Effect Mechanism of Land Consolidation on Soil Bacterial Community: A Case Study in Eastern China
Abstract
1. Introduction
2. Framework and Data Collection
2.1. Research Framework
2.2. Study Area
2.3. Soil Collection and Analysis
2.3.1. Soil Sampling
2.3.2. Soil Basic Physical and Chemical Properties Test
2.3.3. Soil Heavy Metal Content Test
2.3.4. Soil Microbial Properties Determination
- (1)
- Soil microbial biomass determination
- (2)
- DNA extraction and sequencing analysis
2.4. Statistic Analysis
3. Results and Discussion
3.1. Effect of Land Consolidation on Soil Bacterial Community
3.1.1. Changes in Soil Bacterial Diversity
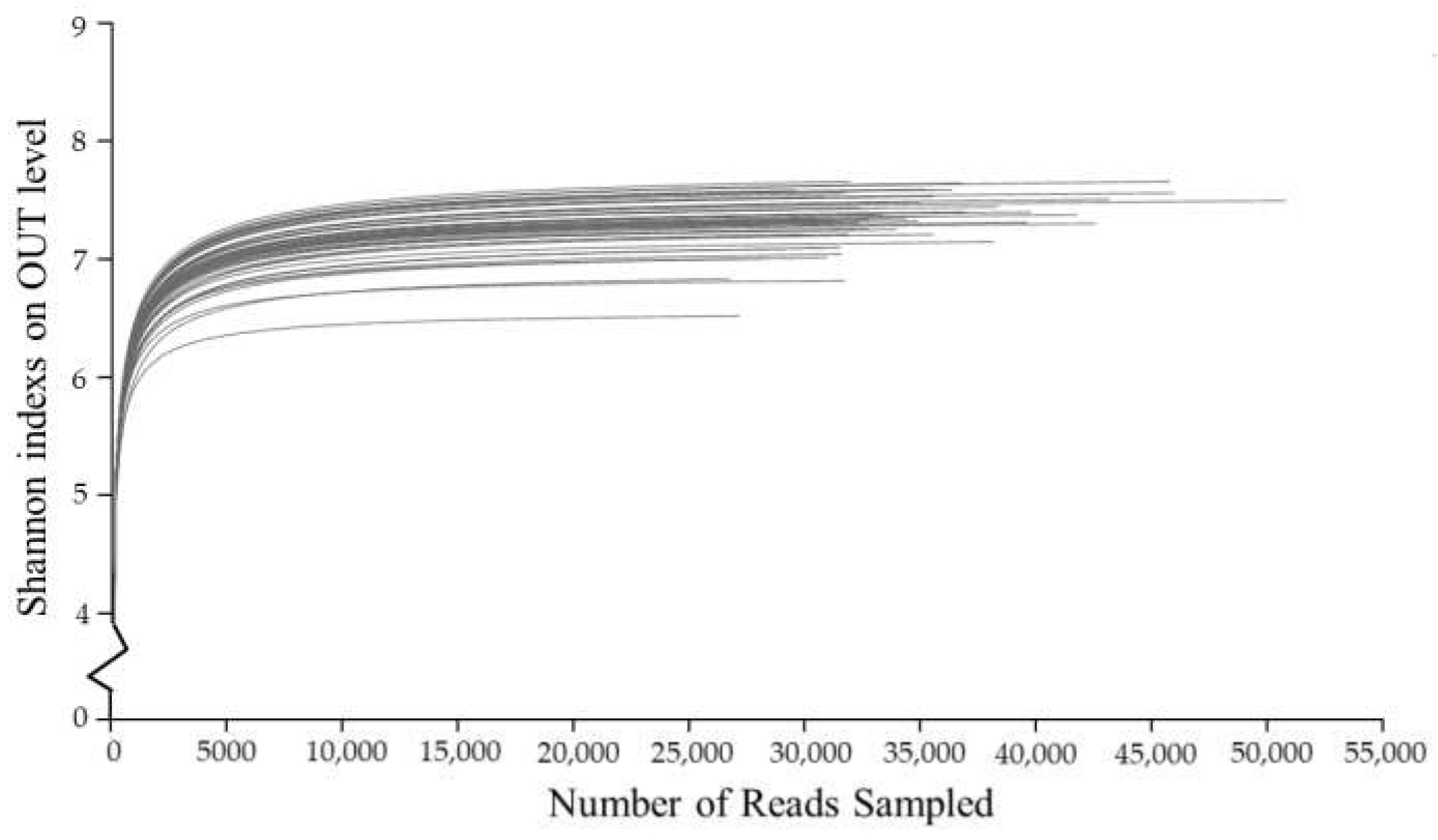
Analysis of α Diversity of Bacterial Community
Analysis of β Diversity of Bacterial Community
3.1.2. Variations in Soil Bacterial Community Structure
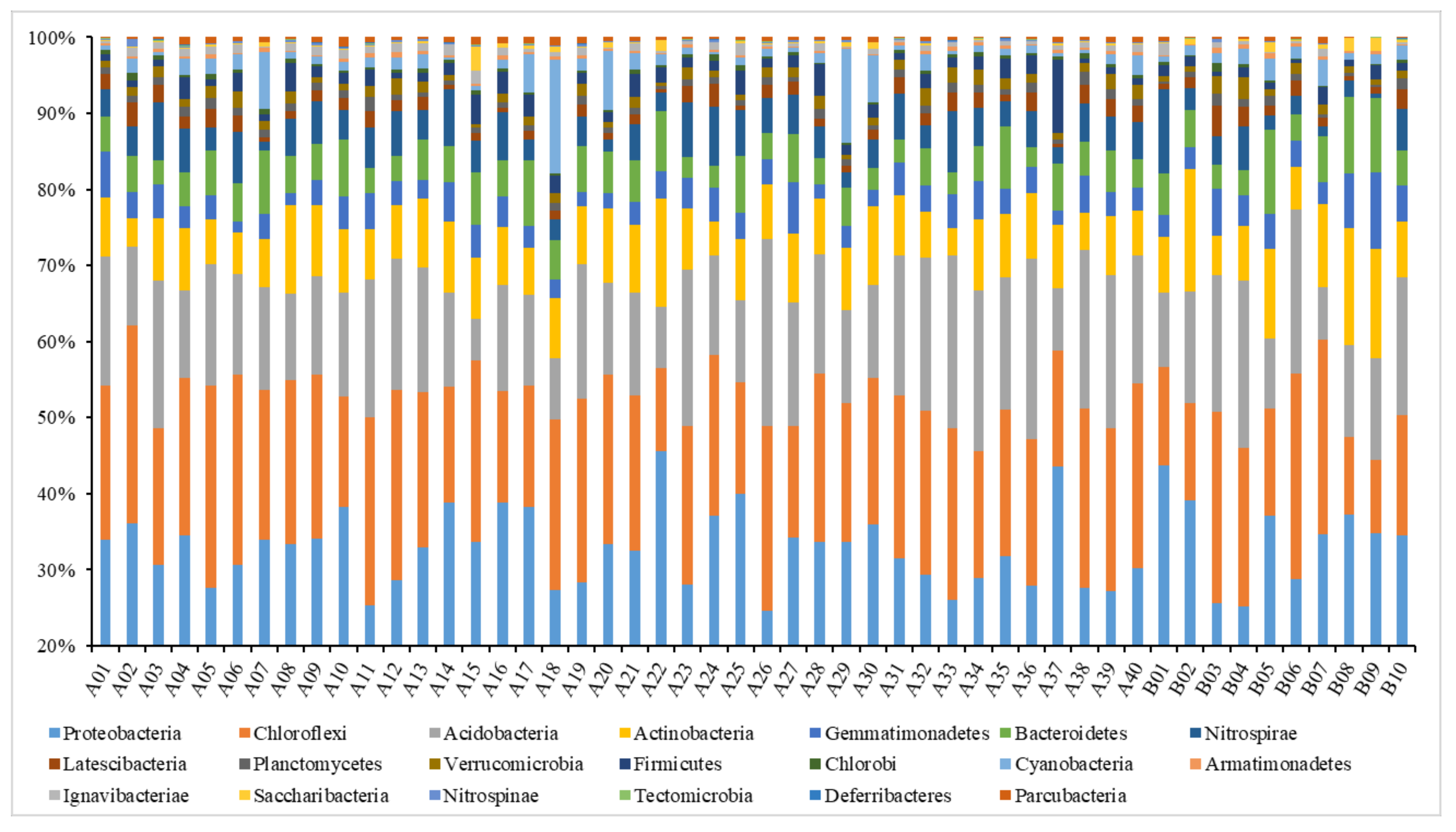
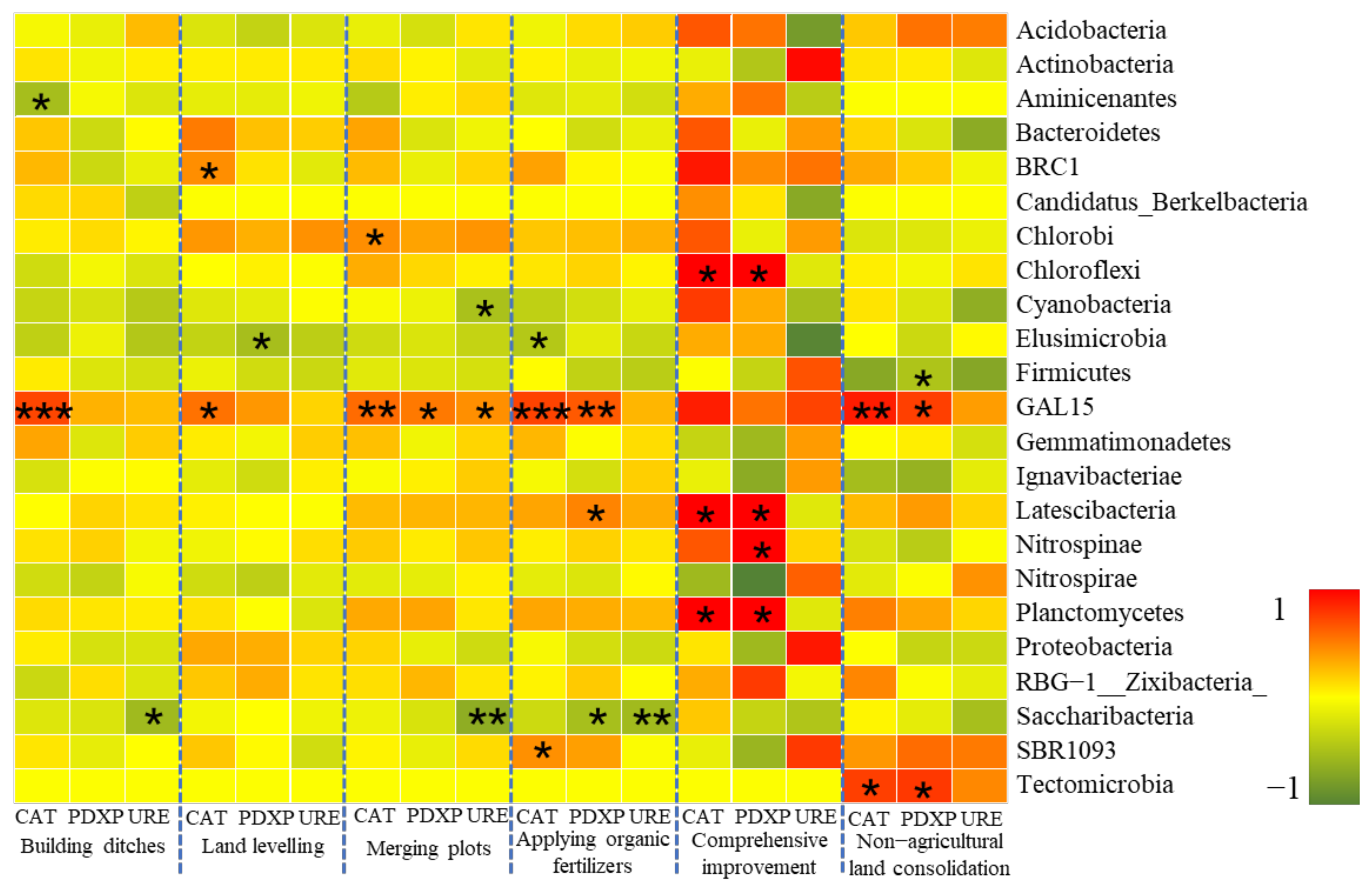
Analysis on the Changes of Bacterial Community at the Phylum Level
Analysis on the Changes of Bacterial Community at the Genus Level
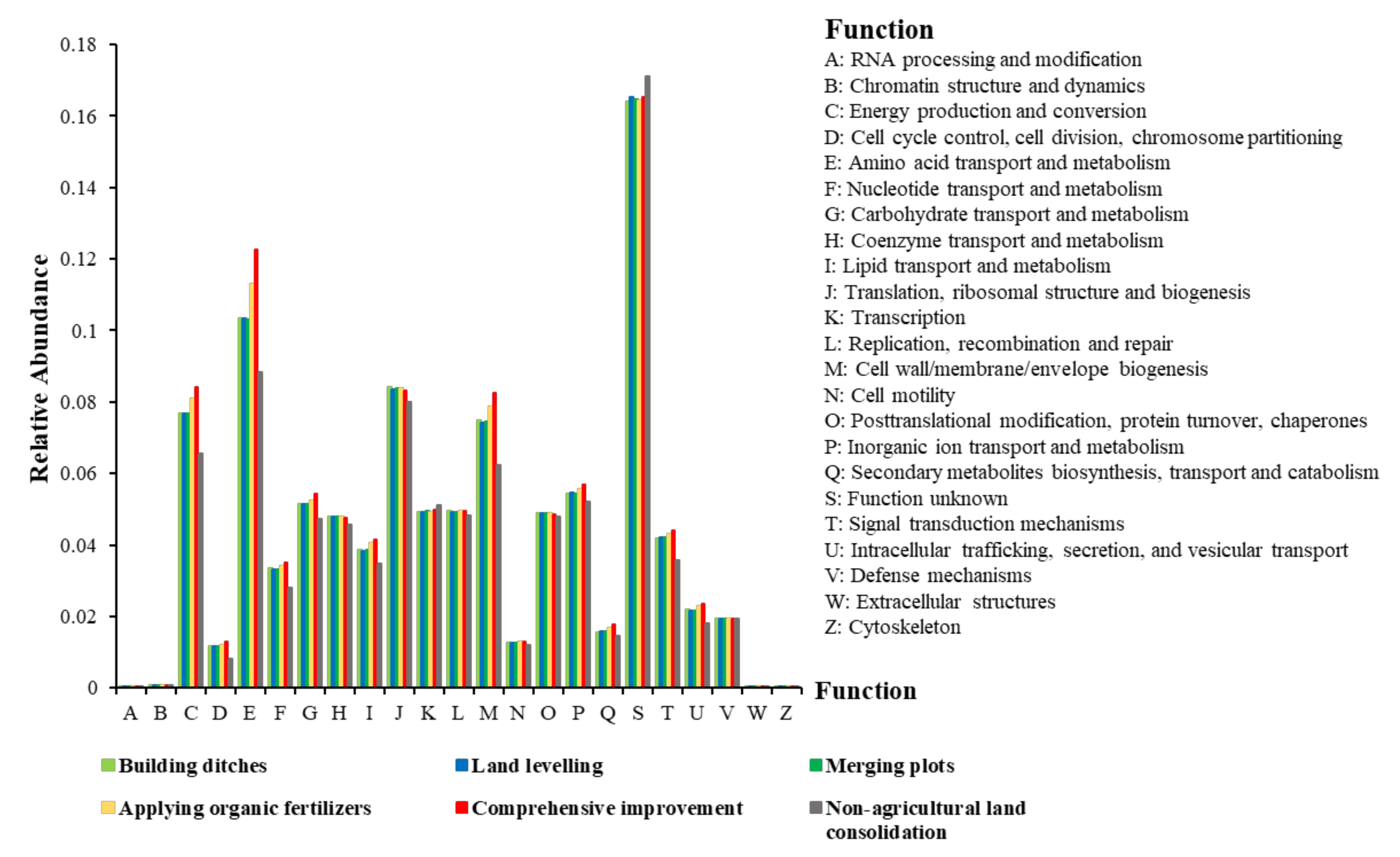
Analysis of Changes in Bacterial Community Function
3.2. Farmland Consolidation Regulates the Basic Physical and Chemical Properties of Soil and Its Mechanism of Action on Bacteria
3.2.1. Farmland Consolidation Promotes Changes in Basic Physical and Chemical Properties of Soil
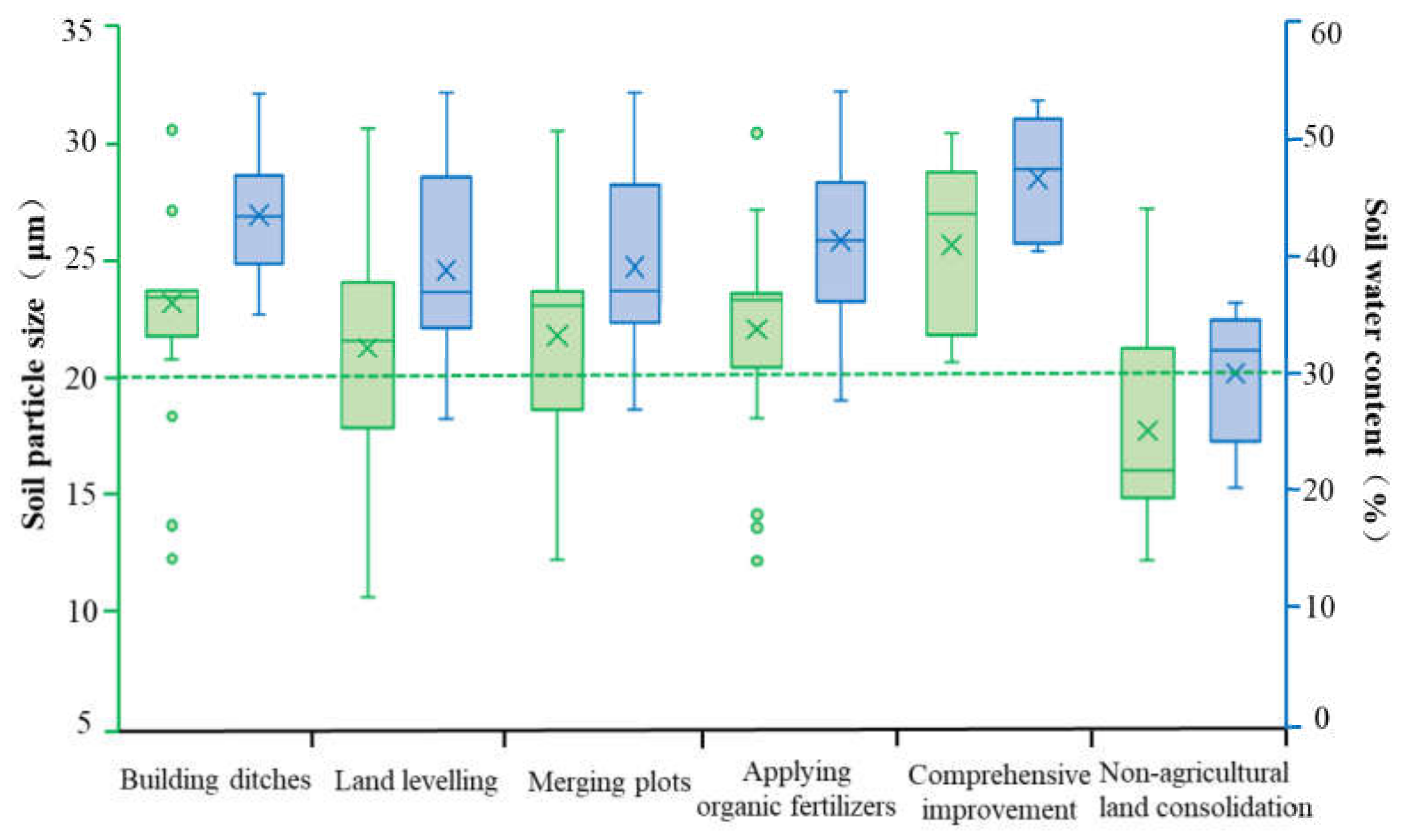
Soil Physical Properties
Soil Chemistry
Soil Enzyme Activity
3.2.2. The Mechanism of Basic Physical and Chemical Properties of Soil on Bacterial Community
Soil Physical Properties and Bacterial Community
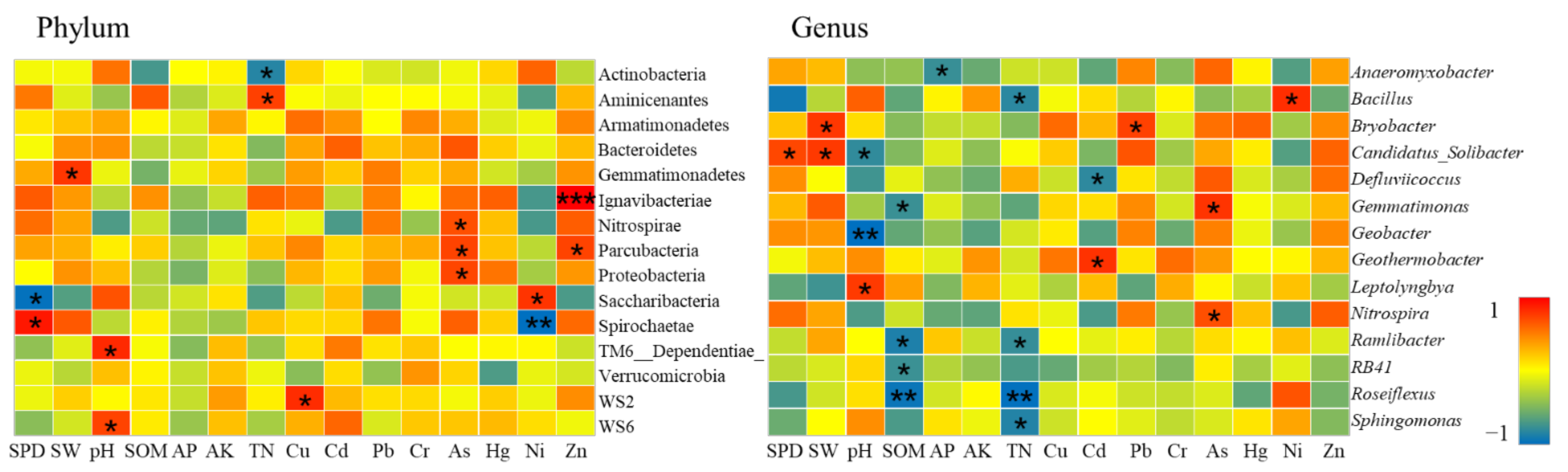
Soil Chemical Properties and Bacterial Communities
Soil Enzyme Activity and Bacterial Community
3.3. Farmland Consolidation Regulates Soil Heavy Metal Content and Its Mechanism of Action on Bacteria
3.3.1. Effects of Farmland Consolidation on Soil Heavy Metal Content
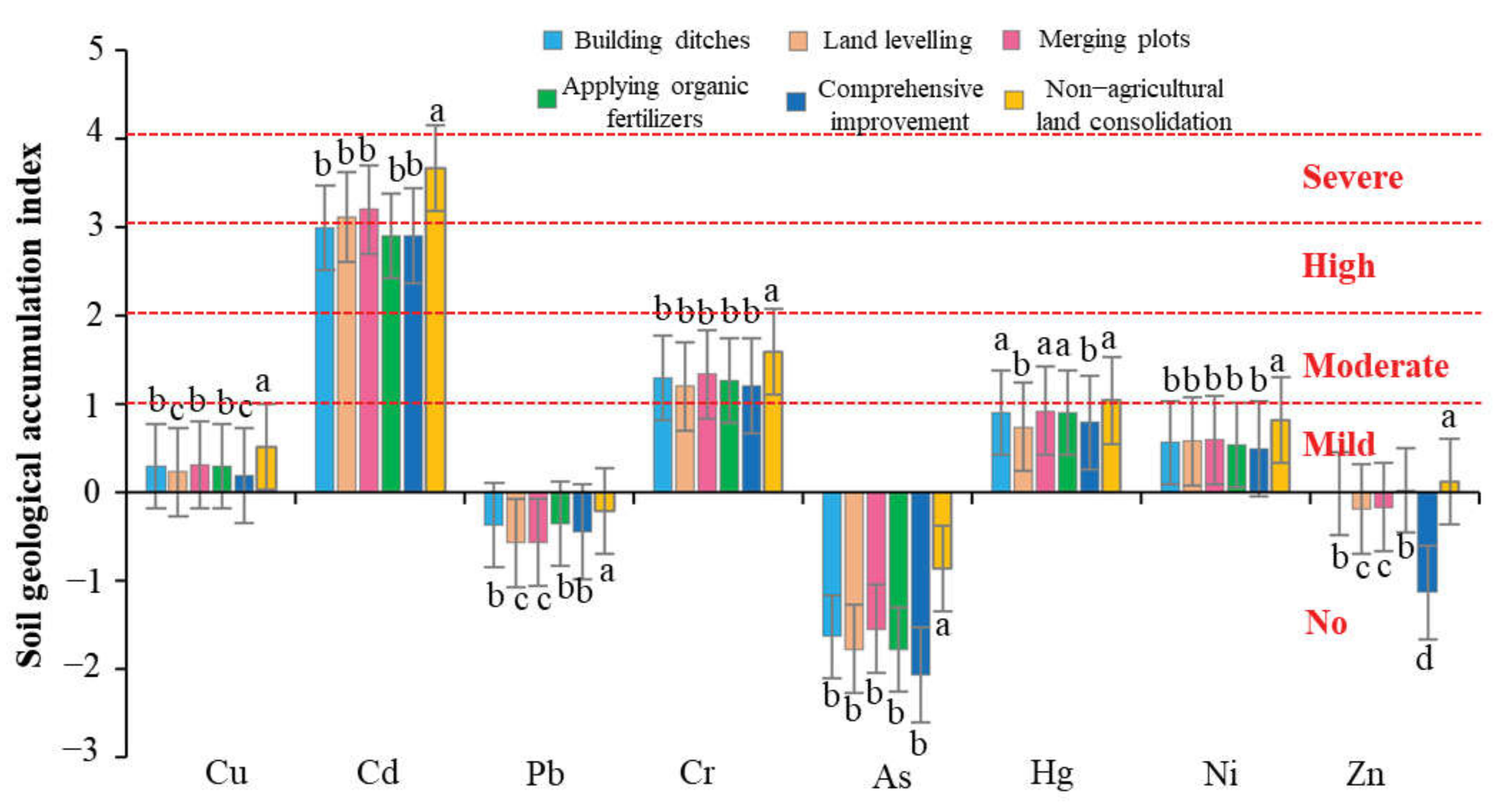
Heavy Metal Content of Farmland Soil
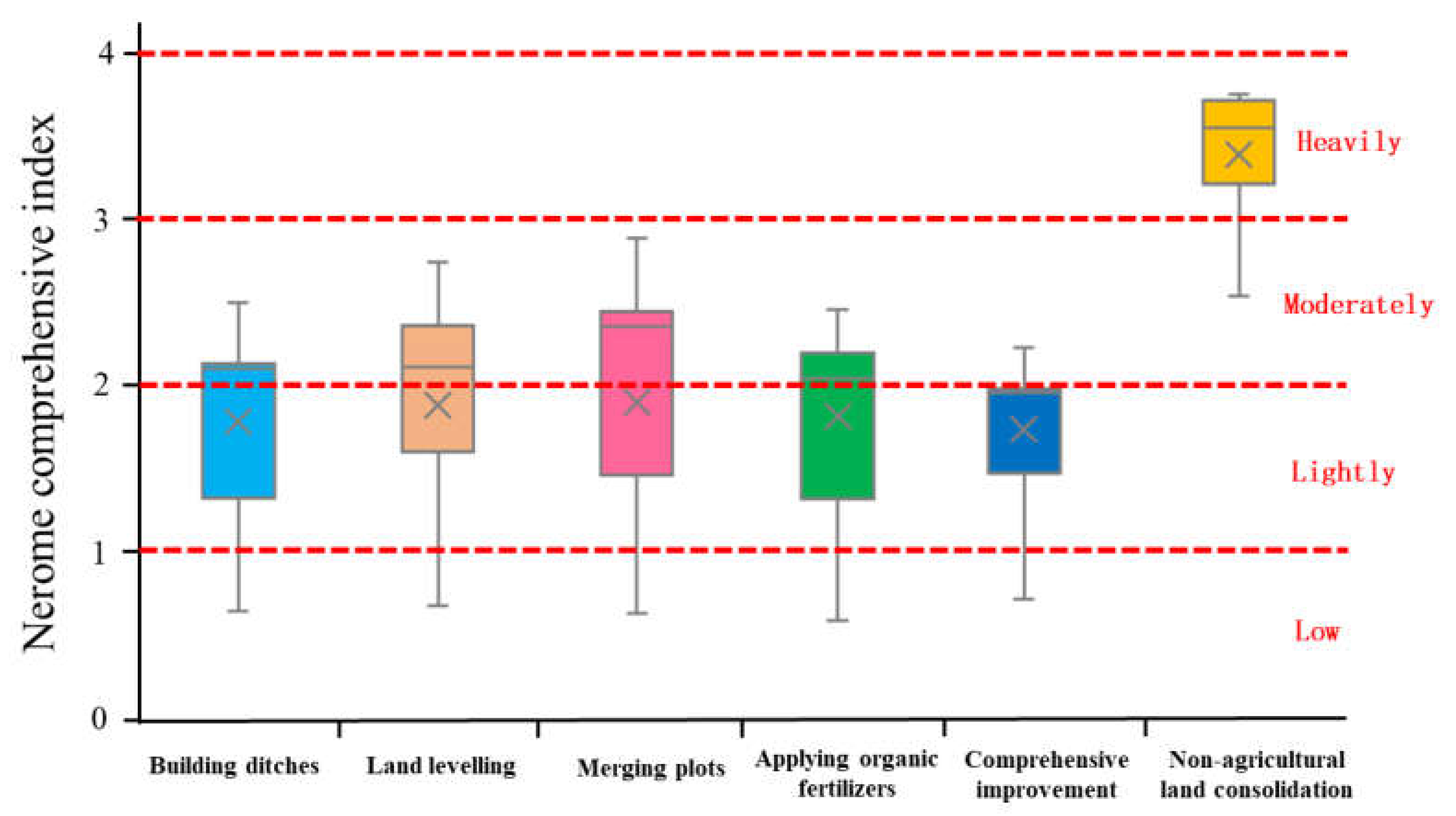
Heavy Metal Pollution Level of Farmland Soil
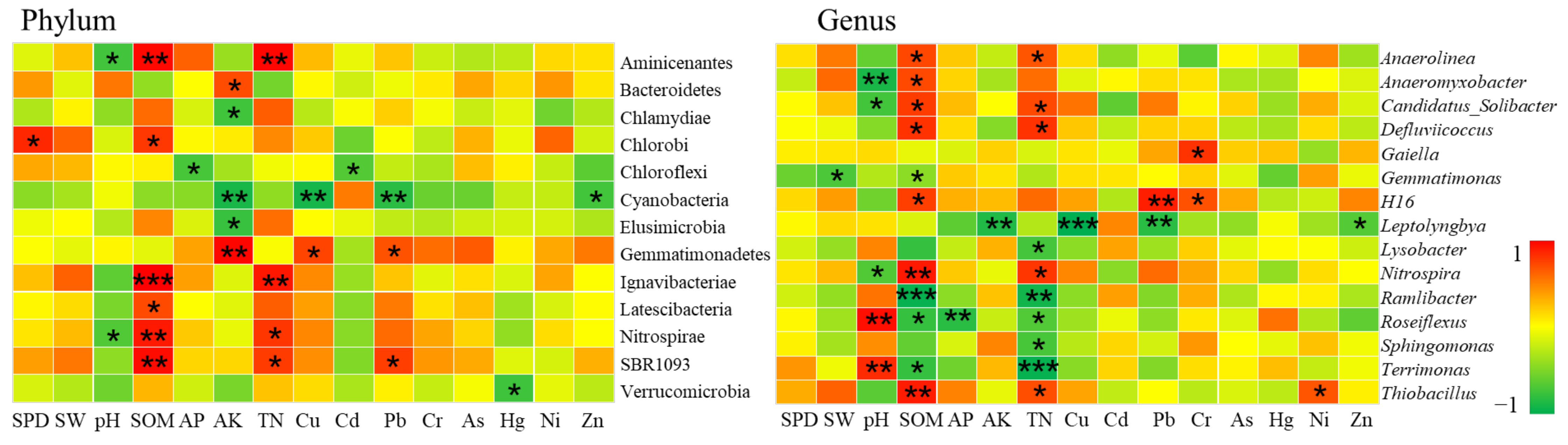
3.3.2. The Mechanism of Soil Heavy Metal Pollution on Bacterial Communities
Bacterial Communities at Low Pollution Levels
Bacterial Communities at Light Pollution Levels
Bacterial Communities at Moderate Pollution Levels
Bacterial Communities under Heavy Pollution Levels
4. Conclusions
- (1)
- Farmland consolidation had a significant impact on soil microbial characteristics, which was mainly manifested in changes in soil microbial biomass, microbial diversity and community structure. The soil microbial biomass carbon and nitrogen in farmland consolidation areas were significantly higher than those in non-agricultural land consolidation areas, and the microbial biomass phosphorus in soil samples from most farmland consolidation areas was significantly higher than that in non-agricultural land consolidation areas. In the study area, the soil bacterial and fungal community richness indexes Sobs and Chao of the cultivated land that had implemented farmland consolidation were significantly higher than the non-agricultural land consolidation areas at the p < 0.05 level. The soil bacterial community diversity indexes Shannon and Invsimpson in farmland consolidation areas were significantly higher than those in non-agricultural land consolidation areas, especially the soil bacterial community diversity index in comprehensive improvement areas was the highest.
- (2)
- Farmland consolidation could have a significant impact on the basic physical and chemical properties of the soil. In the study area, the soil particle size and water content of the agricultural land consolidation area were significantly higher than those on the non-agricultural land consolidation area, and the soil pH value of the non-agricultural land consolidation area was significantly higher than that of the construction ditches, combined plots, application of organic fertilizer, and comprehensive improvement areas where the soil pH was close to neutral. Regarding soil nutrients, the content of organic matter, available phosphorus, available potassium, and total nitrogen in non-agricultural land consolidation areas was also significantly lower than that in farmland consolidation areas, especially the application of organic fertilizer and comprehensive improvement areas had higher soil nutrients. In addition, the soil catalase, phosphatase, and urease activities in farmland consolidation areas were significantly higher than those in non-agricultural land consolidation areas. Our results showed that farmland consolidation had effectively improved the soil environment of farmland by adjusting soil pH, improving soil nutrients, accelerating soil water circulation, improving soil enzyme activity, and creating favorable conditions for the survival and reproduction of soil microorganisms.
- (3)
- Farmland consolidation had an indirect impact on soil bacteria by adjusting the basic physical and chemical properties of the soil. Studies have shown that the effects of different farmland consolidation measures on the relative abundance of soil bacteria were quite different. The soil water content in the farmland through the implementation of construction ditches was significantly improved, and the area with larger soil particle size could accelerate the water cycle, thereby effectively inhibiting the increase in the relative abundance of Cyanobacteria and Elusimicrobia. However, in areas with land levelling, Cyanobacteria was significantly negatively correlated with soil particle size. This was due to the mechanical compaction of soil particle morphology, which resulted in soil voids and poor water ventilation performance, which restricted the reproduction of Cyanobacteria. The larger the soil particle size, the greater the impact it would bear. Important soil nutrients such as SOM, AP, AK, TN also had a greater impact on the structural changes of soil bacteria, but there was a significant negative correlation between soil bacteria and soil nutrients in non-agricultural land consolidation areas. This was probably due to the large-scale application of inorganic fertilizers in non-agricultural land consolidation areas, resulting in soil environmental pollution, destroying the dynamic balance of soil nutrients, and reducing soil bacterial activity. In addition, in farmland consolidation areas, there was a significant positive correlation between soil enzymes and more dominant bacteria in farmland where organic fertilizers were applied and comprehensive improvement was implemented. This was because these two groups of soils had a high content of soil enzymes, and bacteria could reproduce quickly by obtaining nutrients such as carbon, phosphorus, and nitrogen that were decomposed by soil enzymes.
- (4)
- Farmland consolidation had a significant effect on the content of heavy metals in the soil. Among the eight heavy metals tested in the farmland soil of the study area, the average content of seven heavy metals was greater than the background value of the soil, and only the average value of As was slightly lower than the background value. This showed that there was a relatively serious accumulation of heavy metals in farmland soils in this study area. As an artificial measure that strongly disturbed the soil environment, farmland consolidation was an important factor affecting the spatial distribution of soil heavy metal content. There were large differences in the content of heavy metals between farmland with different farmland consolidation measures and farmland in non-agricultural land consolidation areas, and the highest average values of various heavy metal content were in non-agricultural land consolidation areas. The content of heavy metals in farmland where building ditches, merging plots, land levelling, applying organic fertilizers, and comprehensive improvement were implemented was lower than that of non-agricultural land consolidation areas.
- (5)
- The impact of heavy metals on bacterial community structure varied greatly under different levels of heavy metal pollution. Cultivated lands with low pollution levels were all located in farmland consolidation areas. A total of 4 bacterial phyla exhibited strong absorption and transfer functions for heavy metals such as Hg and Ni, and 6 bacterial genera showed a significant positive correlation with heavy metals such as Pb, As, and Ni. Most of the soil samples at the lightly polluted level were located in farmland consolidation areas. Among them, the bacteria Gemmatimonadetes and SBR1093 had strong adsorption and degradation functions on the heavy metals Cu and Pb. The bacteria genera Gaiella, H16, Thiobacillus and the heavy metals Pb, Cr, Ni content were significantly positively correlated. Among the soil samples with moderate pollution levels, 13 samples were located in farmland consolidation areas. A total of 11 bacterial phyla were significantly correlated with the heavy metals Pb, Cr, As, and Cd, respectively. Bacterial genera such as Sphingomonas, Thioalkalispira, and Geothermobacter were significantly positively correlated with the heavy metals Cr and Ni respectively. Among the soil samples with heavily polluted levels, a total of 7 samples were located in non-agricultural land consolidation areas. Among them, the heavy metals Cu, As, Ni, and Zn had significant effects on the 7 bacterial phyla. The bacterial genera Geothermobacter, Bryobacter, Gemmatimonas, Nitrospira, and Bacillus were significantly positively correlated with Cd, Pb, As, and Ni under the condition of consuming a lot of soil nutrients.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Djanibekov, U.; Finger, R. Agricultural risks and farm land consolidation process in transition countries: The case of cotton in Uzbekistan. Agric. Syst. 2018, 164, 223–235. [Google Scholar] [CrossRef]
- Demetriou, D. The assessment of land valuation in land consolidation schemes: The need for a new land valuation framework. Land Use Policy 2016, 54, 487–498. [Google Scholar] [CrossRef]
- Wang, J.; Yan, S.; Guo, Y.; Li, J.; Sun, G. The effects of land consolidation on the ecological connectivity based on ecosystem service value: A case study of Da’an land consolidation project in Jilin province. J. Geogr. Sci. 2015, 25, 603–616. [Google Scholar] [CrossRef]
- Akkaya Aslan, S.T.; Gundogdu, K.S.; Yaslioglu, E.; Kirmikil, M.; Arici, I. Personal, physical and socioeconomic factors affecting farmers’ adoption of land consolidation. Span. J. Agric. Res. SJAR 2007, 5, 204–213. [Google Scholar] [CrossRef]
- Sklenička, P.; Hladík, J.; Střeleček, F.; Kottová, B.; Lososová, J.; Číhal, L.; Šálek, M. Historical, environmental and socio–economic driving forces on land ownership fragmentation, the land consolidation effect and project costs. Agric. Econ. 2009, 55, 571–582. [Google Scholar] [CrossRef][Green Version]
- Legrand, F.; Picot, A.; Cobo–Diaz, J.F.; Carof, M.; Chen, W.; Le Floch, G. Effect of tillage and static abiotic soil properties on microbial diversity. Appl Soil Ecol. 2018, 132, 135–145. [Google Scholar] [CrossRef]
- de Paul Obade, V. Integrating management information with soil quality dynamics to monitor agricultural productivity. Sci. Total Environ. 2019, 651, 2036–2043. [Google Scholar] [CrossRef]
- Malik, A.A.; Puissant, J.; Buckeridge, K.M.; Goodall, T.; Jehmlich, N.; Chowdhury, S.; Gweon, H.S.; Peyton, J.M.; Mason, K.E.; van Agtmaal, M.; et al. Land use driven change in soil pH affects microbial carbon cycling processes. Nat. Commun. 2018, 9, 3591. [Google Scholar] [CrossRef]
- Hadzi, G.Y.; Ayoko, G.A.; Essumang, D.K.; Osae, S.K.D. Contamination impact and human health risk assessment of heavy metals in surface soils from selected major mining areas in Ghana. Environ. Geochem. Health 2019, 41, 2821–2843. [Google Scholar] [CrossRef]
- Wang, J.; Deng, H.; Wu, S.; Deng, Y.; Liu, L.; Han, C.; Jiang, Y.; Zhong, W. Assessment of abundance and diversity of exoelectrogenic bacteria in soil under different land use types. Catena 2019, 172, 572–580. [Google Scholar] [CrossRef]
- Jiang, B.; Adebayo, A.; Jia, J.; Xing, Y.; Deng, S.; Guo, L.; Liang, Y.; Zhang, D. Impacts of heavy metals and soil properties at a Nigerian e–waste site on soil microbial community. J. Hazard. Mater. 2019, 362, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Majeed, A.; Muhammad, Z.; Ahmad, H. Plant growth promoting bacteria: Role in soil improvement, abiotic and biotic stress management of crops. Plant Cell Rep. 2018, 37, 1599–1609. [Google Scholar] [CrossRef]
- Bender, S.F.; Wagg, C.; van der Heijden, M.G.A. An Underground Revolution: Biodiversity and Soil Ecological Engineering for Agricultural Sustainability. Trends Ecol. Evol. 2016, 31, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Simonin, M.; Richaume, A. Impact of engineered nanoparticles on the activity, abundance, and diversity of soil microbial communities: A review. Environ. Sci. Pollut. Res. 2015, 22, 13710–13723. [Google Scholar] [CrossRef]
- Anjos, D.C.; Hernandez, F.F.F.; Bañuelos, G.S.; Dangi, S.R.; Tirado–Corbalá, R.; Da Silva, F.N.; Filho, P.F.M. Microbial community and heavy metals content in soils along the Curu River in Ceará, Brazil. Geoderma Reg. 2018, 14, e173. [Google Scholar] [CrossRef]
- Stańczuk–Gałwiaczek, M.; Sobolewska–Mikulska, K.; Ritzema, H.; van Loon–Steensma, J.M. Integration of water management and land consolidation in rural areas to adapt to climate change: Experiences from Poland and the Netherlands. Land Use Policy 2018, 77, 498–511. [Google Scholar] [CrossRef]
- Bardgett, R.D.; van der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Falkowski, P.G.; Fenchel, T.; Delong, E.F. The microbial engines that drive Earth’s biogeochemical cycles. Science 2008, 320, 1034–1039. [Google Scholar] [CrossRef]
- Frey, S.D.; Lee, J.; Melillo, J.M.; Six, J. The temperature response of soil microbial efficiency and its feedback to climate. Nat. Clim. Change 2013, 3, 395–398. [Google Scholar] [CrossRef]
- Karhu, K.; Auffret, M.D.; Dungait, J.A.; Hopkins, D.W.; Prosser, J.I.; Singh, B.K.; Subke, J.A.; Wookey, P.A.; Agren, G.I.; Sebastia, M.T.; et al. Temperature sensitivity of soil respiration rates enhanced by microbial community response. Nature 2014, 513, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.A.; Da Silva, T.F.; Pylro, V.S.; Salles, J.F.; Andreote, F.D.; Dini–Andreote, F. Soil Microbial Diversity Affects the Plant–Root Colonization by Arbuscular Mycorrhizal Fungi. Microb. Ecol. 2021, 82, 100–103. [Google Scholar] [CrossRef]
- Ke, P.J.; Wan, J. Effects of soil microbes on plant competition: A perspective from modern coexistence theory. Ecol. Monogr. 2020, 90, e01391. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, J.; Jiang, K.; Liu, J.; Du, D. Responses of soil N–fixing bacteria communities to invasive plant species under different types of simulated acid deposition. Sci. Nat. 2017, 104, 43. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, S.; Zhang, Y.; Wang, X.; Wang, R.; Li, J. Tillage system change affects soil organic carbon storage and benefits land restoration on loess soil in North China. Land Degrad. Dev. 2018, 29, 2880–2887. [Google Scholar] [CrossRef]
- Beattie, R.E.; Henke, W.; Campa, M.F.; Hazen, T.C.; McAliley, L.R.; Campbell, J.H. Variation in microbial community structure correlates with heavy–metal contamination in soils decades after mining ceased. Soil Biol. Biochem. 2018, 126, 57–63. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Z.; Yao, Q.; Hu, X.; Zhang, W.; Mi, G.; Chen, X.; Wang, G. Distinct soil bacterial communities in response to the cropping system in a Mollisol of northeast China. Appl. Soil Ecol. 2017, 119, 407–416. [Google Scholar] [CrossRef]
- Muchová, Z.; Konc, Ľ.; Petrovič, F. Land plots valuation in land consolidation in slovakia: A need for a new approach. Int. J. Strateg. Prop. Manag. 2018, 22, 372–380. [Google Scholar] [CrossRef]
- Johansen, P.H.; Ejrnæs, R.; Kronvang, B.; Olsen, J.V.; Præstholm, S.; Schou, J.S. Pursuing collective impact: A novel indicator–based approach to assessment of shared measurements when planning for multifunctional land consolidation. Land Use Policy 2018, 73, 102–114. [Google Scholar] [CrossRef]
- Mirzavand, J.; Moradi–Talebbeigi, R. Relationships between field management, soil compaction, and crop productivity. Arch. Agron Soil Sci. 2020, 144, 1–12. [Google Scholar] [CrossRef]
- He, M.; Wang, Y.; Tong, Y.; Zhao, Y.; Qiang, X.; Song, Y.; Wang, L.; Song, Y.; Wang, G.; He, C. Evaluation of the environmental effects of intensive land consolidation: A field–based case study of the Chinese Loess Plateau. Land Use Policy 2020, 94, 104523. [Google Scholar] [CrossRef]
- Wu, C.; Huang, J.; Zhu, H.; Zhang, L.; Minasny, B.; Marchant, B.P.; McBratney, A.B. Spatial changes in soil chemical properties in an agricultural zone in southeastern China due to land consolidation. Soil Tillage Res. 2019, 187, 152–160. [Google Scholar] [CrossRef]
- Li, P.; Chen, Y.; Hu, W.; Li, X.; Yu, Z.; Liu, Y. Possibilities and requirements for introducing agri–environment measures in land consolidation projects in China, evidence from ecosystem services and farmers’ attitudes. Sci. Total Environ. 2019, 650, 3145–3155. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Ye, Y.; Wu, C.; Yang, J.; Hu, Y.; Shi, H. Comprehensive assessment of paddy soil quality under land consolidation: A novel perspective of microbiology. PeerJ. 2019, 7, e7351. [Google Scholar] [CrossRef]
- Kadlec, V.; Žížala, D.; Novotný, I.; Heřmanovská, D.; Kapička, J.; Tippl, M. Land Consolidations as an Effective Instrument in Soil Conservation. Ekologia 2014, 33, 188–200. [Google Scholar] [CrossRef][Green Version]
- Zhong, L.; Wang, J.; Zhang, X.; Ying, L.; Zhu, C. Effects of agricultural land consolidation on soil conservation service in the Hilly Region of Southeast China–Implications for land management. Land Use Policy 2020, 95, 104637. [Google Scholar] [CrossRef]
- Zhou, J.; Deng, Y.; Shen, L.; Wen, C.; Yan, Q.; Ning, D.; Qin, Y.; Xue, K.; Wu, L.; He, Z.; et al. Temperature mediates continental–scale diversity of microbes in forest soils. Nat. Commun. 2016, 7, 12083. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chen, B.; Zhu, L.; Xing, B. Effects and mechanisms of biochar–microbe interactions in soil improvement and pollution remediation: A review. Environ. Pollut. 2017, 227, 98–115. [Google Scholar] [CrossRef]
- Yu, X.; Yang, L.; Fei, S.; Ma, Z.; Hao, R.; Zhao, Z. Effect of Soil Layer and Plant–Soil Interaction on Soil Microbial Diversity and Function after Canopy Gap Disturbance. Forests 2018, 9, 680. [Google Scholar] [CrossRef]
- Lüneberg, K.; Schneider, D.; Siebe, C.; Daniel, R. Drylands soil bacterial community is affected by land use change and different irrigation practices in the Mezquital Valley, Mexico. Sci. Rep. 2018, 8, 1413. [Google Scholar] [CrossRef]
- Baldoncini, M.; Albéri, M.; Bottardi, C.; Chiarelli, E.; Raptis, K.G.C.; Strati, V.; Mantovani, F. Biomass water content effect on soil moisture assessment via proximal gamma–ray spectroscopy. Geoderma 2019, 335, 69–77. [Google Scholar] [CrossRef]
- Song, X.; Yang, F.; Ju, B.; Li, D.; Zhao, Y.; Yang, J.; Zhang, G. The influence of the conversion of grassland to cropland on changes in soil organic carbon and total nitrogen stocks in the Songnen Plain of Northeast China. Catena 2018, 171, 588–601. [Google Scholar] [CrossRef]
- Vourlitis, G.L.; Kirby, K.; Vallejo, I.; Asaeli, J.; Holloway, J.M. Potential soil extracellular enzyme activity is altered by long–term experimental nitrogen deposition in semiarid shrublands. Appl. Soil Ecol. 2021, 158, 103779. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, S.; Bao, H.; Chen, D.; Wang, C.; Li, B.; Tong, G.; Yuan, Y.; Xu, B. Improving risk management by using the spatial interaction relationship of heavy metals and PAHs in urban soil. J. Hazard. Mater. 2019, 364, 108–116. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Caban, J.R.; Kuppusamy, S.; Kim, J.H.; Yoon, Y.; Kim, S.Y.; Lee, Y.B. Green manure amendment enhances microbial activity and diversity in antibiotic–contaminated soil. Appl. Soil Ecol. 2018, 129, 72–76. [Google Scholar] [CrossRef]
- Nottingham, A.T.; Fierer, N.; Turner, B.L.; Whitaker, J.; Ostle, N.J.; McNamara, N.P.; Bardgett, R.D.; Leff, J.W.; Salinas, N.; Silman, M.R.; et al. Microbes follow Humboldt: Temperature drives plant and soil microbial diversity patterns from the Amazon to the Andes. Ecology 2018, 99, 2455–2466. [Google Scholar] [CrossRef] [PubMed]
- Barnes, A.D.; Allen, K.; Kreft, H.; Corre, M.D.; Jochum, M.; Veldkamp, E.; Clough, Y.; Daniel, R.; Darras, K.; Denmead, L.H.; et al. Direct and cascading impacts of tropical land–use change on multi–trophic biodiversity. Nat. Ecol. Evol. 2017, 1, 1511–1519. [Google Scholar] [CrossRef]
- Wang, D.; Bai, J.; Wang, W.; Zhang, G.; Cui, B.; Liu, X.; Li, X. Comprehensive assessment of soil quality for different wetlands in a Chinese delta. Land Degrad. Dev. 2018, 29, 3783–3794. [Google Scholar] [CrossRef]
- Kong, M.; Zhong, H.; Wu, Y.; Liu, G.; Xu, Y.; Wang, G. Developing and validating intrinsic groundwater vulnerability maps in regions with limited data: A case study from Datong City in China using DRASTIC and Nemerow pollution indices. Environ. Earth Sci. 2019, 78, 262. [Google Scholar] [CrossRef]
- Negahban, S.; Mokarram, M.; Pourghasemi, H.R.; Zhang, H. Ecological risk potential assessment of heavy metal contaminated soils in Ophiolitic formations. Environ. Res. 2021, 192, 110305. [Google Scholar] [CrossRef]
- Keshavarzi, A.; Kumar, V. Ecological risk assessment and source apportionment of heavy metal contamination in agricultural soils of Northeastern Iran. Int. J. Environ. Health Res. 2019, 29, 544–560. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; HUANG, Y.; LEI, M.; HAO, X.; LI, X.; TIE, B.; XIE, J. Soil Contamination and Assessment of Heavy Metals of Xiangjiang River Basin. Environ. Sci. 2012, 33, 260–265. [Google Scholar]
- Dobrovol Skaya, T.G.; Zvyagintsev, D.G.; Chernov, I.Y.; Golovchenko, A.V.; Zenova, G.M.; Lysak, L.V.; Manucharova, N.A.; Marfenina, O.E.; Polyanskaya, L.M.; Stepanov, A.L.; et al. The role of microorganisms in the ecological functions of soils. Eurasian Soil Sci. 2015, 48, 959–967. [Google Scholar] [CrossRef]
- Liang, J.; Liu, J.; Jia, P.; Yang, T.; Zeng, Q.; Zhang, S.; Liao, B.; Shu, W.; Li, J. Novel phosphate–solubilizing bacteria enhance soil phosphorus cycling following ecological restoration of land degraded by mining. ISME J. 2020, 14, 1600–1613. [Google Scholar] [CrossRef]
- Schneider, F.; Don, A.; Hennings, I.; Schmittmann, O.; Seidel, S.J. The effect of deep tillage on crop yield—What do we really know? Soil Tillage Res. 2017, 174, 193–204. [Google Scholar] [CrossRef]
- Vanderlinden, K.; Vereecken, H.; Hardelauf, H.; Herbst, M.; Martínez, G.; Cosh, M.H.; Pachepsky, Y.A. Temporal Stability of Soil Water Contents: A Review of Data and Analyses. Vadose Zone J. 2012, 11, j2011–j2178. [Google Scholar] [CrossRef]
- Li, M.; Nie, S.; Chen, X.; Luo, L.; Zhu, H.; Shi, H.; Ge, T.; Tong, C.; Wu, J. Istribution Characteristics of Rice Photosynthesized Carbon in Soil Aggregates of Different Size and Density. Environ. Sci. 2013, 34, 1568–1575. [Google Scholar]
- Spohn, M. Phosphorus and carbon in soil particle size fractions: A synthesis. Biogeochemistry 2020, 147, 225–242. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, L.; Guo, X.; Li, M.; Yu, S.; Wang, M. Particle component and distribution characteristics of organic carbon of sediments in water and shore soils. J. Soil Water Conserv. 2014, 28, 304–308. [Google Scholar]
- LI, F.; Renkou, X.; Wenfeng, T.; Shungui, Z.; Tongxu, L.; Zhenqing, S.; Liping, F.; Chengshuai, L.; Fanghua, L.; Xiaomin, L.; et al. The Frontier and Perspectives of Soil Chemistry in the New Era. Acta Pedol. Sinica. 2020, 57, 1088–1104. [Google Scholar]
- Laurent, C.; Bravin, M.N.; Crouzet, O.; Pelosi, C.; Tillard, E.; Lecomte, P.; Lamy, I. Increased soil pH and dissolved organic matter after a decade of organic fertilizer application mitigates copper and zinc availability despite contamination. Sci. Total Environ. 2020, 709, 135927. [Google Scholar] [CrossRef]
- Ghosh, A.; Singh, A.B.; Kumar, R.V.; Manna, M.C.; Bhattacharyya, R.; Rahman, M.M.; Sharma, P.; Rajput, P.S.; Misra, S. Soil enzymes and microbial elemental stoichiometry as bio-indicators of soil quality in diverse cropping systems and nutrient management practices of Indian Vertisols. Appl. Soil Ecol. 2020, 145, 103304. [Google Scholar] [CrossRef]
- Kuscu, I.S.K. Changing of soil properties and urease–catalase enzyme activity depending on plant type and shading. Environ. Monit. Assess. 2019, 191, 178. [Google Scholar] [CrossRef] [PubMed]
- Adetunji, A.T.; Lewu, F.B.; Mulidzi, R.; Ncube, B. The biological activities of beta–glucosidase, phosphatase and urease as soil quality indicators: A review. J. Soil Sci. Plant Nutr. 2017, 17, 794–807. [Google Scholar] [CrossRef]
- Chen, X.; Hao, B.; Jing, X.; He, J.; Ma, W.; Zhu, B. Minor responses of soil microbial biomass, community structure and enzyme activities to nitrogen and phosphorus addition in three grassland ecosystems. Plant. Soil. 2019, 444, 21–37. [Google Scholar] [CrossRef]
- Song, D.; Chen, L.; Zhang, S.; Zheng, Q.; Ullah, S.; Zhou, W.; Wang, X. Combined biochar and nitrogen fertilizer change soil enzyme and microbial activities in a 2–year field trial. Eur. J. Soil Biol. 2020, 99, 103212. [Google Scholar] [CrossRef]
- Maxwell, T.L.; Augusto, L.; Bon, L.; Courbineau, A.; Altinalmazis–Kondylis, A.; Milin, S.; Bakker, M.R.; Jactel, H.; Fanin, N. Effect of a tree mixture and water availability on soil nutrients and extracellular enzyme activities along the soil profile in an experimental forest. Soil Biol. Biochem. 2020, 148, 107864. [Google Scholar] [CrossRef]
- Gomez, E.J.; Delgado, J.A.; Gonzalez, J.M. Environmental factors affect the response of microbial extracellular enzyme activity in soils when determined as a function of water availability and temperature. Ecol. Evol. 2020, 10, 10105–10115. [Google Scholar] [CrossRef] [PubMed]
- Díaz, F.J.; Sanchez–Hernandez, J.C.; Notario, J.S. Effects of irrigation management on arid soils enzyme activities. J. Arid Environ. 2021, 185, 104330. [Google Scholar] [CrossRef]
- Bogunovic, I.; Pereira, P.; Galic, M.; Bilandzija, D.; Kisic, I. Tillage system and farmyard manure impact on soil physical properties, CO2 emissions, and crop yield in an organic farm located in a Mediterranean environment (Croatia). Environ. Earth Sci. 2020, 79, 70. [Google Scholar] [CrossRef]
- Mickan, B.S.; Abbott, L.K.; Solaiman, Z.M.; Mathes, F.; Siddique, K.H.M.; Jenkins, S.N. Soil disturbance and water stress interact to influence arbuscular mycorrhizal fungi, rhizosphere bacteria and potential for N and C cycling in an agricultural soil. Biol. Fertil. Soils 2019, 55, 53–66. [Google Scholar] [CrossRef]
- Moyano, F.E.; Manzoni, S.; Chenu, C. Responses of soil heterotrophic respiration to moisture availability: An exploration of processes and models. Soil Biol. Biochem. 2013, 59, 72–85. [Google Scholar] [CrossRef]
- Zornoza, R.; Acosta, J.A.; Martínez–Martínez, S.; Faz, A.; Bååth, E. Main factors controlling microbial community structure and function after reclamation of a tailing pond with aided phytostabilization. Geoderma 2015, 245, 1–10. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Z.; Yao, Q.; Sui, Y.; Shi, Y.; Chu, H.; Tang, C.; Franks, A.E.; Jin, J.; Liu, X.; et al. Ammonia–Oxidizing Archaea Show More Distinct Biogeographic Distribution Patterns than Ammonia–Oxidizing Bacteria across the Black Soil Zone of Northeast China. Front. Microbiol. 2018, 9, 171. [Google Scholar] [CrossRef]
- Gao, S.; Guan, D.; Ma, M.; Zhang, W.; Li, J.; Shen, D. Effects of Fertilization on Bacterial Community Under the Condition of Continuous Soybean Monoculture in Black Soil in Northeast China. Sci. Agric. Sin. 2017, 50, 1271–1281. [Google Scholar]
- Shen, J.; Zhang, L.; Guo, J.; Ray, J.L.; He, J. Impact of long–term fertilization practices on the abundance and composition of soil bacterial communities in Northeast China. Appl. Soil Ecol. 2010, 46, 119–124. [Google Scholar] [CrossRef]
- Zhao, J.; Ni, T.; Li, J.; Lu, Q.; Fang, Z.; Huang, Q.; Zhang, R.; Li, R.; Shen, B.; Shen, Q. Effects of organic–inorganic compound fertilizer with reduced chemical fertilizer application on crop yields, soil biological activity and bacterial community structure in a rice–wheat cropping system. Appl. Soil Ecol. 2016, 99, 1–12. [Google Scholar] [CrossRef]
- Aarti, C.; Khusro, A.; Agastian, P.; Darwish, N.M.; Al Farraj, D.A. Molecular diversity and hydrolytic enzymes production abilities of soil bacteria. Saudi J. Biol. Sci. 2020, 27, 3235–3248. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liu, J.; Lu, X.; Chen, X.; Zhang, B.; Zhang, X.; Zhou, W.; Yang, Y. The application of organic fertilizer improves the activity of the soil enzyme, increases the number and the species variety of bacteria in black soil. Soil Fertil. Sci. China 2020, 4, 50–55. [Google Scholar]
- Zhou, B.; Zhao, L.; Wang, Y.; Sun, Y.; Li, X.; Xu, H.; Weng, L.; Pan, Z.; Yang, S.; Chang, X.; et al. Spatial distribution of phthalate esters and the associated response of enzyme activities and microbial community composition in typical plastic–shed vegetable soils in China. Ecotoxicol. Environ. Saf. 2020, 195, 110495. [Google Scholar] [CrossRef]
- Bhuiyan, M.A.H.; Karmaker, S.C.; Bodrud–Doza, M.; Rakib, M.A.; Saha, B.B. Enrichment, sources and ecological risk mapping of heavy metals in agricultural soils of dhaka district employing SOM, PMF and GIS methods. Chemosphere 2021, 263, 128339. [Google Scholar] [CrossRef] [PubMed]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Ramos–Miras, J.J.; Roca–Perez, L.; Guzman–Palomino, M.; Boluda, R.; Gil, C. Background levels and baseline values of available heavy metals in Mediterranean greenhouse soils (Spain). J. Geochem. Explor. 2011, 110, 186–192. [Google Scholar] [CrossRef]
- Qu, M.; Chen, J.; Huang, B.; Zhao, Y. Source apportionment of soil heavy metals using robust spatial receptor model with categorical land–use types and RGWR–corrected in–situ FPXRF data. Environ. Pollut. 2021, 270, 116220. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, J.; Lu, J.; Sun, Y. Study on the influence of soil microbial community on the long–term heavy metal pollution of different land use types and depth layers in mine. Ecotoxicol. Environ. Saf. 2019, 170, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Kuang, J.L.; Huang, L.N.; Chen, L.X.; Hua, Z.S.; Li, S.J.; Hu, M.; Li, J.T.; Shu, W.S. Contemporary environmental variation determines microbial diversity patterns in acid mine drainage. ISME J. 2013, 7, 1038–1050. [Google Scholar] [CrossRef]
- Rahman, Z.; Singh, V.P. Assessment of heavy metal contamination and Hg–resistant bacteria in surface water from different regions of Delhi, India. Saudi J. Biol. Sci. 2018, 25, 1687–1695. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Meng, D.; Li, J.; Yin, H.; Liu, H.; Liu, X.; Cheng, C.; Xiao, Y.; Liu, Z.; Yan, M. Response of soil microbial communities and microbial interactions to long–term heavy metal contamination. Environ. Pollut. 2017, 231, 908–917. [Google Scholar] [CrossRef]
- Chodak, M.; Gołębiewski, M.; Morawska–Płoskonka, J.; Kuduk, K.; Niklińska, M. Diversity of microorganisms from forest soils differently polluted with heavy metals. Appl. Soil Ecol. 2013, 64, 7–14. [Google Scholar] [CrossRef]









| Land Consolidation Measures | The Community Richness Index | The Community Evenness Index | The Community Diversity Index | The Community Coverage | |||
|---|---|---|---|---|---|---|---|
| Sobs | Chao | Shannoneven | Simpsoneven | Shannon | Invsimpson | Coverage | |
| Comprehensive improvement | 4312.00 ± 452.33a | 5866.69 ± 523.61a | 0.89 ± 0.01a | 0.17 ± 0.02a | 7.44 ± 0.12a | 730.73 ± 118.61a | 0.95 ± 0.01a |
| Applying organic fertilizers | 4318.92 ± 447.21a | 5768.07 ± 530.29a | 0.88 ± 0.01a | 0.15 ± 0.04a | 7.36 ± 0.16a | 658.19 ± 189.31a | 0.96 ± 0.01a |
| Building ditches | 4370.70 ± 443.19a | 5808.02 ± 493.61a | 0.88 ± 0.01a | 0.16 ± 0.03a | 7.39 ± 0.14a | 693.77 ± 161.98a | 0.95 ± 0.01a |
| Merging plots | 4349.68 ± 440.11a | 5823.19 ± 528.11a | 0.88 ± 0.01a | 0.15 ± 0.04a | 7.38 ± 0.15a | 675.83 ± 176.20a | 0.96 ± 0.01a |
| Land levelling | 4405.47 ± 396.52a | 5963.16 ± 483.81a | 0.88 ± 0.01a | 0.16 ± 0.04a | 7.39 ± 0.17a | 696.92 ± 202.67a | 0.96 ± 0.01a |
| Non-agricultural land consolidation | 3338.20 ± 675.30b | 4348.85 ± 922.49b | 0.87 ± 0.01a | 0.13 ± 0.04a | 7.01 ± 0.25b | 439.17 ± 166.72b | 0.96 ± 0.01a |
| Group | pH | Organic Matter (g/kg) | Available Phosphorus (mg/kg) | Available Potassium (μg/mL) | Total Nitrogen (g/kg) |
|---|---|---|---|---|---|
| Building ditches | 7.09 ± 0.63b | 46.63 ± 14.22b | 90.78 ± 83.78a | 29.65 ± 13.31a | 2.41 ± 0.73b |
| Land levelling | 7.23 ± 0.63a | 41.97 ± 15.33b | 72.85 ± 54.01b | 28.99 ± 12.00a | 2.20 ± 0.77b |
| Merging plots | 7.11 ± 0.57b | 41.52 ± 15.30b | 70.24 ± 57.77b | 28.60 ± 15.25a | 2.23 ± 0.77b |
| Applying organic fertilizers | 7.11 ± 0.64b | 52.49 ± 11.59a | 93.67 ± 84.50a | 31.38 ± 14.57a | 2.51 ± 0.64b |
| Comprehensive improvement | 6.93 ± 0.51b | 57.98 ± 11.66a | 79.80 ± 58.37b | 37.78 ± 7.61a | 3.08 ± 0.45a |
| Non-agricultural land consolidation | 7.38 ± 0.71a | 30.21 ± 9.80c | 53.43 ± 37.13c | 24.00 ± 14.93b | 1.69 ± 0.45c |
| Group | Catalase (mg/g) | Phosphatase (mg/g) | Urease (mg/g) |
|---|---|---|---|
| Building ditches | 207.66 ± 38.30a | 16.017 ± 8.44a | 0.25 ± 0.16a |
| Land levelling | 203.62 ± 38.48a | 17.35 ± 10.65a | 0.28 ± 0.22a |
| Merging plots | 201.61 ± 32.37a | 15.28 ± 9.97b | 0.23 ± 0.15a |
| Applying organic fertilizers | 198.61 ± 41.72a | 17.54 ± 9.68a | 0.25 ± 0.16a |
| Comprehensive improvement | 206.72 ± 28.98a | 20.88 ± 10.58a | 0.33 ± 0.16a |
| Non-agricultural land consolidation | 183.58 ± 50.72a | 12.32 ± 11.87c | 0.22 ± 0.30a |
| Unit: mg/kg | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cu | Cd | Pb | Cr | As | Hg | Ni | Zn | |
| Building ditches | 45.95 ± 12.74 | 2.28 ± 0.23 | 43.39 ± 13.23 | 214.65 ± 11.67 | 4.66 ± 3.83 | 0.57 ± 0.28 | 53.15 ± 5.60 | 125.75 ± 26.70 |
| Land levelling | 39.44 ± 7.92 | 2.23 ± 0.27 | 37.54 ± 12.81 | 214.61 ± 11.34 | 4.21 ± 3.51 | 0.45 ± 0.15 | 54.06 ± 7.63 | 110.47 ± 18.39 |
| Merging plots | 41.72 ± 8.83 | 2.27 ± 0.33 | 37.62 ± 12.06 | 212.64 ± 11.22 | 4.99 ± 3.91 | 0.53 ± 0.25 | 54.55 ± 7.44 | 112.51 ± 21.58 |
| Applying organic fertilizers | 46.55 ± 12.25 | 2.27 ± 0.31 | 42.98 ± 10.38 | 216.45 ± 11.12 | 3.89 ± 2.90 | 0.53 ± 0.21 | 55.00 ± 6.86 | 128.36 ± 24.72 |
| Comprehensive improvement | 39.90 ± 9.17 | 2.28 ± 0.13 | 40.29 ± 14.98 | 208.70 ± 6.59 | 2.93 ± 3.94 | 0.47 ± 0.14 | 51.10 ± 6.93 | 117.82 ± 20.45 |
| Non-agricultural land consolidation | 48.97 ± 6.71 | 3.30 ± 0.55 | 46.83 ± 8.81 | 252.96 ± 11.05 | 7.88 ± 5.29 | 0.65 ± 0.37 | 64 ± 10.85 | 136.82 ± 21.17 |
| Maximum | 84 | 3.98 | 77.5 | 268.8 | 14.4 | 1.38 | 80.4 | 198 |
| Minimum | 25 | 1.57 | 20 | 195 | 0.81 | 0.1 | 39 | 70.6 |
| Average value | 45.17 | 2.5 | 41.94 | 223.79 | 5.29 | 0.55 | 56.77 | 123.27 |
| Variation coefficient (%) | 22.38 | 22.86 | 26.4 | 8.72 | 77.91 | 49.99 | 15.96 | 20.7 |
| Background value | 22.6 | 0.17 | 35.7 | 56 | 6.9 | 0.17 | 23.9 | 83.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Ye, Y.; Liu, S.; Wen, J.; Chen, D. Effect Mechanism of Land Consolidation on Soil Bacterial Community: A Case Study in Eastern China. Int. J. Environ. Res. Public Health 2022, 19, 845. https://doi.org/10.3390/ijerph19020845
Lin Y, Ye Y, Liu S, Wen J, Chen D. Effect Mechanism of Land Consolidation on Soil Bacterial Community: A Case Study in Eastern China. International Journal of Environmental Research and Public Health. 2022; 19(2):845. https://doi.org/10.3390/ijerph19020845
Chicago/Turabian StyleLin, Yaoben, Yanmei Ye, Shuchang Liu, Jiahao Wen, and Danling Chen. 2022. "Effect Mechanism of Land Consolidation on Soil Bacterial Community: A Case Study in Eastern China" International Journal of Environmental Research and Public Health 19, no. 2: 845. https://doi.org/10.3390/ijerph19020845
APA StyleLin, Y., Ye, Y., Liu, S., Wen, J., & Chen, D. (2022). Effect Mechanism of Land Consolidation on Soil Bacterial Community: A Case Study in Eastern China. International Journal of Environmental Research and Public Health, 19(2), 845. https://doi.org/10.3390/ijerph19020845





