Adsorption and pH Values Determine the Distribution of Cadmium in Terrestrial and Marine Soils in the Nansha Area, Pearl River Delta
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Soil Sampling and Chemical Analysis
2.3. Batch Experiments
2.3.1. Isothermal Adsorption Experiment
2.3.2. Adsorption Kinetic Experiment
2.4. Analysis
3. Results and Discussion
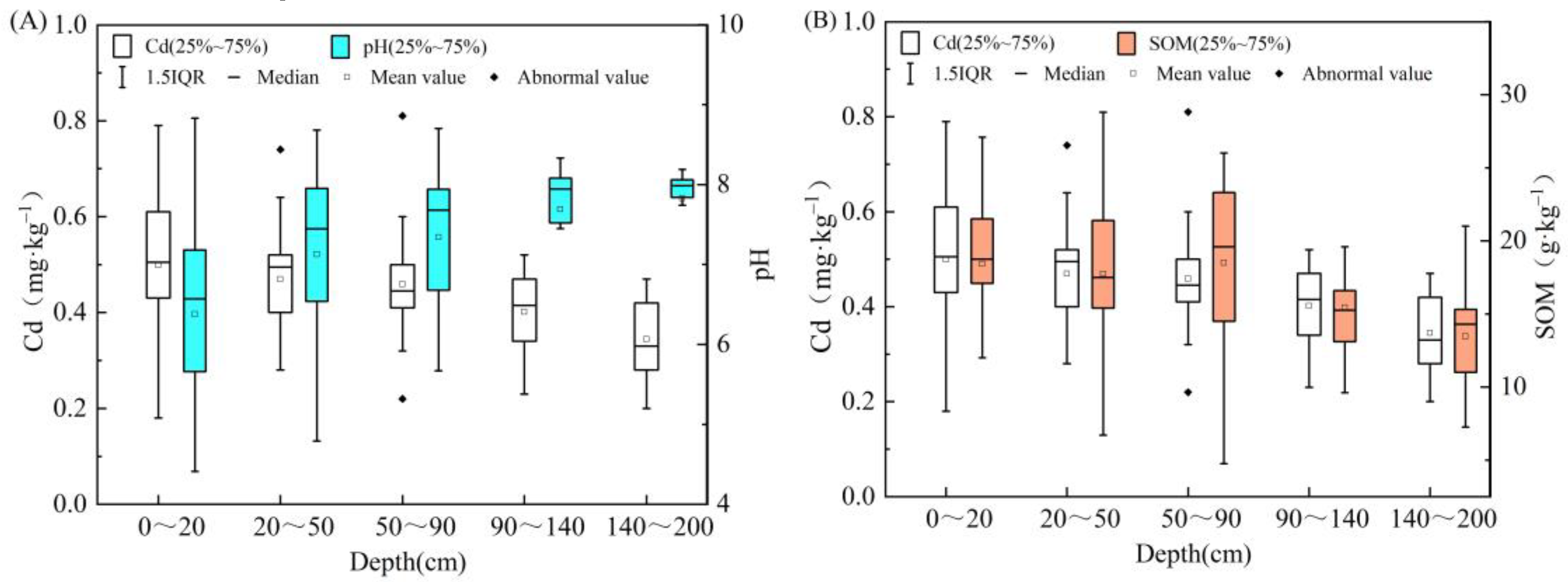
3.1. Physical and Chemical Properties of Soils in Two Sedimentary Environments
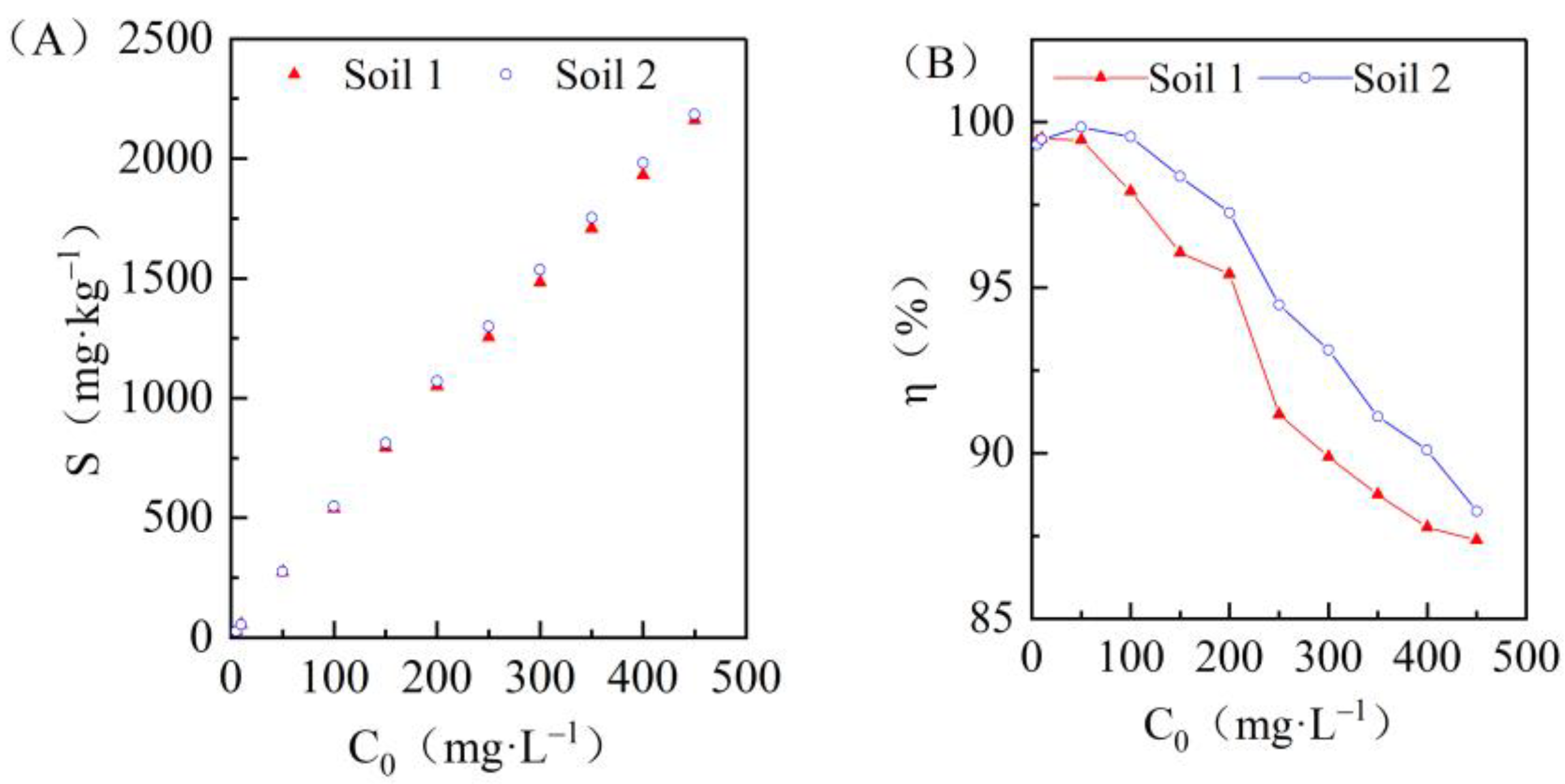
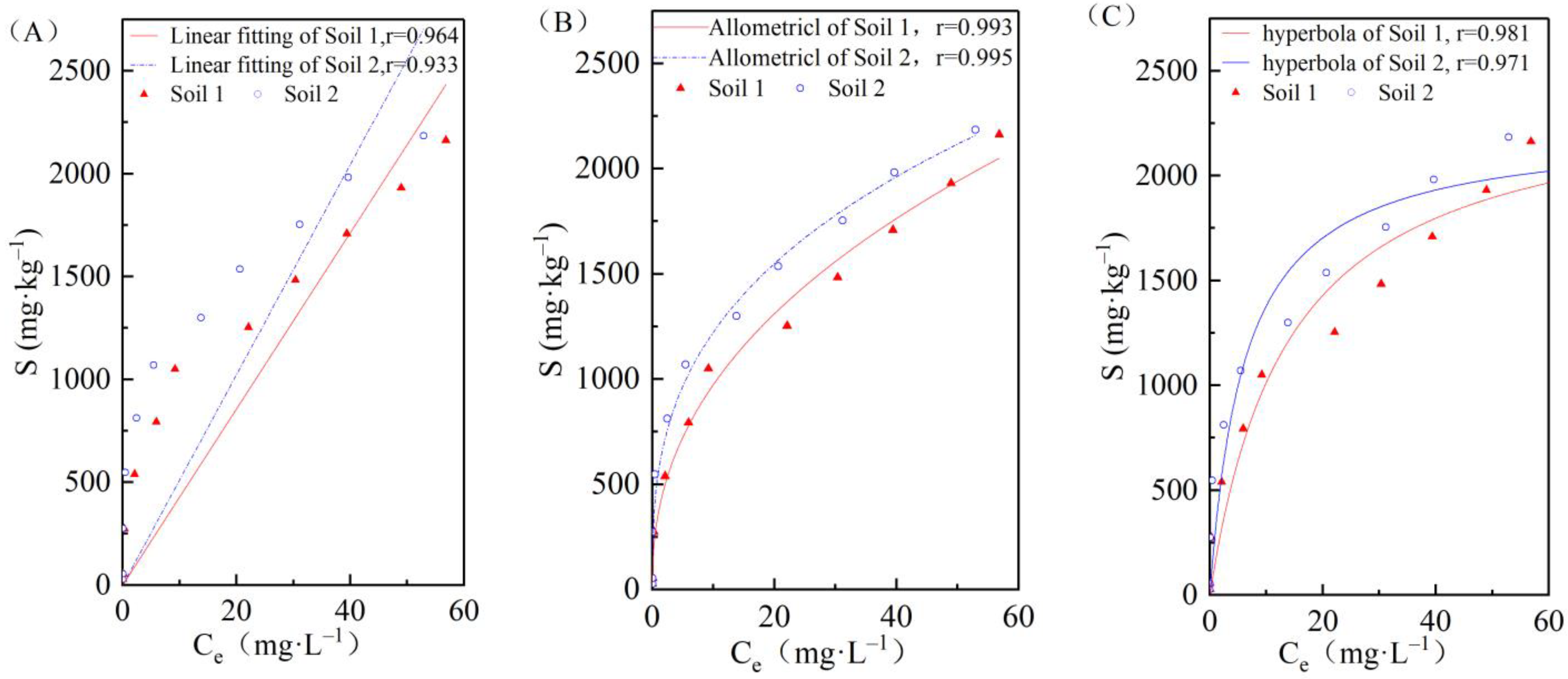
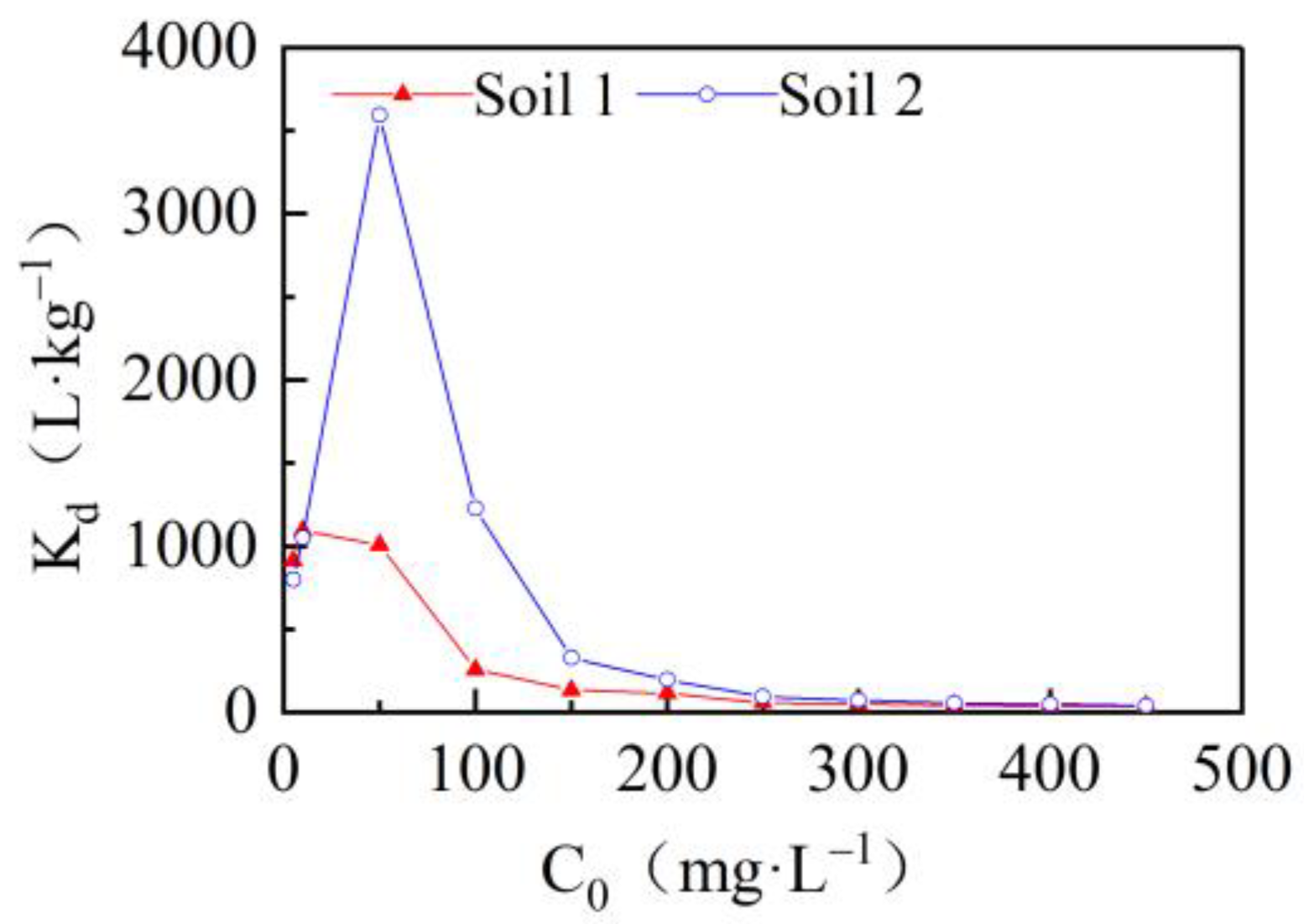
3.2. Effects of Initial Concentrations on Cadmium Adsorption in the Two Soils
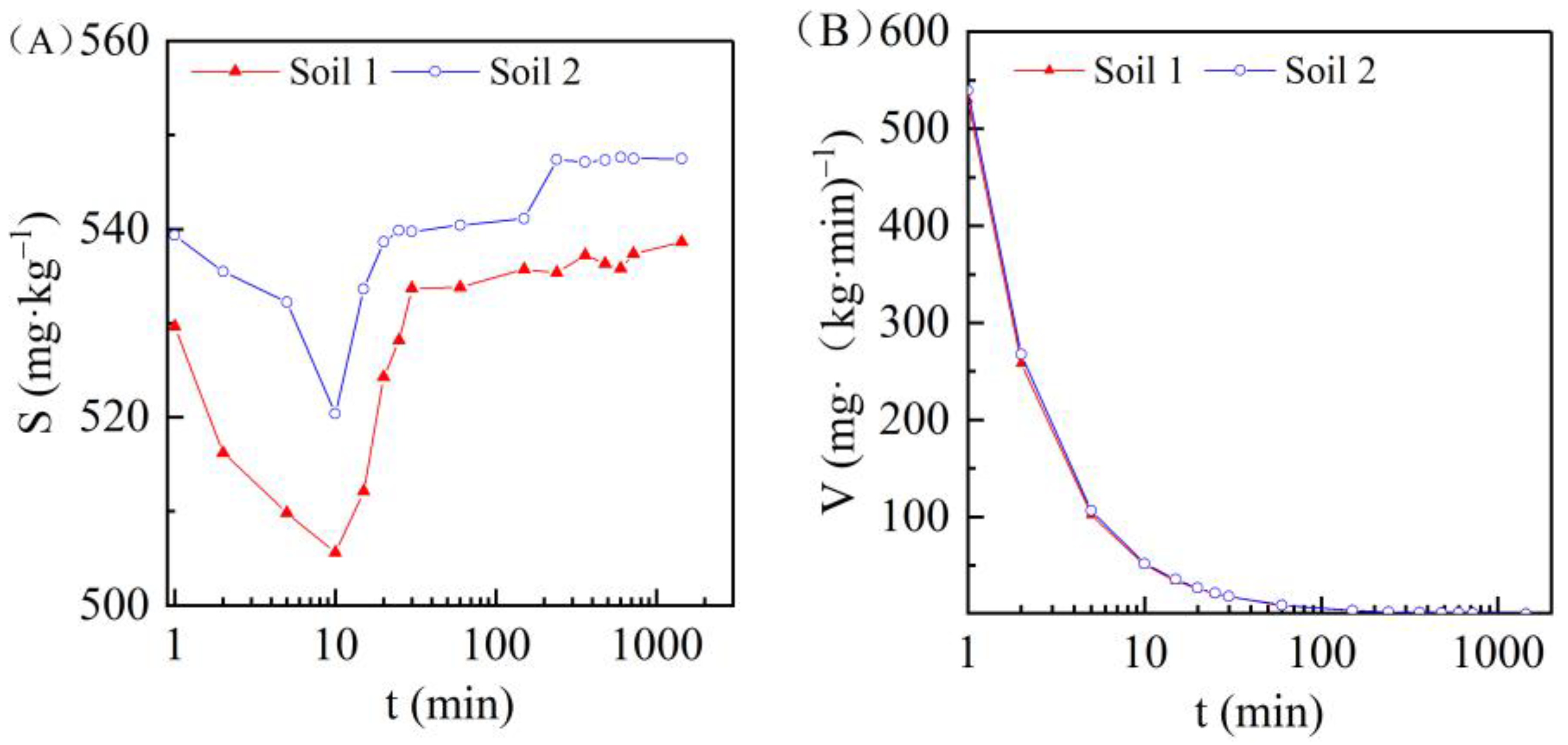
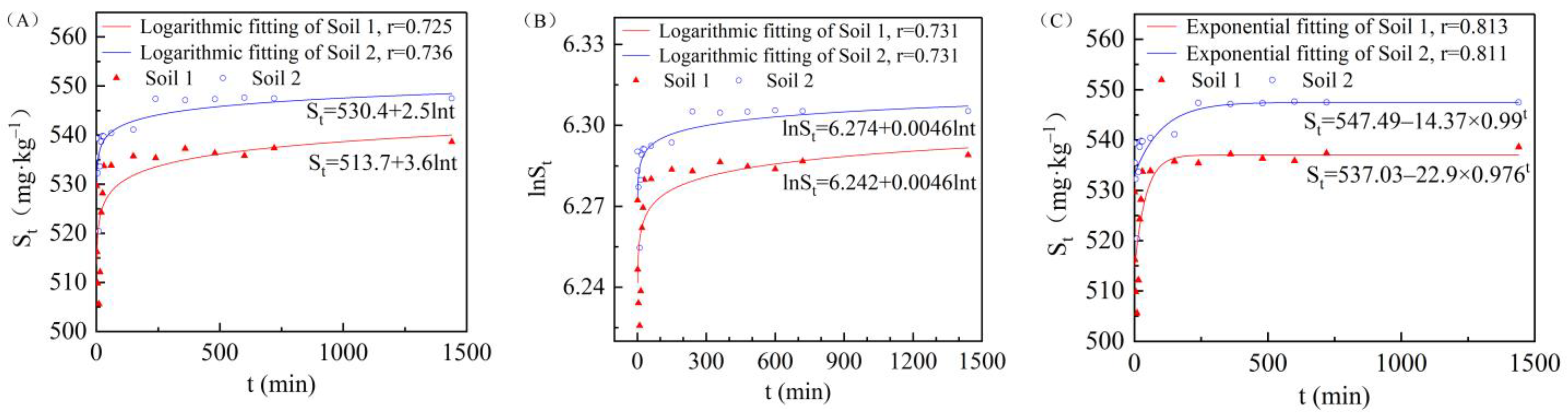
3.3. Effect of Adsorption Time on Cadmium Adsorption in the Two Soils
3.4. Effect of Solution pH Value on Cadmium Adsorption in Two Soils
3.5. Factors Affecting Cadmium Adsorption in Undisturbed Soil Columns
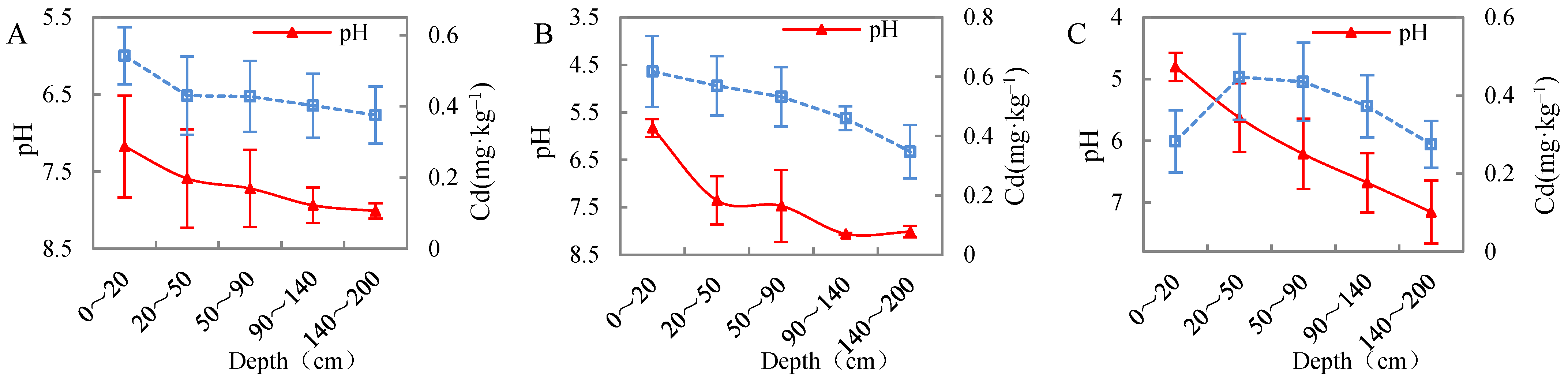
3.5.1. Effect of Soil pH on Cadmium Migration in Undisturbed Soil Columns
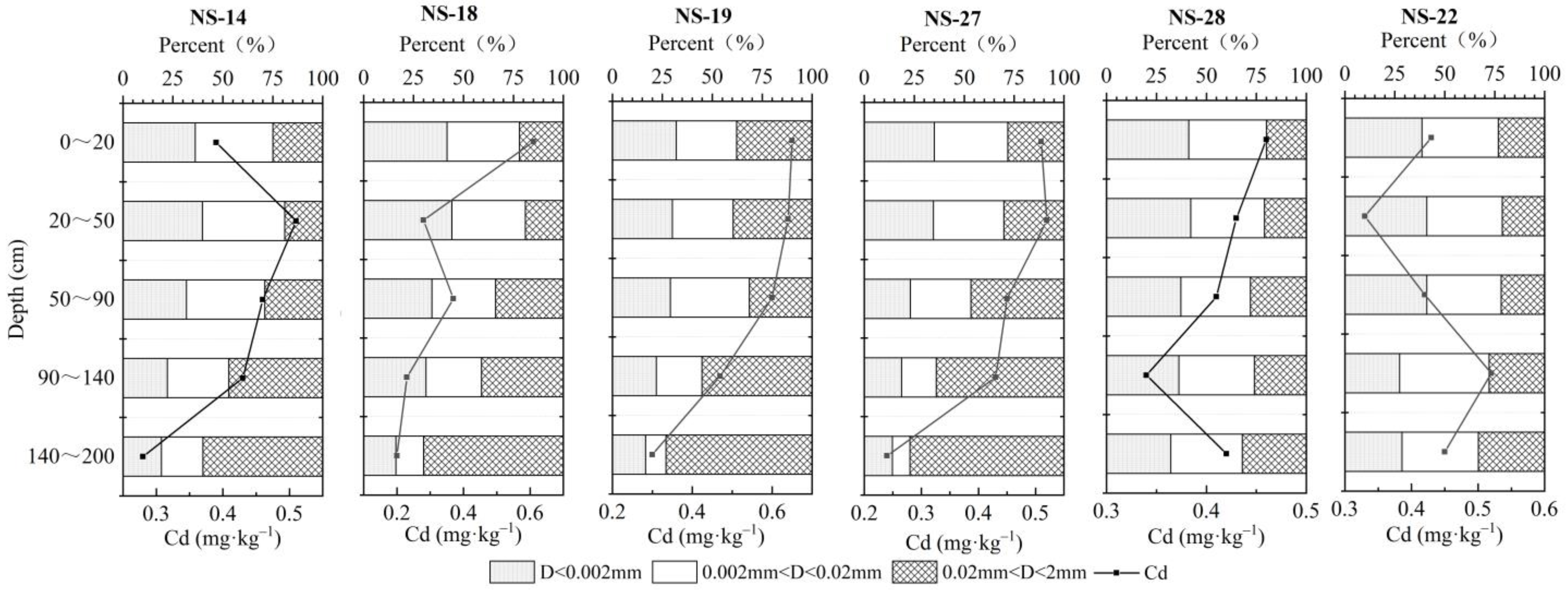
3.5.2. Soil Mechanical Composition
3.5.3. Soil Organic Matter
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Peng, C.; Wang, M.; Chen, W. Modelling cadmium contamination in paddy soils under long-term remediation measures: Model development and stochastic simulations. Environ. Pollut. 2016, 216, 146–155. [Google Scholar] [CrossRef]
- Li, Q.; Wang, C.; Dai, T.; Shi, W.; Zhang, X.; Xiao, Y.; Song, W.; Li, B.; Wang, Y. Prediction of soil cadmium distribution across a typical area of Chengdu Plain, China. Sci. Rep. 2017, 7, 7115. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Jia, Z.; Li, S. Risk assessment and sources identification of soil heavy metals in a typical county of Chongqing Municipality, Southwest China. Process. Saf. Environ. Prot. 2018, 113, 275–281. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, T.; Liu, L.; Ouyang, X. Impact of Soil Heavy Metal Pollution on Food Safety in China. PLoS ONE 2015, 10, e0135182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, Q.H.; Du, H.Y.; Fang, J.W.; Shao, L.B.; Xia, B. Source and cause of regional Cd enrichment in alluvial soils in the pearl river delta plain. J. Agro-Environ. Sci. 2005, 4, 746–750. [Google Scholar]
- Du, H.Y.; Lai, Q.H.; Lin, J.P. Multi-Objective Geochemical Atlas of the People’s Republic of China (Pearl River Delta Economic Zone, Guangdong Province); Geological Publishing House: Beijing, China, 2011. [Google Scholar]
- Zhang, H.; Reynolds, M. Cadmium exposure in living organisms: A short review. Sci. Total Environ. 2019, 678, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Yan, H.; Dai, C.; Xu, W.; Gu, F.; Zhang, F.; Li, T.; Xian, J.; He, X.; Yu, Y.; et al. The systematic exploration of cadmium-accumulation characteristics of maize kernel in acidic soil with different pollution levels in China. Sci. Total Environ. 2020, 729, 138972. [Google Scholar] [CrossRef] [PubMed]
- Elbana, T.A.; Selim, H.M. Cadmium Transport in Alkaline and Acidic Soils: Miscible Displacement Experiments. Soil Sci. Soc. Am. J. 2010, 74, 1956–1966. [Google Scholar] [CrossRef]
- Li, P.; Lang, M.; Wang, X.-X.; Zhang, T.-L. Sorption and Desorption of Copper and Cadmium in a Contaminated Soil Affected by Soil Amendments. CLEAN Soil Air, Water 2016, 44, 1547–1556. [Google Scholar] [CrossRef]
- Ardestani, M.M.; van Gestel, C.A.M. Sorption and pH determine the long-term partitioning of cadmium in natural soils. Environ. Sci. Pollut. Res. 2016, 23, 18492–18501. [Google Scholar] [CrossRef]
- Shen, B.; Wang, X.; Zhang, Y.; Zhang, M.; Wang, K.; Xie, P.; Ji, H. The optimum pH and Eh for simultaneously minimizing bioavailable cadmium and arsenic contents in soils under the organic fertilizer application. Sci. Total Environ. 2020, 711, 135229. [Google Scholar] [CrossRef] [PubMed]
- Hamid, Y.; Tang, L.; Hussain, B.; Usman, M.; Lin, Q.; Rashid, M.S.; He, Z.; Yang, X. Organic soil additives for the remediation of cadmium contaminated soils and their impact on the soil-plant system: A review. Sci. Total Environ. 2020, 707, 136121. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Ye, X.; Jia, J.; Zhang, G.; Zhao, Q.; Cui, B.; Liu, X. Phosphorus sorption-desorption and effects of temperature, pH and salinity on phosphorus sorption in marsh soils from coastal wetlands with different flooding conditions. Chemosphere 2017, 188, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, L.; Wen, H.; Wu, C.; Zhang, Y. Evaluation of the Polarimetric-Radar Quantitative Precipitation Estimates of an Extremely Heavy Rainfall Event and Nine Common Rainfall Events in Guangzhou. Atmosphere 2018, 9, 330. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.; Sun, H.; Liu, H.; Wang, X.; Wang, B.; Zheng, D. Atmospheric Arsenic Deposition in the Pearl River Delta Region, South China: Influencing Factors and Speciation. Environ. Sci. Technol. 2018, 52, 2506–2516. [Google Scholar] [CrossRef]
- Rengarajan, R.; Sarma, V. Submarine groundwater discharge and nutrient addition to the coastal zone of the Godavari estuary. Mar. Chem. 2015, 172, 57–69. [Google Scholar] [CrossRef]
- Liu, J.A. Evaluating Submarine Groundwater Discharge and Its Effects on Eco-Environment in Estuarine and Coastal Waters by Radium Isotopes. Ph.D. Thesis, East China Normal University, Shanghai, China, 2019. [Google Scholar]
- Li, Y.; Zhang, H.; Chen, X.; Tu, C.; Luo, Y.; Christie, P. Distribution of heavy metals in soils of the Yellow River Delta: Concentrations in different soil horizons and source identification. J. Soils Sediments 2014, 14, 1158–1168. [Google Scholar] [CrossRef]
- Bai, J.; Xiao, R.; Zhang, K.; Gao, H. Arsenic and heavy metal pollution in wetland soils from tidal freshwater and salt marshes before and after the flow-sediment regulation regime in the Yellow River Delta, China. J. Hydrol. 2012, 450-451, 244–253. [Google Scholar] [CrossRef]
- Tang, Z.M. Effect of Pearl River System on Environmental Quality of Alluvial Plain Soil. Mater’s Thesis, China University of Geosciences, Beijing, China, 2017. [Google Scholar]
- Zhu, H.; Chen, C.; Xu, C.; Zhu, Q.; Huang, D. Effects of soil acidification and liming on the phytoavailability of cadmium in paddy soils of central subtropical China. Environ. Pollut. 2016, 219, 99–106. [Google Scholar] [CrossRef]
- Zhou, T.; Wu, L.; Luo, Y.; Christie, P. Effects of organic matter fraction and compositional changes on distribution of cadmium and zinc in long-term polluted paddy soils. Environ. Pollut. 2018, 232, 514–522. [Google Scholar] [CrossRef]
- Zeng, H.; Chen, L.; Zhou, X.; Zeng, Q. Cadmium accumulation in winter crops and the assessment of paddy soil phytoremediation in southern China. Environ. Sci. Pollut. Res. 2019, 26, 17173–17182. [Google Scholar] [CrossRef]
- Diagboya, P.N.; Olu-Owolabi, B.I.; Adebowale, K.O. Effects of time, soil organic matter, and iron oxides on the relative retention and redistribution of lead, cadmium, and copper on soils. Environ. Sci. Pollut. Res. 2015, 22, 10331–10339. [Google Scholar] [CrossRef] [Green Version]
- Furuya, M.; Hashimoto, Y.; Yamaguchi, N. Time-Course Changes in Speciation and Solubility of Cadmium in Reduced and Oxidized Paddy Soils. Soil Sci. Soc. Am. J. 2016, 80, 870–877. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Huang, C.; Chen, Z.; Bao, K. Distribution, Ecological Risk Assessment, and Bioavailability of Cadmium in Soil from Nansha, Pearl River Delta, China. Int. J. Environ. Res. Public Health 2019, 16, 3637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Q. Climatic Indicators and Sedimentary Environment Studies Inferred from Ransgressive and Egressive Sediments of Core LZ908, South Bohai Sea. Ph.D. Thesis, The Institute of Ceanology, Chinese Academy of Sciences, Qingdao, China, 2014. [Google Scholar]
- People’s Government of Nansha District in Guangzhou. Climate of Nansha; People’s Government of Nansha District in Guangzhou: Guangzhou, China, 2021.
- DZ/T0279.1-2016; Analysis Methods for Regional Geochemical Sample-Part 1: Determination of 24 Components Including Aluminum Oxide etc. by Pressed Power Pellets-X-ray Fluorescence Spectrometry. Geological and Mineral Industry Standards of the People’s Republic of China. Ministry of Land and Resources: Beijing, China, 2016.
- NY/T1121.6-2006; Soil Testing Part 6: Method for Determination of Soil Organic Matter. Agricultural Industry Standard of the People’s Republic of China. Ministry of Agriculture of the PRC: Beijing, China, 2006.
- GB/T14506.30-2010; Methods for Chemical Analysis of Silicate Rocks—Part 30: Determination of 44 Elements. Agricultural Industry Standard of the People’s Republic of China. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2010.
- NY/T1121.3-2006; Soil Testing Part 3: Method for Determination of Soil Mechanical Composition. Agricultural Industry Standard of the People’s Republic of China. Ministry of Agriculture of the PRC: Beijing, China, 2006.
- Duan, Y.; Wang, B.G.; Wang, H.M.; Jiao, T.L.; Qin, Y.X.; Ci, R.Z.M. Adsorption characteristics of Cd in alluvial and lacustrine soils—A case study in Dangtu county, Anhui. Earth Sci. 2021, 46, 1490–1504. [Google Scholar]
- DB44/T1415-2014; Risk Screening Values for Soil Heavy Metal The Pearl River Delta Area. Local Standard of Guangdong, China. Bureau of Quality and Technical Supervision of Guangdong, China: Guangzhou, China, 2014.
- Dou, L.; Du, H.Y.; Huang, Y.H.; Lai, Q.H.; You, Y.H. Main research achievements of agro-geological and eco-geochemical research in pearl river delta economic zone, Guangdong province, China. Geol. Surv. China 2015, 2, 47–55. [Google Scholar]
- Houng, K.-H.; Lee, D.-Y. Comparisons of Linear and Nonlinear Langmuir and Freundlich Curve-Fit in the study of Cu, Cd and Pb adsorption On Taiwan Soils. Soil Sci. 1998, 163, 115–121. [Google Scholar] [CrossRef]
- Arabi, S.; Sohrabi, M.R. Removal of methylene blue, a basic dye, from aqueous solutions using nano-zerovalent iron. Water Sci. Technol. 2014, 70, 24–31. [Google Scholar] [CrossRef]
- Barrow, J. Modelling the effects of pH on phosphate sorption by soils. Eur. J. Soil Sci. 1984, 35, 283–297. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, R.D.; Zhang, J.Y.; Pan, R. Effects of physical and chemical characteristics of soils on the adsorption of Cd. J. Irrig. Drain. 2012, 31, 19–22. [Google Scholar]
- Sauvé, S.; Manna, S.; Turmel, M.-C.; Roy, A.G.; Courchesne, F. Solid−Solution Partitioning of Cd, Cu, Ni, Pb, and Zn in the Organic Horizons of a Forest Soil. Environ. Sci. Technol. 2003, 37, 5191–5196. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Dang, X.L.; Zhang, Y.L. Effect of the freezing-thawing on kinetics of adsorption-desorption of the soil cadmium. Acta Sci. Circumst. 2012, 32, 721–725. [Google Scholar]
- Davis, J.; O Leckie, J. Surface ionization and complexation at the oxide/water interface II. Surface properties of amorphous iron oxyhydroxide and adsorption of metal ions. J. Colloid Interface Sci. 1978, 67, 90–107. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Igarashi, T.; Villacorte-Tabelin, M.; Park, I.; Opiso, E.M.; Ito, M.; Hiroyoshi, N. Arsenic, selenium, boron, lead, cadmium, copper, and zinc in naturally contaminated rocks: A review of their sources, modes of enrichment, mechanisms of release, and mitigation strategies. Sci. Total Environ. 2018, 645, 1522–1553. [Google Scholar] [CrossRef]
- Chen, D.; Wang, X.; Wang, X.; Feng, K.; Su, J.; Dong, J. The mechanism of cadmium sorption by sulphur-modified wheat straw biochar and its application cadmium-contaminated soil. Sci. Total Environ. 2020, 714, 136550. [Google Scholar] [CrossRef] [PubMed]
- Inyang, H.I.; Onwawoma, A.; Bae, S. The Elovich equation as a predictor of lead and cadmium sorption rates on contaminant barrier minerals. Soil Tillage Res. 2016, 155, 124–132. [Google Scholar] [CrossRef]
- Covelo, E.; Andrade, M.L.; Vega, F. Heavy metal adsorption by humic umbrisols: Selectivity sequences and competitive sorption kinetics. J. Colloid Interface Sci. 2004, 280, 1–8. [Google Scholar] [CrossRef]
- Ren, B.; Wu, Y.; Deng, D.; Tang, X.; Li, H. Effect of multiple factors on the adsorption of Cd in an alluvial soil from Xiba, China. J. Contam. Hydrol. 2020, 232, 103605. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Böttcher, J.; Utermann, J. Evaluation of field-scale variability of heavy metal sorption in soils by scale factors—Scaling approach and statistical analysis. Geoderma 2015, 241–242, 115–125. [Google Scholar] [CrossRef]
- Bashir, S.; Adeel, M.; Gulshan, A.B.; Iqbal, J.; Khan, S.; Rehman, M.; Azeem, M. Effects of Organic and Inorganic Passivators on the Immobilization of Cadmium in Contaminated Soils: A Review. Environ. Eng. Sci. 2019, 36, 986–998. [Google Scholar] [CrossRef]
- Vranova, V.; Rejsek, K.; Formanek, P. Aliphatic, Cyclic, and Aromatic Organic Acids, Vitamins, and Carbohydrates in Soil: A Review. Sci. World J. 2013, 2013, 524239. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Soil Mechanical Composition Ratio (%) | SOM | CEC | pH | Cd | |||
|---|---|---|---|---|---|---|---|
| D < 0.002 mm | 0.002 mm < D < 0.02 mm | 0.02 mm < D < 2 mm | g/kg | cmol/kg | mg/kg | ||
| Soil 1 | 35 | 36.8 | 28.2 | 18.0 | 15.2 | 6.40 | 0.52 |
| Soil 2 | 18.6 | 17.4 | 64 | 15.8 | 10.5 | 8.08 | 0.48 |
| Adsorption Model | Parameter | Unit | Soil 1 | Soil 2 |
|---|---|---|---|---|
| Henry equation | Kd | L/kg | 42.81 | 51.11 |
| Freundlich equation | KF | - | 363.04 | 558.21 |
| 1/n | - | 0.43 | 0.34 | |
| Langmiur equation | KL | - | 0.072 | 0.162 |
| Sm | mg/kg | 2419.83 | 2228.25 |
| Soil | pH | Expression | COD (R2) | Sm (mg/kg) |
|---|---|---|---|---|
| Soil 1 | pH = 3.5 | S = 536.27 − 12.13 × 0.968 t | 0.899 | 536.27 |
| pH = 4.5 | S = 538.85 − 11.11 × 0.959 t | 0.948 | 538.85 | |
| pH = 5.5 | S = 537.18 − 9.69 × 0.852 t | 0.864 | 537.18 | |
| pH = 9.5 | S = 540.70 − 5.64 × 0.977 t | 0.906 | 540.70 | |
| Soil 2 | pH = 3.5 | S = 545.32 − 6.84 × 0.933 t | 0.883 | 545.32 |
| pH = 4.5 | S = 546.27 − 5.83 × 0.933 t | 0.875 | 546.27 | |
| pH = 5.5 | S = 546.14 − 5.24 × 0.994 t | 0.801 | 546.14 | |
| pH = 9.5 | S = 547.48 − 4.22 × 0.994 t | 0.915 | 547.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Bao, K.; Huang, C.; Zhao, X.; Han, W.; Yin, Z. Adsorption and pH Values Determine the Distribution of Cadmium in Terrestrial and Marine Soils in the Nansha Area, Pearl River Delta. Int. J. Environ. Res. Public Health 2022, 19, 793. https://doi.org/10.3390/ijerph19020793
Wang F, Bao K, Huang C, Zhao X, Han W, Yin Z. Adsorption and pH Values Determine the Distribution of Cadmium in Terrestrial and Marine Soils in the Nansha Area, Pearl River Delta. International Journal of Environmental Research and Public Health. 2022; 19(2):793. https://doi.org/10.3390/ijerph19020793
Chicago/Turabian StyleWang, Fangting, Ke Bao, Changsheng Huang, Xinwen Zhao, Wenjing Han, and Zhibin Yin. 2022. "Adsorption and pH Values Determine the Distribution of Cadmium in Terrestrial and Marine Soils in the Nansha Area, Pearl River Delta" International Journal of Environmental Research and Public Health 19, no. 2: 793. https://doi.org/10.3390/ijerph19020793
APA StyleWang, F., Bao, K., Huang, C., Zhao, X., Han, W., & Yin, Z. (2022). Adsorption and pH Values Determine the Distribution of Cadmium in Terrestrial and Marine Soils in the Nansha Area, Pearl River Delta. International Journal of Environmental Research and Public Health, 19(2), 793. https://doi.org/10.3390/ijerph19020793





