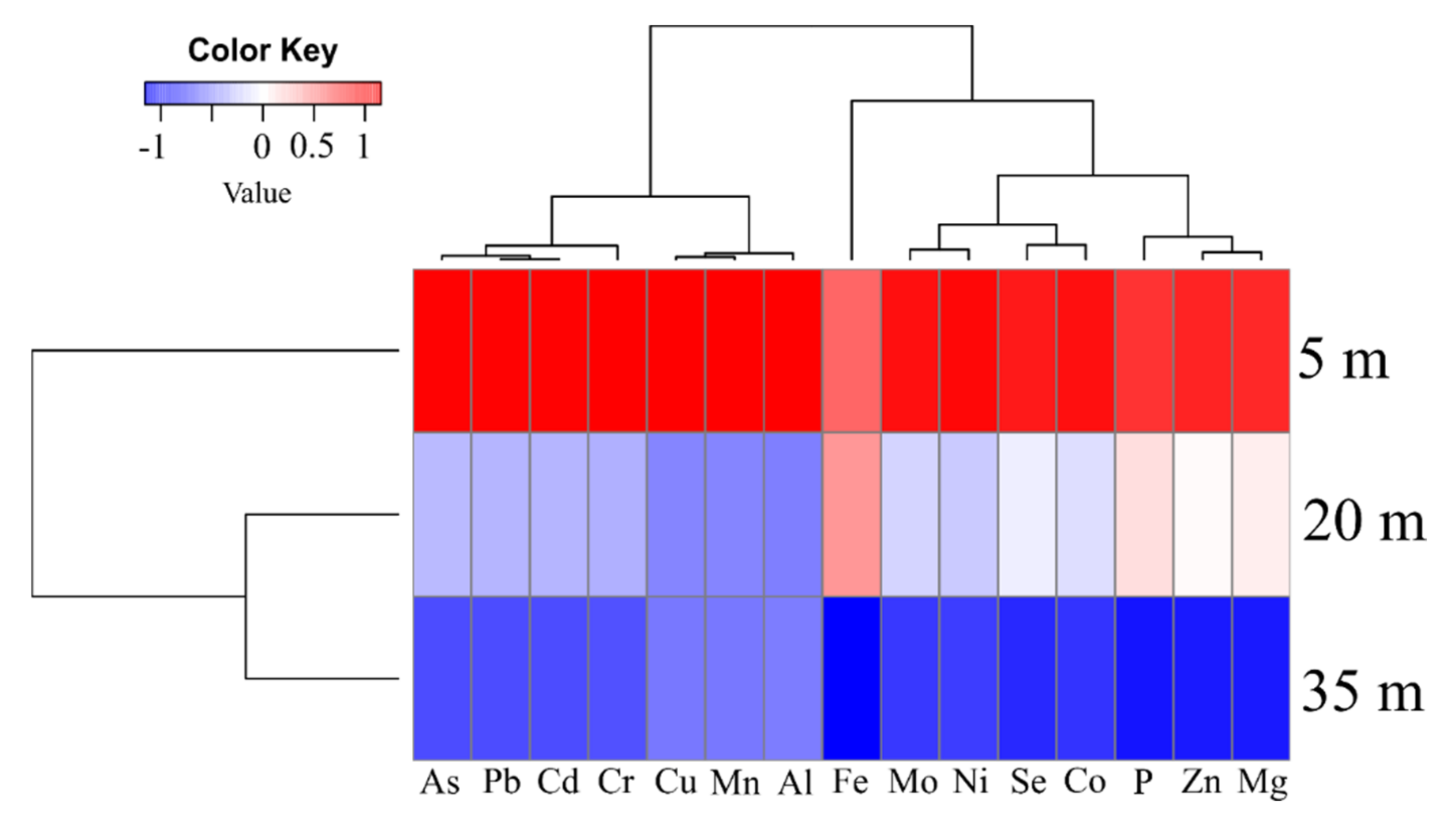
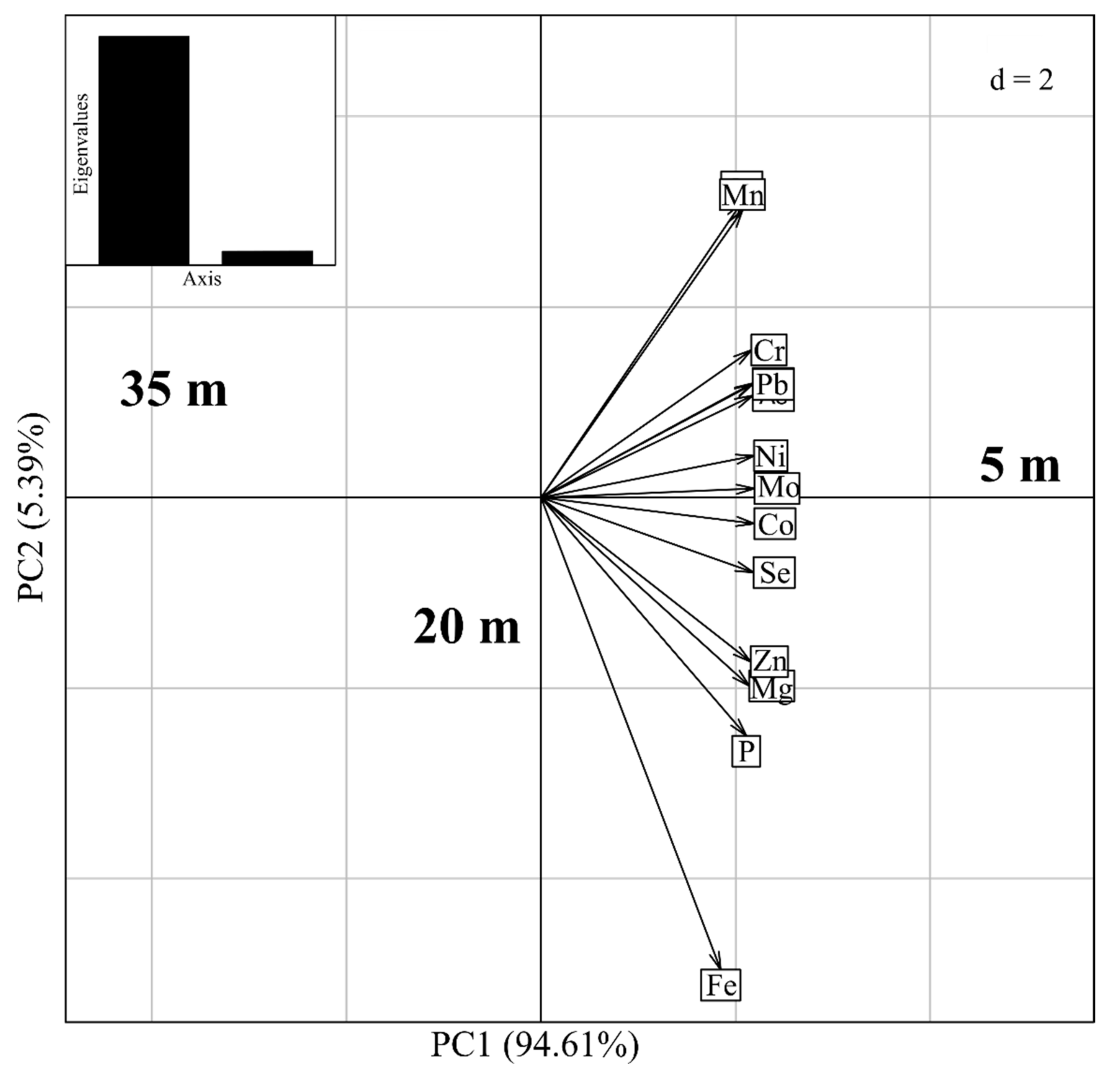
| Element | Concentrations of Elements in Soil: (Distances from the Sampling Sites to Highway Edge) (mg/kg·dw) | Conama/Brazil (mg/kg) | China (mg/kg) | USA (mg/kg) | ||
|---|---|---|---|---|---|---|
| 5 m | 20 m | 35 m | ||||
| Al | 79.56|6.35 a | 54.65|1.87 a | 54.44|1.50 a | * | 6.4 | 4.7 |
| As | 20.85|0.71 a | 15.84|0.75 b | 14.10|0.94 b | 15 | 9.2 | 5.2 |
| Cd | 24.26|1.16 a | 16.06|0.91 b | 12.88|0.88 b | 1.3 | 0.07 | *** |
| Co | 15.12|0.20 a | 13.27|0.22 b | 12.09|0.12 b | 35 | 11 | 6.7 |
| Cr | 28.47|0.46 a | 20.28|0.44 b | 18.11|0.18 b | * | 54 | 37 |
| Cu | 160.33|0.88 a | 73.16|0.48 b | 70.65|0.56 b | 200 | 20 | 17 |
| Fe | 188.00|0.88 a | 183.76|6.03 a | 152.30|0.27 a | * | 2.8 | 1.8 |
| Mg | 230.01|0.50 a | 220.81|0.51 b | 209.40|0.17 b | * | 0.67 | 0.44 |
| Mn | 118.10|0.13 a | 45.17|0.27 b | 42.24|0.24 b | * | 432 | 333 |
| Mo | 45.42|0.28 a | 28.16|0.56 b | 18.64|1.35 b | 30 | 1.2 | 0.59 |
| Ni | 49.86|0.54 a | 40.22|0.29 b | 35.64|0.50 b | 30 | 23 | 13 |
| P | 20.57|0.58 a | 18.60|0.58 b | 15.54|0.70 b | * | ** | 0.02 |
| Se | 14.10|0.87 a | 13.00|0.02 b | 12.14|0.18 b | * | 0.22 | 0.26 |
| Zn | 178.85|1.27 a | 165.40|0.12 b | 150.28|0.07 b | 300 | 67 | 48 |
| Pb | 12.44|0.51 a | 7.08|1.33 b | 5.64|0.45 b | 72 | 24 | 16 |
| Elements | Trunk Xylem Sap (mg/L) | Leaves (mg/L) |
|---|---|---|
| Al | 21.40 ± 0.20 | 3.86 ± 0.39 |
| As | <LOD | 0.06 ± 0.07 |
| Cd | <LOD | 0.42 ± 0.10 |
| Co | <LOD | <LOD |
| Cr | <LOD | 0.34 ± 0.16 |
| Cu | 0.60 ± 0.01 | 0.07 ± 0.05 |
| Fe | 6.60 ± 0.30 | 6.50 ± 0.52 |
| Mg | 341.00 ± 2.40 | 31.89 ± 1.19 |
| Mn | 52.20 ± 0.50 | 5.51 ± 0.24 |
| Mo | <LOD | <LOD |
| Ni | <LOD | <LOD |
| P | 266.90 ± 0.40 | 41.07 ± 0.16 |
| Se | 8.30 ± 0.40 | 0.29 ± 0.14 |
| V | <LOD | <LOD |
| Zn | 1.40 ± 0.10 | 0.44 ± 0.03 |
| Element | Transfer Factor of Concentrations of Elements in Soil to Trunk Xylem Sap of the Plant | ||
|---|---|---|---|
| Distances from the Sampling Sites to Highway Edge | |||
| 5 m | 20 m | 35 m | |
| Al | 0.28 | 0.39 | 0.40 |
| Cu | 0.00 | 0.00 | 0.00 |
| Fe | 0.03 | 0.04 | 0.04 |
| Mg | 1.48 | 1.54 | 1.63 |
| Mn | 0.44 | 1.15 | 1.23 |
| P | 12.97 | 14.35 | 17.17 |
| Se | 0.58 | 0.64 | 0.68 |
| Zn | 0.00 | 0.00 | 0.00 |
| Element | Transfer Factor of Concentrations of Elements in Soil to Leaves of the Plant | ||
|---|---|---|---|
| Distances from the Sampling Sites to Highway Edge | |||
| 5 m | 20 m | 35 m | |
| Al | 0.05 | 0.07 | 0.07 |
| As | 0.00 | 0.00 | 0.00 |
| Cd | 0.02 | 0.02 | 0.03 |
| Cr | 0.01 | 0.01 | 0.02 |
| Cu | 0.00 | 0.00 | 0.00 |
| Fe | 0.03 | 0.03 | 0.04 |
| Mg | 0.14 | 0.14 | 0.15 |
| Mn | 0.04 | 0.12 | 0.13 |
| P | 1.99 | 2.20 | 2.64 |
| Se | 0.02 | 0.02 | 0.02 |
| Zn | 0.00 | 0.00 | 0.00 |
| Elements | Adults (Female/Male) 50 Years (70 kg) | Adolescents (Female/Male) 12 Years (40 kg) | ||
|---|---|---|---|---|
| Estimated Daily Intake (mg/kg/day) | Upper Level of Tolerable Intake (UL) (mg/day) | Estimated Daily Intake (mg/kg/day) | Upper Level of Tolerable Intake (UL) (mg/day) | |
| Al | 0.07 ± 7.1 × 10−4 | ND | 0.03 ± 2.5 × 10−4 | ND |
| Cu | 2.14 × 10−3 ± 3.5 × 10−5 | 10 | 7.5 × 10−4 ± 1.0 × 10−5 | 5 |
| Fe | 0.02 ± 0.011 | 45 | 8.25 × 10−3 ± 0.04 | 40 |
| Mg | 1.28 ± 8.57 × 10−3 | 350 | 0.43 ± 3.0 × 10−3 | 350 |
| Mn | 0.18 ± 1.78 × 10−3 | 11 | 0.06 ± 6.25 × 10−4 | 6 |
| P | 0.95 ± 1.42 × 10−3 | 4000 | 0.3 ± 5.0 × 10−4 | 4000 |
| Se | 0.03 ± 1.42 × 10−3 | 0.4 | 0.01 ± 5.0 × 10−4 | 0.28 |
| Zn | 5.0 × 10−3 ± 3.5 × 10−4 | 40 | 1.75 × 10−3 ± 1.25 × 10−4 | 23 |
| Elements | Adults 50 Years (70 kg) | Children 12 Years (40 kg) |
|---|---|---|
| HQ | HQ | |
| Al | 1.88 × 10−2 ± 1.80 × 10−4 | 6.59 × 10−3 ± 6.16 × 10−5 |
| Cu | 0.01 ± 2.15 × 10−4 | 4.4 × 10−3 ± 7.3 × 10−5 |
| Fe | 8.27 × 10−3 ± 3.76 × 10−3 | 2.9 × 10−3 ± 1.3 × 10−3 |
| Mg | 0.03 ± 1.92 × 10−4 | 9.55 × 10−3 ± 6.72 × 10−5 |
| Mn | 1.91 ± 0.0182 | 0.68 ± 6.42 × 10−3 |
| P | 11,751.90 ± 17.50 | 4105.47 ± 6.16 |
| Se | 1.43 ± 0.07 | 0.507 ± 0.024 |
| Zn | 4.10 × 10−3 ± 2.93 × 10−4 | 1.43 × 10−3 ± 1.02 × 10−4 |
| Chronic hazard index (HI) | 11,755.31 ± 17.59 | 4106.60 ± 6.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosa, A.C.G.; Melo, E.S.d.P.; Junior, A.S.A.; Gondim, J.M.S.; de Sousa, A.G.; Cardoso, C.A.L.; Viana, L.F.; Carvalho, A.M.A.; Machate, D.J.; do Nascimento, V.A. Transfer of Metal(loid)s from Soil to Leaves and Trunk Xylem Sap of Medicinal Plants and Possible Health Risk Assessment. Int. J. Environ. Res. Public Health 2022, 19, 660. https://doi.org/10.3390/ijerph19020660
Rosa ACG, Melo ESdP, Junior ASA, Gondim JMS, de Sousa AG, Cardoso CAL, Viana LF, Carvalho AMA, Machate DJ, do Nascimento VA. Transfer of Metal(loid)s from Soil to Leaves and Trunk Xylem Sap of Medicinal Plants and Possible Health Risk Assessment. International Journal of Environmental Research and Public Health. 2022; 19(2):660. https://doi.org/10.3390/ijerph19020660
Chicago/Turabian StyleRosa, Ana C. Gomes, Elaine S. de Pádua Melo, Ademir S. A. Junior, Jacqueline M. S. Gondim, Alexsandro G. de Sousa, Claudia A. L. Cardoso, Lucilene F. Viana, Alexandra M. A. Carvalho, David J. Machate, and Valter Aragão do Nascimento. 2022. "Transfer of Metal(loid)s from Soil to Leaves and Trunk Xylem Sap of Medicinal Plants and Possible Health Risk Assessment" International Journal of Environmental Research and Public Health 19, no. 2: 660. https://doi.org/10.3390/ijerph19020660
APA StyleRosa, A. C. G., Melo, E. S. d. P., Junior, A. S. A., Gondim, J. M. S., de Sousa, A. G., Cardoso, C. A. L., Viana, L. F., Carvalho, A. M. A., Machate, D. J., & do Nascimento, V. A. (2022). Transfer of Metal(loid)s from Soil to Leaves and Trunk Xylem Sap of Medicinal Plants and Possible Health Risk Assessment. International Journal of Environmental Research and Public Health, 19(2), 660. https://doi.org/10.3390/ijerph19020660







