Spatiotemporal Associations between Local Safety Level Index and COVID-19 Infection Risks across Capital Regions in South Korea
Abstract
:1. Introduction
2. Data and Methodology
2.1. Background
2.2. Data Description
2.2.1. Study Region
2.2.2. Distribution of COVID-19 Confirmed Cases
2.2.3. Data Adopted in This Study
2.3. Methodology
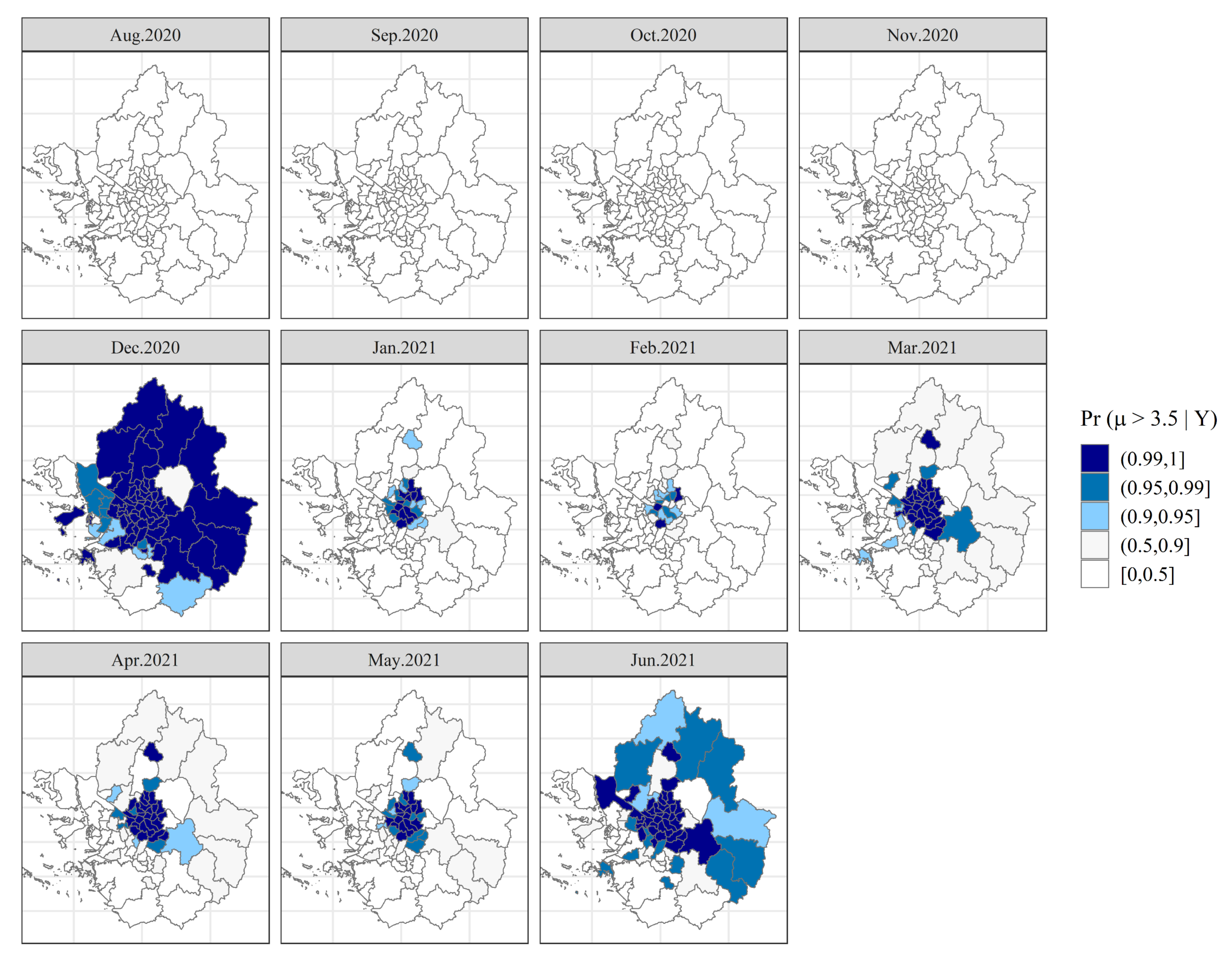
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Coronavirus Disease 19 (COVID-19) First Report: Pneumonia of Unknown Cause-China. Available online: www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/ (accessed on 31 October 2021).
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 31 October 2021).
- KCDC. Cases in Korea by City/Province. Available online: http://ncov.mohw.go.kr/en/bdBoardList.do?brdId=16&brdGubun=162&dataGubun=&ncvContSeq=&contSeq=&board_id=&gubun= (accessed on 31 October 2021).
- Kim, J.Y.; Choe, P.G.; Oh, Y.; Oh, K.J.; Kim, J.; Park, S.J.; Park, J.H.; Na, H.K.; Oh, M. The First Case of 2019 Novel Coronavirus Pneumonia Imported into Korea from Wuhan, China: Implication for Infection Prevention and Control Measures. J. Korean Med Sci. 2020, 35, e61. [Google Scholar] [CrossRef] [PubMed]
- The Pioneer. South Korea has 100th day of 1,000-plus cases. Available online: https://www.dailypioneer.com/2021/trending-news/south-korea-has-100th-day-of-1-000-plus-cases.html (accessed on 31 October 2021).
- World Health Organization. Coronavirus disease (COVID-19). Available online: https://www.who.int/health-topics/coronavirus#tab=tab_2 (accessed on 31 October 2021).
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Sannigrahi, S.; Pilla, F.; Basu, B.; Basu, A.S.; Molter, A. Examining the association between socio-demographic composition and COVID-19 fatalities in the European region using spatial regression approach. Sustain. Cities Soc. 2020, 62, 102418. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.F.; Ma, B.; Bilal; Komal, B.; Bashir, M.A.; Tan, D.; Bashir, M. Correlation between climate indicators and COVID-19 pandemic in New York, USA. Sci. Total Environ. 2020, 728, 138835. [Google Scholar] [CrossRef]
- Wu, X.; Nethery, R.C.; Sabath, B.M.; Braun, D.; Dominici, F. Exposure to air pollution and COVID-19 mortality in the United States: A nationwide cross-sectional study. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Briz-Redón, L.; Serrano-Aroca, N. A spatio-temporal analysis for exploring the effect of temperature on COVID-19 early evolution in Spain. Sci. Total Environ. 2020, 728, 138811. [Google Scholar] [CrossRef]
- Upshaw, T.L.; Brown, C.; Smith, R.; Perri, M.; Ziegler, C.; Pinto, A.D. Social determinants of COVID-19 incidence and outcomes: A rapid review. PLoS ONE 2020, 16, e0248336. [Google Scholar] [CrossRef]
- Kim, S.; Castro, M.C. Spatiotemporal pattern of COVID-19 and government response in South Korea (as of 31 May 2020). Int. J. Infect. Dis. 2020, 98, 328–333. [Google Scholar] [CrossRef]
- Shim, E.; Tariq, A.; Chowell, G. Spatial variability in reproduction number and doubling time across two waves of the COVID-19 pandemic in South Korea, February to July 2020. Int. J. Infect. Dis. 2021, 102, 1–9. [Google Scholar] [CrossRef]
- Lee, W.; Hwang, S.-S.; Song, I.; Park, C.; Kim, H.; Song, I.-K.; Choi, H.M.; Prifti, K.; Kwon, Y.; Kim, J.; et al. COVID-19 in South Korea: Epidemiological and spatiotemporal patterns of the spread and the role of aggressive diagnostic tests in the early phase. Int. J. Epidemiol. 2020, 49, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Lym, Y.; Kim, K.J. Exploring the effects of PM2.5 and temperature on COVID-19 transmission in Seoul, South Korea. Environ. Res. 2021, 203, 111810. [Google Scholar] [CrossRef] [PubMed]
- MOIS. Disaster and Safety Management> Best Practices> Local Safety Diagnosis System. Available online: https://www.mois.go.kr/eng/sub/a03/bestPractices4/screen.do (accessed on 31 October 2021).
- KCDC. Press Release. 27 August 2020. Available online: http://ncov.mohw.go.kr/en/tcmBoardView.do?brdId=12&brdGubun=125&dataGubun=&ncvContSeq=3598&contSeq=3598&board_id=&gubun=# (accessed on 31 October 2021).
- KCDC. Press Release. 25 December 2020. Available online: http://ncov.mohw.go.kr/en/tcmBoardView.do?brdId=12&brdGubun=125&dataGubun=&ncvContSeq=4506&contSeq=4506&board_id=&gubun=# (accessed on 31 October 2021).
- KOSIS. Statistics Korea. COVID-19. Available online: https://kosis.kr/covid_eng/covid_index.do (accessed on 31 October 2021).
- KCDC. Cases in Korea. Available online: http://ncov.mohw.go.kr/en/bdBoardList.do?brdId=16&brdGubun=161&dataGubun=&ncvContSeq=&contSeq=&board_id=&gubun= (accessed on 4 November 2021).
- KCDC. COVID-19 Response. Available online: http://ncov.mohw.go.kr/en/baroView.do?brdId=11&brdGubun=111&dataGubun=&ncvContSeq=&contSeq=&board_id=&gubun= (accessed on 31 October 2021).
- National Spatial Data Infrastructure Portal (NSDI) Open Market. Available online: http://www.nsdi.go.kr/lxportal/?menuno=3085 (accessed on 12 October 2021).
- KOSIS. KOrean Statistical Information Service. Statistical Database. Available online: https://kosis.kr/eng/ (accessed on 2 November 2021).
- MOIS Ministry of the Interior and Safety. Available online: https://www.mois.go.kr/frt/sub/a06/b10/safetyIndex/screen.do (accessed on 2 November 2021).
- SGIS Statistical Geographic Information Service. Available online: https://sgis.kostat.go.kr/view/index (accessed on 2 November 2021).
- Saez, M.; Tobias, A.; Barceló, M.A. Effects of long-term exposure to air pollutants on the spatial spread of COVID-19 in Catalonia, Spain. Environ. Res. 2020, 191, 110177. [Google Scholar] [CrossRef] [PubMed]
- Coccia, M. The relation between length of lockdown, numbers of infected people and deaths of COVID-19, and economic growth of countries: Lessons learned to cope with future pandemics similar to COVID-19 and to constrain the deterioration of economic system. Sci. Total Environ. 2021, 775, 145801. [Google Scholar] [CrossRef]
- Raymundo, C.E.; Oliveira, M.C.; Eleuterio, T.D.A.; André, S.R.; da Silva, M.G.; Queiroz, E.R.D.S.; Medronho, R.D.A. Spatial analysis of COVID-19 incidence and the sociodemographic context in Brazil. PLoS ONE 2021, 16, e0247794. [Google Scholar] [CrossRef] [PubMed]
- Lawson, A. Bayesian Disease Mapping: Hierarchical Modeling in Spatial Epidemiology; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Congdon, P.D. Bayesian Hierarchical Models: With Applications Using R, 2nd ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2019. [Google Scholar]
- Martinez-Beneito, M.A.; Botella-Rocamora, P. Disease Mapping: From Foundations to Multidimensional Modeling, 1st ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2019. [Google Scholar]
- Di Maggio, C.; Klein, M.; Berry, C.; Frangos, S. Black/African American Communities are at highest risk of COVID-19: Spatial modeling of New York City ZIP Code-level testing results. Ann. Epidemiol. 2020, 51, 7–13. [Google Scholar] [CrossRef]
- Giuliani, D.; Dickson, M.M.; Espa, G.; Santi, F. Modelling and predicting the spatio-temporal spread of COVID-19 in Italy. BMC Infect. Dis. 2020, 20, 700. [Google Scholar] [CrossRef]
- Briz-Redón, Á. The impact of MODELLING choices on modelling outcomes: A spatio-temporal study of the association BETWEEN COVID-19 spread and environmental conditions in Catalonia (Spain). Stoch. Environ. Res. Risk Assess. 2021, 35, 1701–1713. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.P.; Ravi, N.; Braneon, C.V. Spatiotemporal Associations between Social Vulnerability, Environmental Measurements, and COVID-19 in the Conterminous United States. GeoHealth 2021, 5, e2021GH000423. [Google Scholar] [CrossRef]
- Besag, J.; York, J.; Mollié, A. Bayesian image restoration, with two applications in spatial statistics. Ann. Inst. Stat. Math. 1991, 43, 1–20. [Google Scholar] [CrossRef]
- Blangiardo, M.; Cameletti, M.; Pirani, M.; Corsetti, G.; Battaglini, M.; Baio, G. Estimating weekly excess mortality at sub-national level in Italy during the COVID-19 pandemic. PLoS ONE 2020, 15, e0240286. [Google Scholar] [CrossRef]
- D’Angelo, N.; Abbruzzo, A.; Adelfio, G. Spatio-Temporal Spread Pattern of COVID-19 in Italy. Mathematics 2021, 9, 2454. [Google Scholar] [CrossRef]
- Simpson, D.; Rue, H.; Riebler, A.; Martins, T.G.; Sørbye, S.H. Penalising Model Component Complexity: A Principled, Practical Approach to Constructing Priors. Stat. Sci. 2017, 32, 1–28. [Google Scholar] [CrossRef]
- Fuglstad, G.-A.; Simpson, D.; Lindgren, F.; Rue, H. Constructing Priors that Penalize the Complexity of Gaussian Random Fields. J. Am. Stat. Assoc. 2019, 114, 445–452. [Google Scholar] [CrossRef] [Green Version]
- Riebler, A.; Sørbye, S.H.; Simpson, D.; Rue, H. An intuitive Bayesian spatial model for disease mapping that accounts for scaling. Stat. Methods Med. Res. 2016, 25, 1145–1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moraga, P. Geospatial Health Data: Modeling and Visualization with R-INLA and Shiny, 1st ed.; Chapman & Hall/CRC Biostatistics Series; Chapman and Hall/CRC: Boca Raton, FL, USA, 2019. [Google Scholar]
- Blangiardo, M.; Cameletti, M. Spatial and Spatio-Temporal Bayesian Models with R—INLA; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Lym, Y. Exploring dynamic process of regional shrinkage in Ohio: A Bayesian perspective on population shifts at small-area levels. Cities 2021, 115, 103228. [Google Scholar] [CrossRef]
- Rue, H.; Riebler, A.; Sørbye, S.H.; Illian, J.B.; Simpson, D.P.; Finn, K.L. Bayesian Computing with INLA: A Review. Annu. Rev. Stat. Appl. 2017, 4, 395–421. [Google Scholar] [CrossRef] [Green Version]
- Spiegelhalter, D.J.; Best, N.G.; Carlin, B.P.; Linde, A.V. Bayesian measures of model complexity and fit. J. R. Stat. Soc. Ser. B Methodol. 2002, 64, 583–639. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yue, R.Y.; Faraway, J.J. Bayesian Regression Modeling with INLA (Chapman & Hall/CRC Computer Science & Data Analysis), 1st ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2018. [Google Scholar]
- Guglielmi, N.; Iacomini, E.; Viguerie, A. Delay differential equations for the spatially-resolved simulation of epidemics with specific application to COVID-19. arXiv 2021, arXiv:2103.01102. [Google Scholar]
- Bontempi, E.; Coccia, M.; Vergalli, S.; Zanoletti, A. Can commercial trade represent the main indicator of the COVID-19 diffusion due to human-to-human interactions? A comparative analysis between Italy, France, and Spain. Environ. Res. 2021, 201, 111529. [Google Scholar] [CrossRef]
- Jalilian, A.; Mateu, J. A hierarchical spatio-temporal model to analyze relative risk variations of COVID-19: A focus on Spain, Italy and Germany. Stoch. Environ. Res Risk Assess. 2021, 35, 797–812. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Cabreros, C.; Mal, J.; Ballesteros, F.; Sillanpää, M.; Tripathi, V.; Bontempi, E. Novel coronavirus disease 2019 (COVID-19) pandemic: From transmission to control with an interdisciplinary vision. Environ. Res. 2021, 197, 111126. [Google Scholar] [CrossRef] [PubMed]
- Maleki, M.; Anvari, E.; Hopke, P.K.; Noorimotlagh, Z.; Mirzaee, S.A. An updated systematic review on the association between atmospheric particulate matter pollution and prevalence of SARS-CoV-2. Environ. Res. 2021, 195, 110898. [Google Scholar] [CrossRef] [PubMed]
- Marquès, M.; Domingo, J.L. Positive association between outdoor air pollution and the incidence and severity of COVID-19. A review of the recent scientific evidences. Environ. Res. 2022, 203, 111930. [Google Scholar] [CrossRef]
- Shah, A.S.; Gribben, C.; Bishop, J.; Hanlon, P.; Caldwell, D.; Wood, R.; Reid, M.; McMenamin, J.; Goldberg, D.; Stockton, D.; et al. Effect of Vaccination on Transmission of SARS-CoV-2. N. Engl. J. Med. 2021, 385, 1718–1720. [Google Scholar] [CrossRef] [PubMed]
- Eyre, D.W.; Taylor, D.; Purver, M.; Chapman, D.; Fowler, T.; Pouwels, K.B.; Walker, A.W.; Peto, T. The impact of SARS-CoV-2 vaccination on Alpha & Delta variant transmission. medRxiv 2021. [Google Scholar] [CrossRef]
- Levine-Tiefenbrun, M.; Yelin, I.; Katz, R.; Herzel, E.; Golan, Z.; Schreiber, L.; Wolf, T.; Nadler, V.; Ben-Tov, A.; Kuint, J.; et al. Decreased SARS-CoV-2 viral load following vaccination. medRxiv 2021. [Google Scholar] [CrossRef]
- COVID-19 Vaccination. Available online: https://ncv.kdca.go.kr/eng/ (accessed on 4 November 2021).

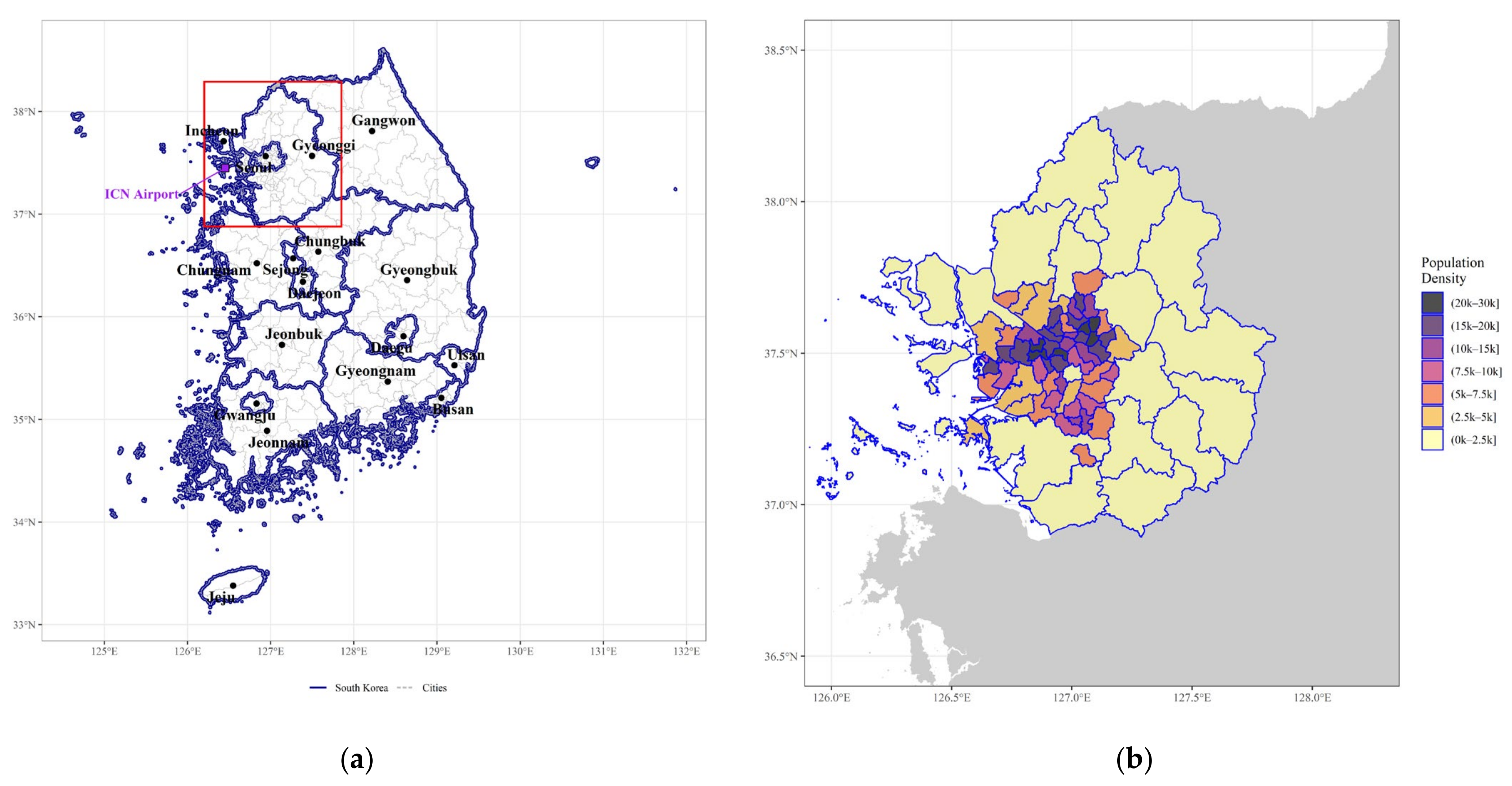
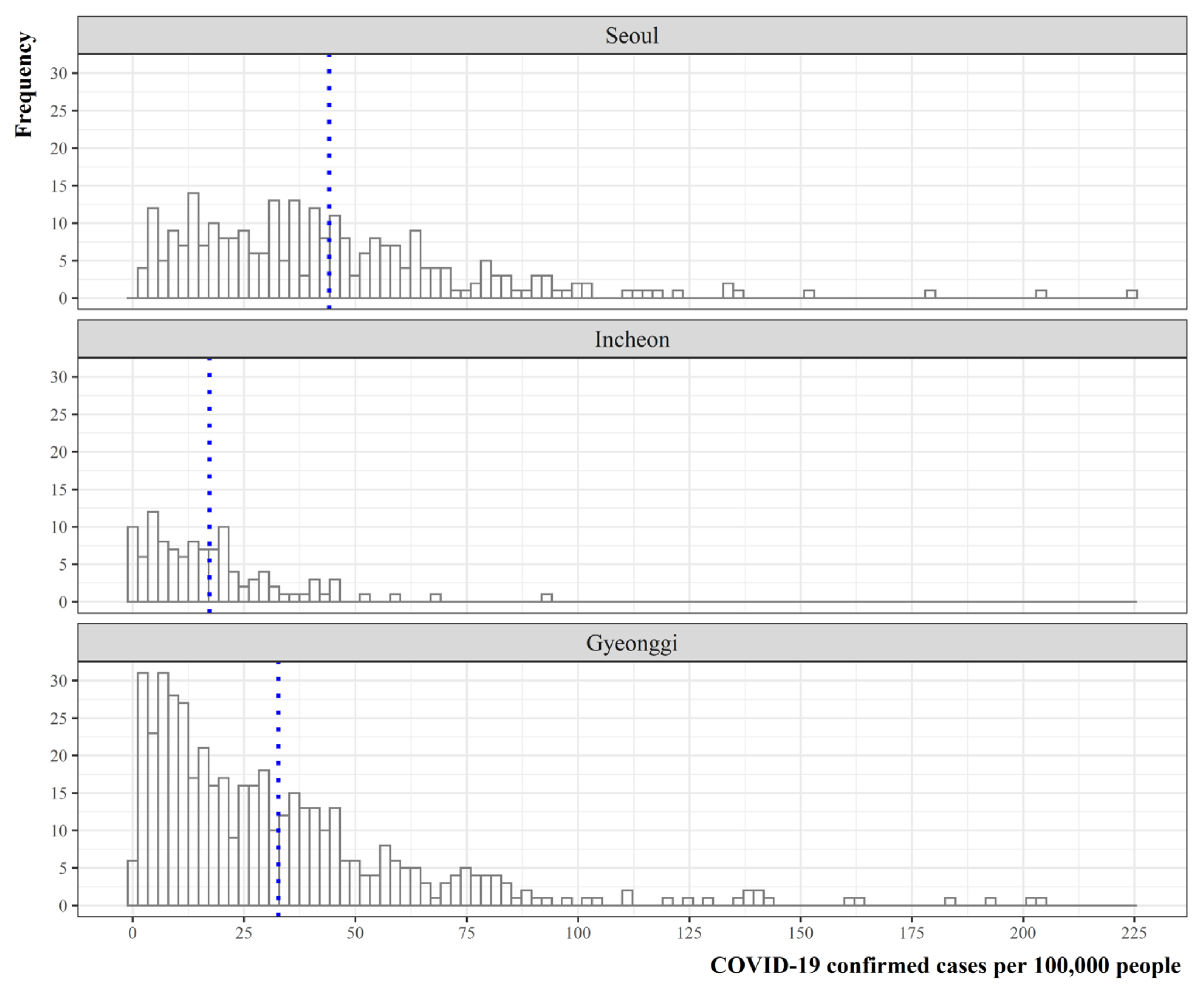

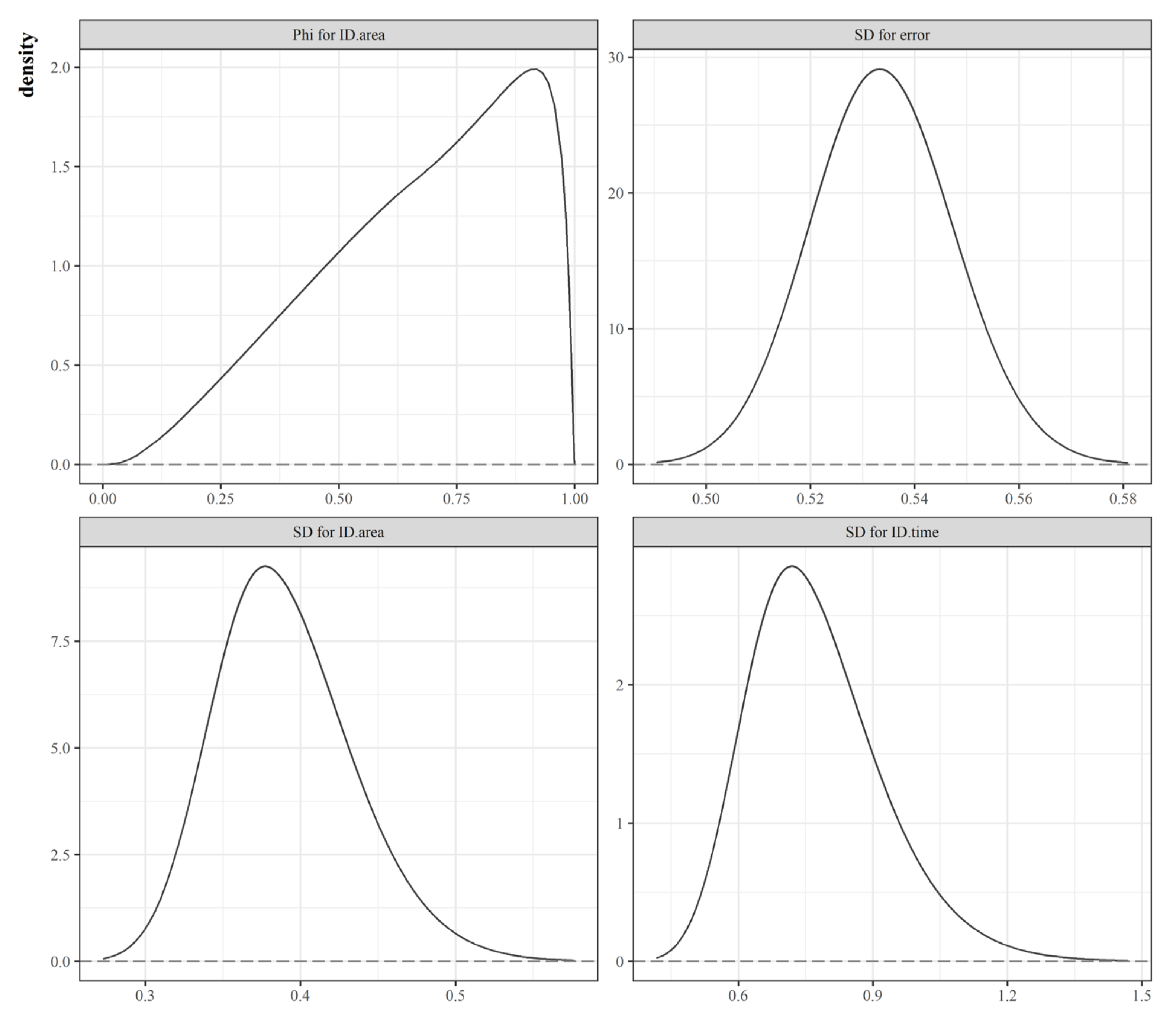
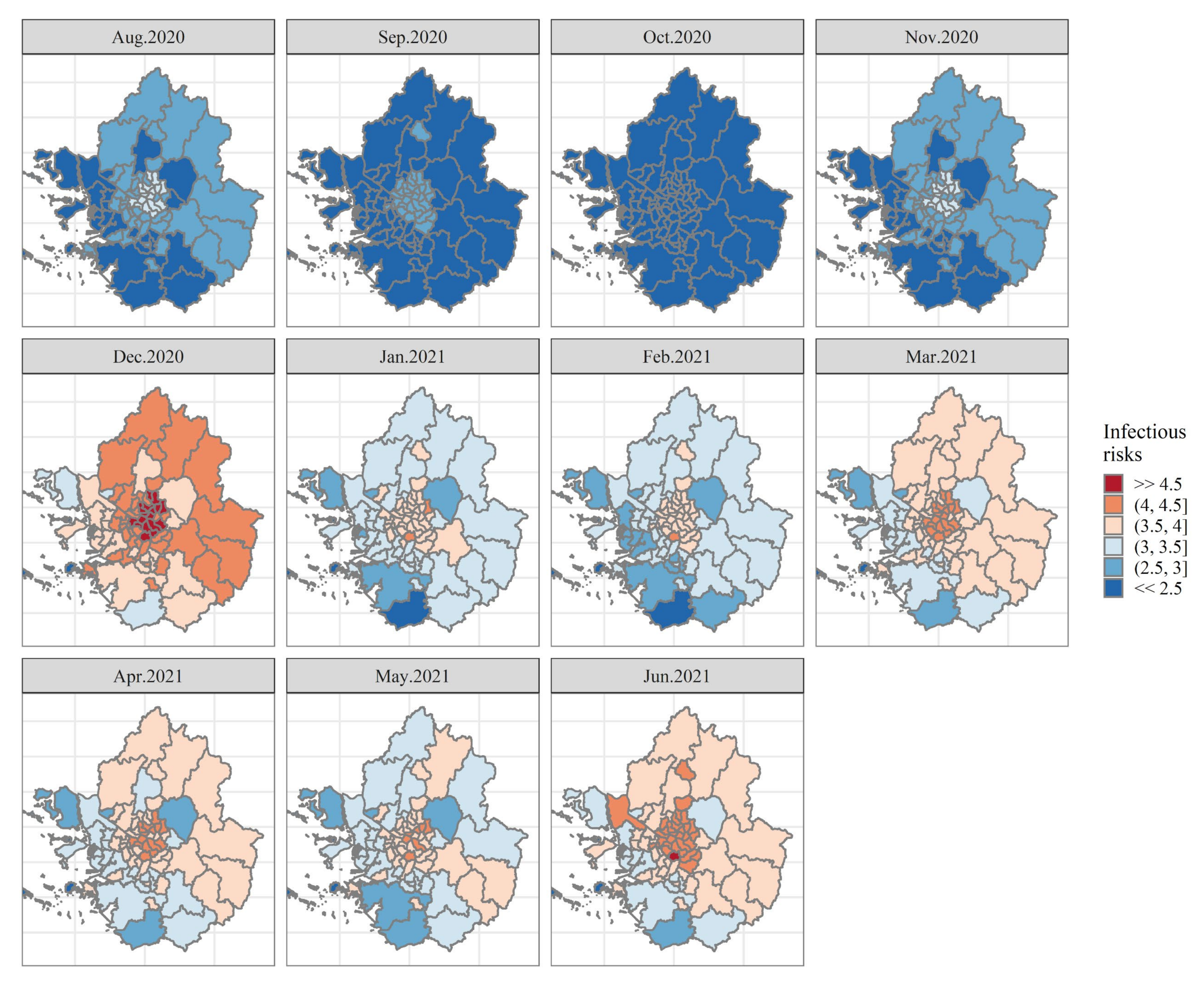
| Data attribute | Description | Temporal Dimension | Sources |
|---|---|---|---|
| COVID-19 cases | Number of confirmed cases | Daily (1 August 2020~30 June 2021) | Each municipality |
| Population | Population count | Monthly (August 2020~June 2021) | KOSIS 1 |
| Population density | Population per km2 | Monthly (August 2020~June 2021) | KOSIS 1 |
| Age 10–19 | Percent of population aged 10–19 | Census 2020 | KOSIS 1 |
| Age 20–29 | Percent of population aged 20–29 | Census 2020 | KOSIS 1 |
Local safety level index
| Index value (Levels 1–5) | 2019 | MOIS 2 |
| Spatial data | Geographical boundaries | Census boundary 2020 | SGIS 3 |
| Dependent Variable: The Natural Log of Monthly Aggregates of COVID-19 per 100,000 | ||||||
|---|---|---|---|---|---|---|
| Model 1 3 | Model 2 3 | Model 3 3 | ||||
| Mean (S.D.) | 90% C.I. | Mean (S.D.) | 90% C.I. | Mean (S.D.) | 90% C.I. | |
| Fixed effects | ||||||
| Intercept | 3.083 (0.118) | (2.889, 3.277) | 3.106 (0.079) | (2.975, 3.236) | 2.984 (0.169) | (2.702, 3.259) |
| Age cohort 10–19 | 0.076 (0.046) | (0.001, 0.151) | 0.076 (0.031) | (0.025, 0.126) | 0.100 (0.062) | (−0.003, 0.202) |
| Age cohort 20–29 | 0.057 (0.047) | (−0.021, 0.135) | 0.067 (0.032) | (0.015, 0.119) | 0.055 (0.063) | (−0.049, 0.157) |
| Population density Q2 1 | 0.427 (0.118) | (0.233, 0.621) | 0.354 (0.079) | (0.224, 0.485) | 0.476 (0.148) | (0.234, 0.723) |
| Population density Q3 | 0.312 (0.117) | (0.120, 0.504) | 0.285 (0.078) | (0.156, 0.414) | 0.524 (0.164) | (0.260, 0.799) |
| Population density Q4 | 0.400 (0.136) | (0.176, 0.624) | 0.356 (0.091) | (0.206, 0.506) | 0.510 (0.202) | (0.182, 0.845) |
| Living safety Normal 2 | 0.314 (0.119) | (0.118, 0.510) | 0.317 (0.080) | (0.186, 0.448) | 0.207 (0.168) | (−0.068, 0.484) |
| Living safety Good | 0.269 (0.115) | (0.080, 0.458) | 0.285 (0.077) | (0.159, 0.412) | 0.098 (0.170) | (−0.182, 0.377) |
| Infectious disease Normal 2 | −0.267 (0.104) | (−0.438, −0.096) | −0.265 (0.070) | (−0.380, −0.151) | −0.201 (0.143) | (−0.435, 0.034) |
| Infectious disease Good | −0.730 (0.112) | (−0.915, −0.544) | −0.723 (0.075) | (−0.847, −0.599) | −0.489 (0.167) | (−0.764, −0.213) |
| Random effects | ||||||
| (measurement error) | 1.07 (0.052) | (0.986, 1.16) | 2.38 (0.117) | (2.19, 2.57) | 3.515 (0.181) | (3.225, 3.821) |
| (precision of RW1) | 1.89 (0.7385) | (0.91, 3.26) | 1.862 (0.722) | (0.907, 3.207) | ||
| (marginal precision) | 6.818 (1.547) | (4.513, 9.565) | ||||
| (mixing parameter) | 0.673 (0.216) | (0.269, 0.958) | ||||
| Goodness of fit measure | ||||||
| DIC | 2360.61 | 1693.89 | 1418.29 | |||
| WAIC | 2360.87 | 1696.58 | 1423.78 | |||
| Marginal log-Likelihood | −1238.04 | −930.13 | −775.52 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lym, Y.; Lym, H.; Kim, K.; Kim, K.-J. Spatiotemporal Associations between Local Safety Level Index and COVID-19 Infection Risks across Capital Regions in South Korea. Int. J. Environ. Res. Public Health 2022, 19, 824. https://doi.org/10.3390/ijerph19020824
Lym Y, Lym H, Kim K, Kim K-J. Spatiotemporal Associations between Local Safety Level Index and COVID-19 Infection Risks across Capital Regions in South Korea. International Journal of Environmental Research and Public Health. 2022; 19(2):824. https://doi.org/10.3390/ijerph19020824
Chicago/Turabian StyleLym, Youngbin, Hyobin Lym, Keekwang Kim, and Ki-Jung Kim. 2022. "Spatiotemporal Associations between Local Safety Level Index and COVID-19 Infection Risks across Capital Regions in South Korea" International Journal of Environmental Research and Public Health 19, no. 2: 824. https://doi.org/10.3390/ijerph19020824
APA StyleLym, Y., Lym, H., Kim, K., & Kim, K.-J. (2022). Spatiotemporal Associations between Local Safety Level Index and COVID-19 Infection Risks across Capital Regions in South Korea. International Journal of Environmental Research and Public Health, 19(2), 824. https://doi.org/10.3390/ijerph19020824






