Complex Contact Network of Patients at the Beginning of an Epidemic Outbreak: An Analysis Based on 1218 COVID-19 Cases in China
Abstract
:1. Introduction
2. Theoretical Background
2.1. Social Science Research during the Epidemic
2.2. Social Networks and Disease Transmission
3. Data and Method
3.1. Data
3.2. Variables
4. Results
4.1. Description Statistics
4.2. Contact Networks of COVID-19
4.2.1. Contact Networks Structure and Visualization
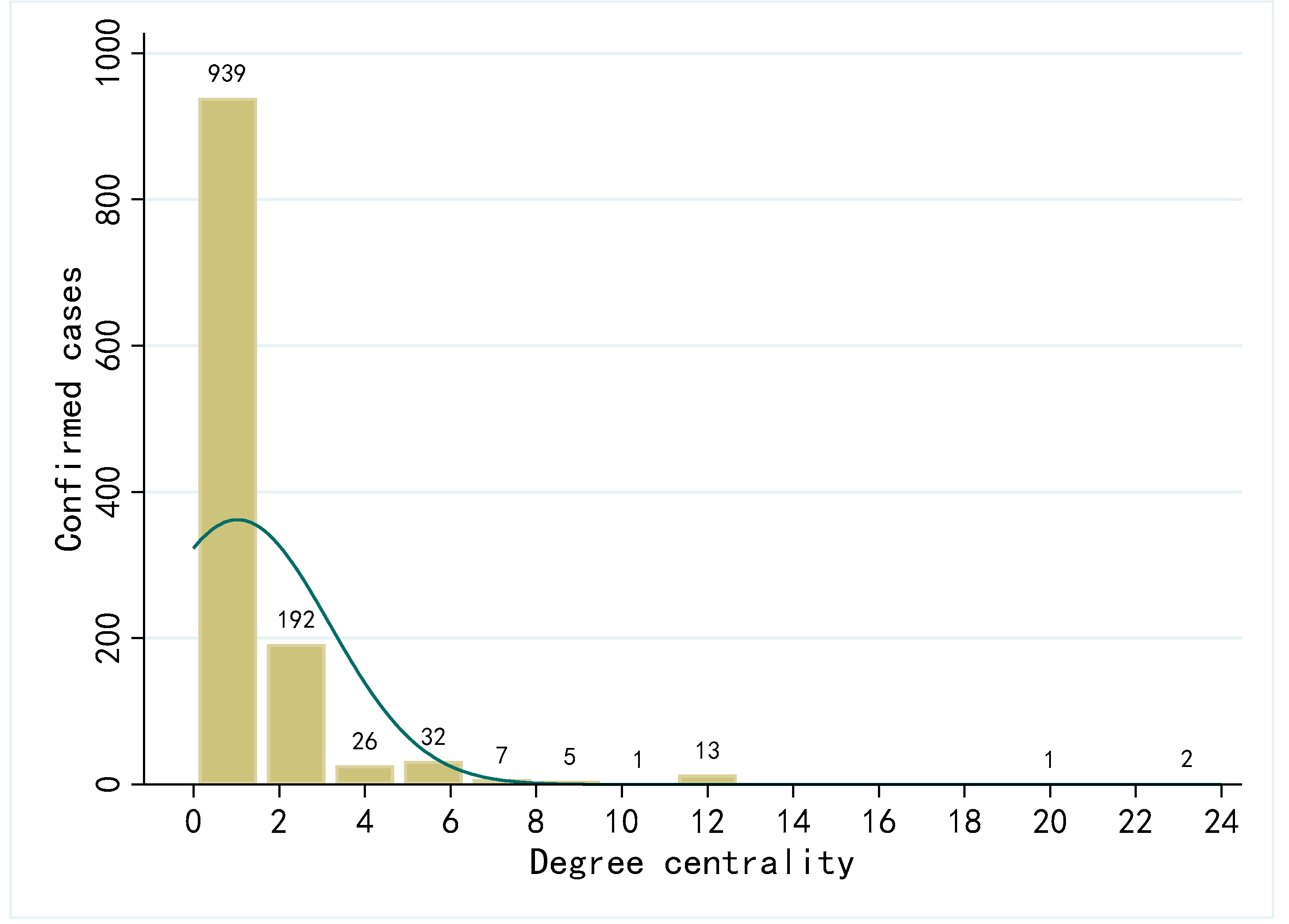
4.2.2. Contact Networks Centrality Analysis
4.2.3. Simulation of Quarantine Policy in Contact Networks
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of COVID-19 Vaccines against the B.1.617.2 (Delta) variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Pulliam, J.R.; Van Schalkwyk, C.; Govender, N.; Von Gottberg, A.; Cohen, C.; Groome, M.J.; Dushoff, J.; Mlisana, K.; Moultrie, H. Increased risk of SARS-CoV-2 reinfection associated with emergence of the Omicron variant in South Africa. MedRxiv 2021. [Google Scholar] [CrossRef]
- Planas, D.; Veyer, D.; Baidaliuk, A.; Staropoli, I.; Guivel-Benhassine, F.; Rajah, M.M.; Planchais, C.; Porrot, F.; Robillard, N.; Puech, J.; et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 2021, 596, 276. [Google Scholar] [CrossRef]
- Muik, A.; Wallisch, A.-K.; Saenger, B.; Swanson, K.A.; Muehl, J.; Chen, W.; Cai, H.; Maurus, D.; Sarkar, R.; Tuereci, O.; et al. Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine-elicited human sera. Science 2021, 371, 1152. [Google Scholar] [CrossRef] [PubMed]
- Collier, D.A.; De Marco, A.; Ferreira, I.A.T.M.; Meng, B.; Datir, R.P.; Walls, A.C.; Kemp, S.A.; Bassi, J.; Pinto, D.; Silacci-Fregni, C.; et al. Sensitivity of SARS-CoV-2 B.1.1.7 to mRNA vaccine-elicited antibodies. Nature 2021, 593, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Eubank, S.; Guclu, H.; Kumar, V.S.A.; Marathe, M.V.; Srinivasan, A.; Toroczkai, Z.; Wang, N. Modelling disease outbreaks in realistic urban social networks. Nature 2004, 429, 180–184. [Google Scholar] [CrossRef]
- Yang, Z. Analysis of dynamic contact network of patients with COVID-19 in Shaanxi Province of China. Sci. Rep. 2021, 11, 4889. [Google Scholar] [CrossRef]
- Moon, S.A.; Scoglio, C.M. Contact tracing evaluation for COVID-19 transmission in the different movement levels of a rural college town in the USA. Sci. Rep. 2021, 11, 4891. [Google Scholar] [CrossRef] [PubMed]
- Selinger, C.; Choisy, M.; Alizon, S. Predicting COVID-19 incidence in French hospitals using human contact network analytics. Int. J. Infect. Dis. 2021, 111, 100–107. [Google Scholar] [CrossRef]
- Luo, T.; Cao, Z.; Wang, Y.; Zeng, D.; Zhang, Q. Role of asymptomatic COVID-19 cases in viral transmission: Findings from a hierarchical community contact network model. IEEE Trans. Autom. Sci. Eng. 2021, 9, 1–10. [Google Scholar] [CrossRef]
- Demongeot, J.; Griette, Q.; Magal, P. SI epidemic model applied to COVID-19 data in mainland China. R. Soc. Open Sci. 2020, 7, 201878. [Google Scholar] [CrossRef]
- Matouk, A. Complex dynamics in susceptible-infected models for COVID-19 with multi-drug resistance. Chaos Solitons Fractals 2020, 140, 110257. [Google Scholar] [CrossRef] [PubMed]
- Malavika, B.; Marimuthu, S.; Joy, M.; Nadaraj, A.; Asirvatham, E.S.; Jeyaseelan, L. Forecasting COVID-19 epidemic in India and high incidence states using SIR and logistic growth models. Clin. Epidemiol. Glob. Health 2021, 9, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, J.; Xiao, C.; Yang, P. Numerical solution and parameter estimation for uncertain SIR model with application to COVID-19. Fuzzy Optim. Decis. Mak. 2021, 20, 189–208. [Google Scholar] [CrossRef]
- Anderson, R.M.; May, R.M. Infectious Diseases of Humans: Dynamics and Control; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Shi, Y.; Huang, R.; Cui, H. Prediction and analysis of tourist management strategy based on the SEIR model during the COVID-19 period. Int. J. Environ. Res. Public Health 2021, 18, 10548. [Google Scholar] [CrossRef]
- López, L.; Rodó, X. A modified SEIR model to predict the COVID-19 outbreak in Spain and Italy: Simulating control scenarios and multi-scale epidemics. Results Phys. 2020, 21, 103746. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Zhu, J.; Sun, Y.; Zhou, K.; Gu, J. The inflection point about COVID-19 may have passed. Sci. Bull. 2020, 65, 865–867. [Google Scholar] [CrossRef]
- Hafner, C.M. The spread of the COVID-19 pandemic in time and space. Int. J. Environ. Res. Public Health 2020, 17, 3827. [Google Scholar] [CrossRef]
- Grefenstette, J.J.; Brown, S.T.; Rosenfeld, R.; DePasse, J.; Stone, N.T.B.; Cooley, P.C.; Wheaton, W.D.; Fyshe, A.; Galloway, D.D.; Sriram, A.; et al. FRED (A Framework for Reconstructing Epidemic Dynamics): An open-source software system for modeling infectious diseases and control strategies using census-based populations. BMC Public Health 2013, 13, 940. [Google Scholar] [CrossRef] [Green Version]
- Newman, M.E.J. Spread of epidemic disease on networks. Phys. Rev. E 2002, 66, 016128. [Google Scholar] [CrossRef] [Green Version]
- Bearman, P.S.; Moody, J.; Stovel, K. Chains of affection: The structure of adolescent romantic and sexual networks. Am. J. Sociol. 2004, 110, 44–91. [Google Scholar] [CrossRef] [Green Version]
- Marvel, S.A.; Martin, T.; Doering, C.R.; Lusseau, D.; Newman, M.E. The small-world effect is a modern phenomenon. arXiv 2013, arXiv:1310.2636. [Google Scholar]
- Firth, J.A.; Hellewell, J.; Klepac, P.; Kissler, S.; Kucharski, A.J.; Spurgin, L.G.; Jit, M.; Atkins, K.E.; Clifford, S.; Villabona-Arenas, C.J.; et al. Using a real-world network to model localized COVID-19 control strategies. Nat. Med. 2020, 26, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; An, Q.; He, Z.; Fang, W. A literature review of social network analysis in epidemic prevention and control. Complexity 2021, 2021, 3816221. [Google Scholar] [CrossRef]
- Brodeur, A.; Gray, D.; Islam, A.; Bhuiyan, S. A literature review of the economics of COVID-19. J. Econ. Surv. 2021, 35, 1007–1044. [Google Scholar] [CrossRef]
- Aristovnik, A.; Ravšelj, D.; Umek, L. A bibliometric analysis of COVID-19 across science and social science research landscape. Sustainability 2020, 12, 9132. [Google Scholar] [CrossRef]
- Bian, Y.; Miao, X.; Lu, X.; Ma, X.; Guo, X. The emergence of a COVID-19 related social capital: The case of China. Int. J. Sociol. 2020, 50, 419–433. [Google Scholar] [CrossRef]
- Xiao, Y.; Bian, Y.; Zhang, L. Mental health of Chinese online networkers under COVID-19: A sociological analysis of survey data. Int. J. Environ. Res. Public Health 2020, 17, 8843. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Z.F. Impact of the COVID-19 pandemic on mental health and quality of life among local residents in Liaoning Province, China: A cross-sectional study. Int. J. Environ. Res. Public Health 2020, 17, 2381. [Google Scholar] [CrossRef] [Green Version]
- Klinenberg, E. Denaturalizing disaster: A social autopsy of the 1995 Chicago heat wave. Theory Soc. 1999, 28, 239–295. [Google Scholar] [CrossRef]
- Yavorsky, J.E.; Qian, Y.; Sargent, A.C. The gendered pandemic: The implications of COVID-19 for work and family. Sociol. Compass 2021, 15, e12881. [Google Scholar] [CrossRef]
- Wu, X.; Li, X.; Lu, Y.; Hout, M. Two tales of one city: Unequal vulnerability and resilience to COVID-19 by socioeconomic status in Wuhan, China. Res. Soc. Strat. Mobil. 2021, 72, 100584. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Zhang, Y.; Li, C.; Li, X. How online social ties influence the epidemic spreading of a multiplex network? In Proceedings of the 2020 IFIP Networking Conference (Networking), Paris, France, 22–26 June 2020; IEEE: Piscataway Township, NJ, USA, 2020.
- Keeling, M.J.; Woolhouse, M.E.J.; May, R.M.; Davies, G.; Grenfell, B.T. Modelling vaccination strategies against foot-and-mouth disease. Nature 2002, 421, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, E.C.; De Arroyabe, J.C.F.; Arranz, N. Preventing internal COVID-19 outbreaks within businesses and institutions: A methodology based on social networks analysis for supporting occupational health and safety services decision making. Sustainability 2020, 12, 4655. [Google Scholar] [CrossRef]
- Yie, K.-Y.; Chien, T.-W.; Yeh, Y.-T.; Chou, W.; Su, S.-B. Using social network analysis to identify spatiotemporal spread patterns of COVID-19 around the world: Online dashboard development. Int. J. Environ. Res. Public Health 2021, 18, 2461. [Google Scholar] [CrossRef]
- Jaffe, H.W. The early days of the HIV-AIDS epidemic in the USA. Nat. Immunol. 2008, 9, 1201–1203. [Google Scholar] [CrossRef] [PubMed]
- Meyers, L.A.; Pourbohloul, B.; Newman, M.; Skowronski, D.M.; Brunham, R.C. Network theory and SARS: Predicting outbreak diversity. J. Theor. Biol. 2005, 232, 71–81. [Google Scholar] [CrossRef]
- Leung, A.; Ding, X.; Huang, S.; Rabbany, R. Contact graph epidemic modelling of COVID-19 for transmission and intervention strategies. arXiv 2020, arXiv:2010.03081. [Google Scholar]
- Liu, F.; Li, X.; Zhu, G. Using the contact network model and Metropolis-Hastings sampling to reconstruct the COVID-19 spread on the “Diamond Princess”. Sci. Bull. 2020, 65, 1297–1305. [Google Scholar] [CrossRef]
- Watts, D.J.; Strogatz, S.H. Collective dynamics of ‘small-world’ networks. Nature 1998, 393, 440–442. [Google Scholar] [CrossRef]
- Kuperman, M.; Abramson, G.; Newman, M.; Barabási, A.-L.; Watts, D.J. Small world effect in an epidemiological model. In The Structure and Dynamics of Networks; Princeton University Press: Princeton, NJ, USA, 2011; pp. 489–492. [Google Scholar]
- Gosak, M.; Duh, M.; Markovič, R.; Perc, M. Community lockdowns in social networks hardly mitigate epidemic spreading. New J. Phys. 2021, 23, 043039. [Google Scholar] [CrossRef]
- Xia, L.; Jiang, G.; Song, Y.; Song, B. An improved local immunization strategy for scale-free networks with a high degree of clustering. Eur. Phys. J. B 2017, 90, 2. [Google Scholar] [CrossRef]
- Chatterjee, A.; Ramadurai, G.; Jagannathan, K. Contagion processes on urban bus networks in Indian cities. Complexity 2016, 21, 451–458. [Google Scholar] [CrossRef] [Green Version]
- Azad, S.; Devi, S. Tracking the spread of COVID-19 in India via social networks in the early phase of the pandemic. J. Travel Med. 2020, 27, 130. [Google Scholar] [CrossRef]
- Jo, W.; Chang, D.; You, M.; Ghim, G.-H. A social network analysis of the spread of COVID-19 in South Korea and policy implications. Sci. Rep. 2021, 11, 8581. [Google Scholar] [CrossRef]
- Du, Z.; Xu, X.; Wang, L.; Fox, S.J.; Cowling, B.J.; Galvani, A.P.; Meyers, L.A. Effects of proactive social distancing on COVID-19 outbreaks in 58 cities, China. Emerg. Infect. Dis. 2020, 26, 2267–2269. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, Y.; Jin, X.; Lu, Y.; Yu, X.; Li, L.; Zhang, Y. Preliminary assessment of Chinese strategy in controlling reemergent local outbreak of COVID-19. Front. Public Health 2021, 9, 650672. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.; Yu, Y.; Xin, M.; She, R.; Luo, S.; Li, L.; Wang, S.; Ma, L.; Tao, F.; Zhang, J.; et al. Adoption of preventive measures during the very early phase of the COVID-19 outbreak in China: National cross-sectional survey study. JMIR Public Health Surveill. 2021, 7, e26840. [Google Scholar] [CrossRef]
- Kraemer, M.U.G.; Yang, C.-H.; Gutierrez, B.; Wu, C.-H.; Klein, B.; Pigott, D.M.; Open COVID-19 Data Working Group; du Plessis, L.; Faria, N.R.; Li, R.; et al. The effect of human mobility and control measures on the COVID-19 epidemic in China. Science 2020, 368, 493–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granovetter, M.S. The Strength of weak ties. Am. J. Sociol. 1973, 78, 1360–1380. [Google Scholar] [CrossRef] [Green Version]
- Li, P.P.; Zhou, S.S.; Zhou, A.J.; Yang, Z. Reconceptualizing and redirecting research on Guanxi: ‘Guan-Xi’ interaction to form a multicolored Chinese Knot. Manag. Organ. Rev. 2019, 15, 643–677. [Google Scholar] [CrossRef] [Green Version]
- Gang, Y.; Tao, Z.; Jie, W.; Zhong-Qian, F.; Bing-Hong, W. Epidemic spread in weighted scale-free networks. Chin. Phys. Lett. 2005, 22, 510–513. [Google Scholar] [CrossRef]
- Mogi, R.; Spijker, J. The influence of social and economic ties to the spread of COVID-19 in Europe. J. Popul. Res. 2021, 4, 1–17. [Google Scholar] [CrossRef]
- Köhler, B. Weak ties, modern epidemics, and world society. Connections 2004, 26, 9–10. [Google Scholar]
- Jia, J.S.; Lu, X.; Yuan, Y.; Xu, G.; Jia, J.; Christakis, N.A. Population flow drives spatio-temporal distribution of COVID-19 in China. Nature 2020, 582, 389–394. [Google Scholar] [CrossRef]
- Xu, G.; Wang, W.; Lu, D.; Lu, B.; Qin, K.; Jiao, L. Geographically varying relationships between population flows from Wuhan and COVID-19 cases in Chinese cities. Geo-Spat. Inf. Sci. 2021, 9, 1–11. [Google Scholar] [CrossRef]
- Mu, X.; Yeh, A.G.-O.; Zhang, X. The interplay of spatial spread of COVID-19 and human mobility in the urban system of China during the Chinese New Year. Environ. Plan. B Urban Anal. City Sci. 2021, 48, 1955–1971. [Google Scholar] [CrossRef]
- Lam, T.-S.; Wong, C.-H.; Lam, W.-H.; Lam, H.-Y.; Lam, Y.C.-K.; Leung, E.C.-M.; Chuang, S.-K. Coronavirus disease 2019 (COVID-19) outbreak during a Chinese New Year dinner in a restaurant, Hong Kong Special Administrative Region SAR (China), 2020. West. Pac. Surveill. Response 2021, 12, 32–34. [Google Scholar] [CrossRef] [PubMed]
- Barabási, A.-L.; Albert, R. Emergence of scaling in random networks. Science 1999, 286, 509–512. [Google Scholar] [CrossRef] [Green Version]
- Manzo, G. Complex social networks are missing in the dominant COVID-19 epidemic models. Sociologica 2020, 14, 31–49. [Google Scholar]
- Contact Network of COVID-19 in China (2020 Jan & Feb). Available online: https://www.youtube.com/watch?v=Cle2A76i2TQ (accessed on 7 January 2022).




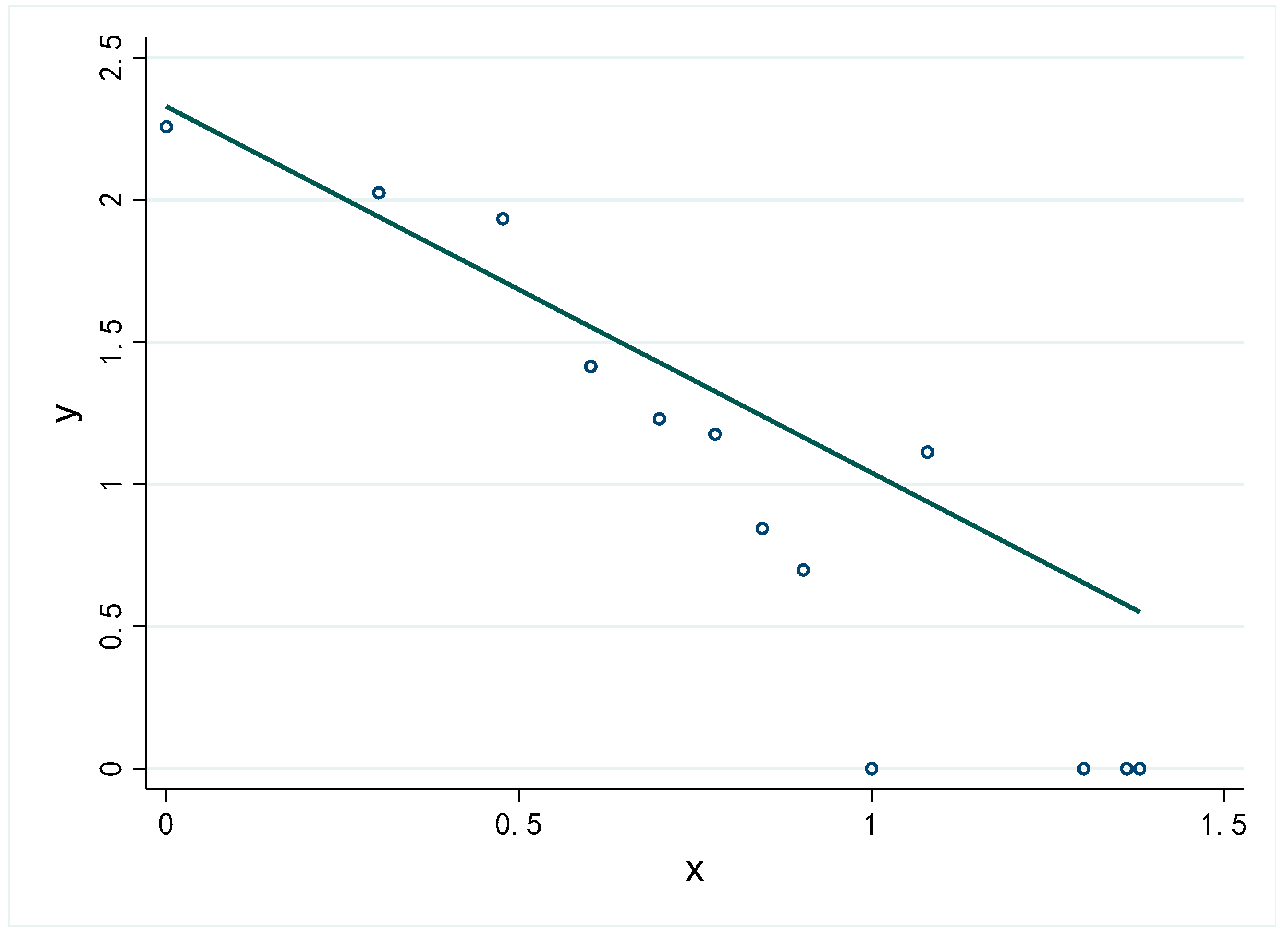
| Indicator | Definition | Equation | Meaning in Contact Network |
|---|---|---|---|
| Degree Centrality | Number of nodes in the network that are directly connected to a focal node | Number of contacts with other patients of a focal patient | |
| Closeness Centrality | Proximity of a node to all other nodes in the network | Proximity of one patient to other patients, with larger values indicating that the epidemic is spreading with fewer intermediate patients and at a faster rate | |
| Betweenness Centrality | The ability of a node to lie on a geodesic path between other pairs of nodes in the network | j ≠ k ≠ i, j < k | The ability of a patient to act as a bridge in the transmission of the virus, such as the position of B in an A-B-C transmission route |
| PageRank Scores | The centrality of a node in the whole network rather than ego network by iterative computation | The degree to which a patient is central to the whole contact network | |
| Number of component | A sub-network of a network in which there are paths between any nodes, but there is no any connections between other sub-networks | — | The more components, the sparser the contact network |
| Density | How closely the network is connected | In a low-density contact network, virus spread becomes difficulty |
| Variables | Items | Frequency | Percentage |
|---|---|---|---|
| Gender | Male | 546 | 50.32% |
| Female | 539 | 49.68% | |
| Infection source | Inside region | 642 | 57.17% |
| Outside region | 481 | 42.83% |
| Variables | Items | Frequency | Percentage |
|---|---|---|---|
| Is there a possibility of being infected by a stranger? | Yes | 684 | 64.65% |
| No | 374 | 35.35% | |
| Is there a possibility of being infected by weak ties? | Yes | 461 | 43.57% |
| No | 597 | 56.43% | |
| Is there a possibility of being infected by strong ties? | Yes | 695 | 65.69% |
| No | 363 | 34.31% | |
| Is there a family member being infected? | Yes | 418 | 39.62% |
| No | 637 | 60.38% |
| Variables | Areas | Mean | S. D. | Min. | Max. |
|---|---|---|---|---|---|
| Degree centrality | Gansu | 0.867 | 1.309 | 0 | 6 |
| Guizhou | 1.288 | 2.241 | 0 | 10 | |
| Hainan | 1.506 | 1.777 | 0 | 6 | |
| Heilongjiang | 0.340 | 0.985 | 0 | 5 | |
| Inner Mongolia | 0.722 | 0.996 | 0 | 3 | |
| Shanxi | 0.681 | 0.783 | 0 | 3 | |
| Tianjin | 2.339 | 3.517 | 0 | 24 | |
| Yunnan | 1.345 | 3.187 | 0 | 12 | |
| Closeness centrality | Gansu | 0.304 | 0.391 | 0 | 1 |
| Guizhou | 0.412 | 0.457 | 0 | 1 | |
| Hainan | 0.479 | 0.459 | 0 | 1 | |
| Heilongjiang | 0.130 | 0.331 | 0 | 1 | |
| Inner Mongolia | 0.366 | 0.452 | 0 | 1 | |
| Shanxi | 0.426 | 0.448 | 0 | 1 | |
| Tianjin | 0.470 | 0.331 | 0 | 1 | |
| Yunnan | 0.320 | 0.459 | 0 | 1 | |
| Betweenness centrality | Gansu | 0.0002 | 0.001 | 0 | 0.0077 |
| Guizhou | <0.001 | <0.001 | 0 | 0.0043 | |
| Hainan | <0.001 | <0.001 | 0 | 0.0022 | |
| Heilongjiang | <0.001 | <0.001 | 0 | 0.00002 | |
| Inner Mongolia | <0.001 | <0.001 | 0 | 0.0020 | |
| Shanxi | <0.001 | <0.001 | 0 | 0.0039 | |
| Tianjin | 0.001 | 0.005 | 0 | 0.0385 | |
| Yunnan | <0.001 | <0.001 | 0 | 0.0003 | |
| PageRank | Gansu | 0.011 | 0.012 | 0.003 | 0.072 |
| Guizhou | 0.007 | 0.007 | 0.002 | 0.064 | |
| Hainan | 0.006 | 0.004 | 0.001 | 0.019 | |
| Heilongjiang | 0.002 | 0.003 | 0.001 | 0.014 | |
| Inner Mongolia | 0.014 | 0.012 | 0.004 | 0.049 | |
| Shanxi | 0.021 | 0.017 | 0.005 | 0.053 | |
| Tianjin | 0.008 | 0.007 | 0.001 | 0.046 | |
| Yunnan | 0.006 | 0.006 | 0.002 | 0.022 |
| Indicators | Areas | Original Network | Removing Nodes That Degree ≥ 3 | Removing Nodes That Degree ≥ 2 |
|---|---|---|---|---|
| Number of component | Gansu | 60 | 73 | 66 |
| Guizhou | 97 | 106 | 102 | |
| Hainan | 96 | 98 | 86 | |
| Heilongjiang | 368 | 363 | 362 | |
| Inner Mongolia | 52 | 54 | 52 | |
| Shanxi | 32 | 33 | 34 | |
| Tianjin | 43 | 61 | 53 | |
| Yunnan | 132 | 131 | 128 | |
| Density | Gansu | 0.010 | 0.003 | <0.001 |
| Guizhou | 0.009 | 0.003 | 0.002 | |
| Hainan | 0.009 | 0.004 | 0.002 | |
| Heilongjiang | 0.001 | <0.001 | <0.001 | |
| Inner Mongolia | 0.010 | 0.007 | 0.003 | |
| Shanxi | 0.015 | 0.013 | 0.008 | |
| Tianjin | 0.019 | 0.006 | 0.003 | |
| Yunnan | 0.008 | 0.002 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Zhang, J.; Gao, S.; Wang, H. Complex Contact Network of Patients at the Beginning of an Epidemic Outbreak: An Analysis Based on 1218 COVID-19 Cases in China. Int. J. Environ. Res. Public Health 2022, 19, 689. https://doi.org/10.3390/ijerph19020689
Yang Z, Zhang J, Gao S, Wang H. Complex Contact Network of Patients at the Beginning of an Epidemic Outbreak: An Analysis Based on 1218 COVID-19 Cases in China. International Journal of Environmental Research and Public Health. 2022; 19(2):689. https://doi.org/10.3390/ijerph19020689
Chicago/Turabian StyleYang, Zhangbo, Jiahao Zhang, Shanxing Gao, and Hui Wang. 2022. "Complex Contact Network of Patients at the Beginning of an Epidemic Outbreak: An Analysis Based on 1218 COVID-19 Cases in China" International Journal of Environmental Research and Public Health 19, no. 2: 689. https://doi.org/10.3390/ijerph19020689
APA StyleYang, Z., Zhang, J., Gao, S., & Wang, H. (2022). Complex Contact Network of Patients at the Beginning of an Epidemic Outbreak: An Analysis Based on 1218 COVID-19 Cases in China. International Journal of Environmental Research and Public Health, 19(2), 689. https://doi.org/10.3390/ijerph19020689





