MiXie, an Online Tool for Better Health Assessment of Workers Exposed to Multiple Chemicals
Abstract
:1. Introduction
2. MiXie
2.1. History
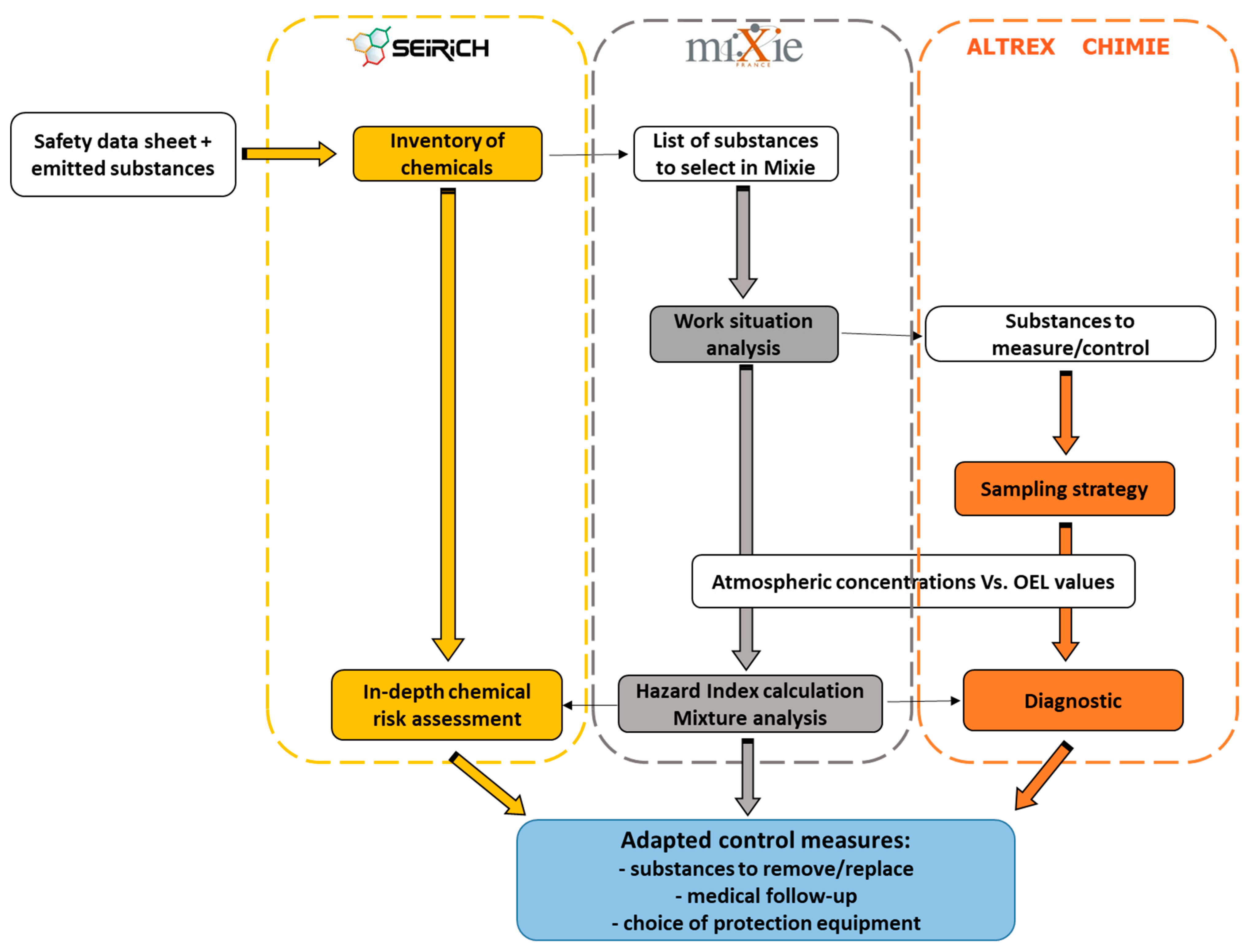
2.2. How MiXie Works
3. Results/Examples of Application Using the French Version of MiXie
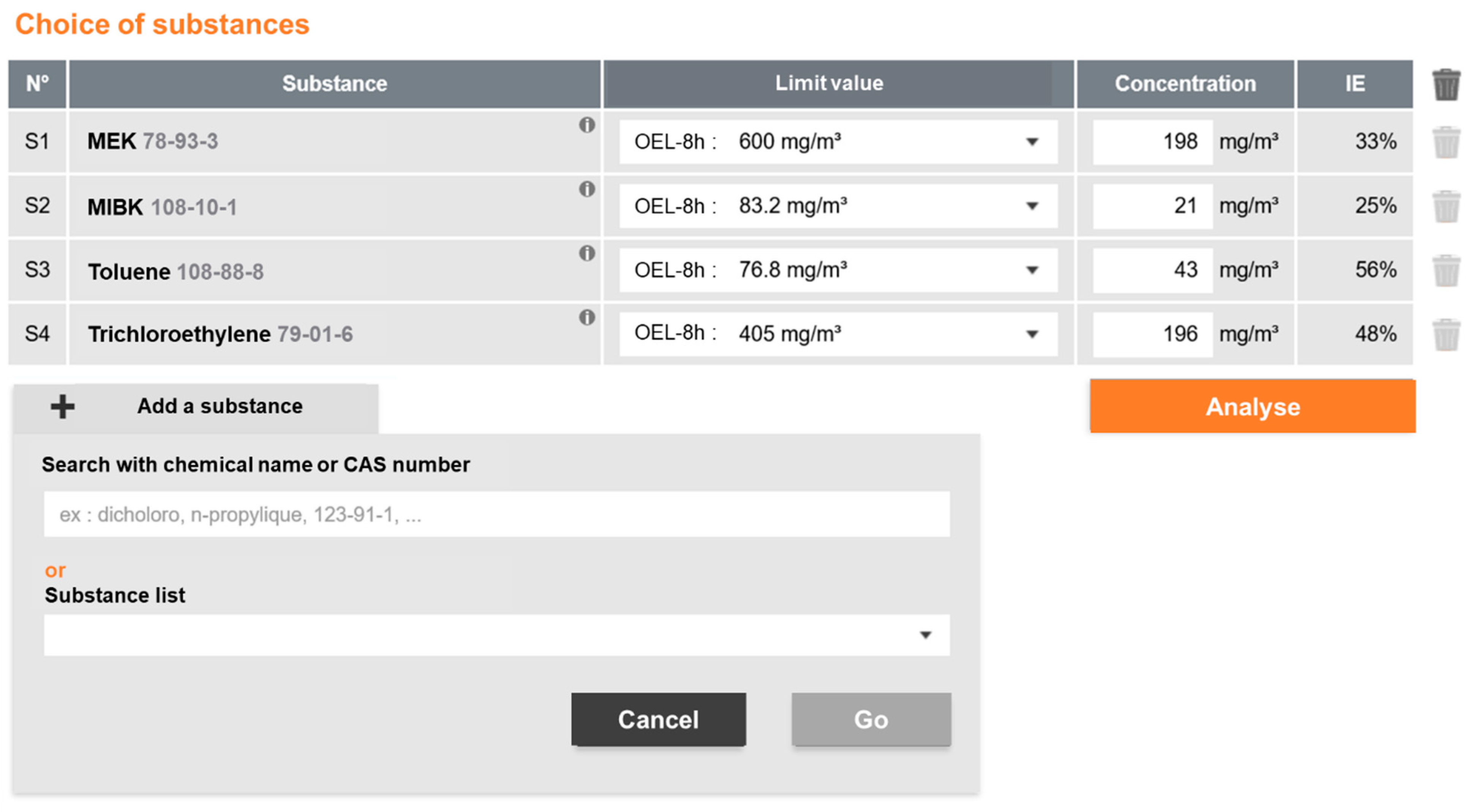
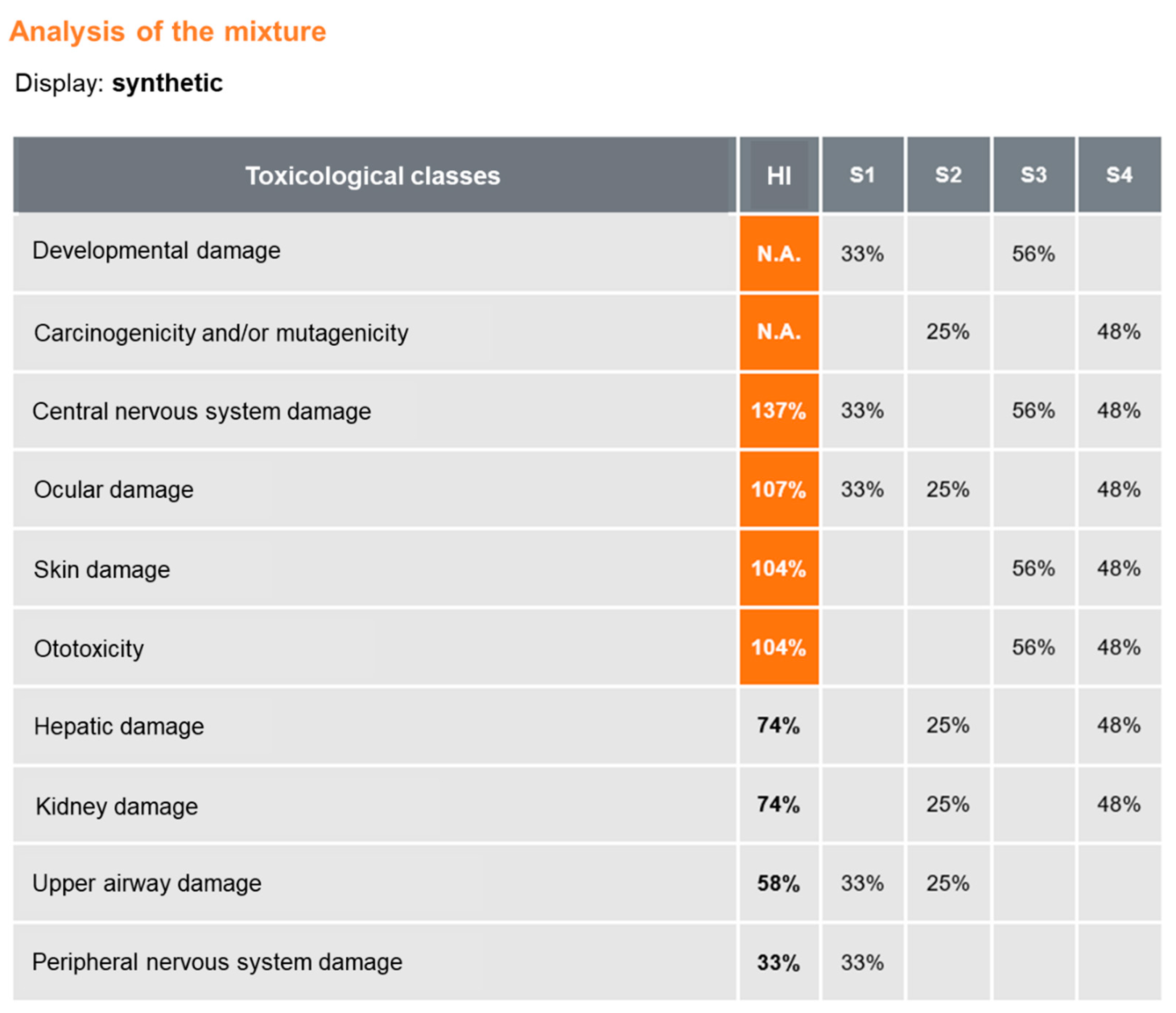
3.1. MiXie in Quantitative Mode: Case Study of a Printing Workstation
3.2. MiXie in Qualitative Mode: Case Study of Nail Salons
- Four substances affect development of the foetus, embryo, and/or child.
- One substance is carcinogenic and/or mutagenic.
- One substance is a sensitizing chemical.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melnick, R.; Lucier, G.; Wolfe, M.; Hall, R.; Stancel, G.; Prins, G. Summary of the National Toxicology Program’s report of the Endocrine Disruptors low-dose peer review. Environ. Health Perspect. 2002, 110, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Delfosse, V.; Huet, T.; Harrus, D.; Granell, M.; Bourguet, M.; Gardia-Parège, C.; Chiavarina, B.; Grimaldi, M.; Le Mével, S.; Blanc, P. Mechanistic insights into the synergistic activation of the RXR-PXR heterodimer by endocrine disruptor mixtures. Proc. Natl. Acad. Sci. USA 2021, 118, 1–10. [Google Scholar] [CrossRef]
- Memmi, S.; Rosankis, E.; Sandret, N.; Duprat, P.; Leonard, M.; Morand, S.; Tassy, V. Premiers résultats de l’enquête SUMER 2017: Comment ont évolué les expositions des salariés aux risques professionnels sur les vingt dernières années? RST 2019, 159, 53–78. Available online: https://www.inrs.fr/media.html?refINRS=TF%20273 (accessed on 22 December 2021).
- Havet, N.; Penot, A.; Morelle, M.; Perrier, L.; Charbotel, B.; Fervers, B. Varied exposure to carcinogenic, mutagenic, and reprotoxic (CMR) chemicals in occupational settings in France. Int. Arch. Occup. Environ. Health 2017, 90, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Clerc, F.; Bertrand, N.J.H.; La Rocca, B. Taking Multiple Exposure Into Account Can Improve Assessment of Chemical Risks. Ann. Work Expo. Health 2017, 62, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Bosson-Rieutort, D.; Sarazin, P.; Bicout, D.J.; Ho, V.; Lavoué, J. Occupational Co-exposures to Multiple Chemical Agents from Workplace Measurements by the US Occupational Safety and Health Administration. Ann. Work Expo. Health 2020, 64, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Labrèche, F.; Busque, M.A.; Roberge, B.; Champoux, D.; Duguay, P. Exposition des Travailleurs Québécois à des Cancérogènes: Industries et Groupes Professionnels; Rapport R-964; Institut de recherche Robert-Sauvé en santé et en sécurité du travail: Montréal, QC, Canada, 2017. [Google Scholar]
- Semple, S. Dermal exposure to chemicals in the workplace: Just how important is skin absorption? Occup. Environ. Med. 2004, 61, 376–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maddi, S.; Goud, T.; Srivastava, P. Cytochrome P450 enzymes, drug transporters and their role in pharmacokinetic drug-drug interactions of xenobiotics: A comprehensive review. Peertechz J. Med. Chem. Res. 2017, 3, 001–011. [Google Scholar] [CrossRef] [Green Version]
- Gerber, W.; Steyn, J.D.; Kotzé, A.F.; Hamman, J.H. Beneficial pharmacokinetic drug interactions: A tool to improve the bioavailability of poorly permeable drugs. Pharmaceutics 2018, 10, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Conference of Governmental Industrial Hygienists (ACGIH®). TLV/BEI Guidelines—ACGIH. Available online: https://www.acgih.org/science/tlv-bei-guidelines/ (accessed on 12 October 2021).
- U.S. Environmental Protection Agency (U.S EPA). Exposure Assessment Tools by Tiers and Types—Aggregate and Cumulative. Available online: https://www.epa.gov/expobox/exposure-assessment-tools-tiers-and-types-aggregate-and-cumulative (accessed on 12 October 2021).
- Schaaper, J.E.; Hertzberg, R.C.; Simmons, J.E.; Mumtaz, M.M.; Rice, G.E. Chemical mixtures: Toxicologic interactions and risk assessment. In Information Resources in Toxicology, 5th ed.; Wexler, P., Ed.; Elsevier: Houston, TX, USA, 2020; pp. 401–414. [Google Scholar]
- Hernandez, A.F.; Tsatsakis, A.M. Human exposure to chemical mixtures: Challenges for the integration of toxicology with epidemiology data in risk assessment. Food Chem. Toxicol. 2017, 103, 188–193. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Note Intention 2021/Request for Support to the Impact Assessment on the Planned Revision of the REACH Regulation; European Commission: Brussels, Belgium, 2021. [Google Scholar]
- Hernandez, A.F.; Buha, A.; Constantin, C.; Wallace, D.R. Critical assessment and integration of separate lines of evidence for risk assessment of chemical mixtures. Arch. Toxicol. 2019, 93, 2741–2757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Règlement sur la santé et la sécurité du travail (Chapitre S-2.1, r. 13). Règlement sur la Santé et la Sécurité du Travail. Available online: http://www.legisquebec.gouv.qc.ca/fr/document/rc/S-2.1,%20r.%2013 (accessed on 12 October 2021).
- Langlois, E.; Mélin, S.; Oury, B.; Redaelli, M. Soin et décoration des ongles: État des lieux des expositions au risque chimique. HST 2018, 251, 54–60. [Google Scholar]
- Niedhammer, I.; Lesuffleur, T.; Labarthe, G.; Chastang, J.F. Role of working conditions in the explanation of occupational inequalities in work injury: Findings from the national French SUMER survey. BMC Public Health 2018, 18, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Bertin, M.; Nguyen, T.H.Y.; Bonvallot, N.; Bodin, J. Occupational co-exposure to biomechanical factors and neurotoxic chemicals in a representative sample of French employees. J. Occup. Health 2020, 62, 12090–12096. [Google Scholar] [CrossRef] [PubMed]
- Rietjens, S.J.; de Lange, D.W.; Meulenbelt, J. Ethylene glycol or methanol intoxication: Which antidote should be used, fomepizole or ethanol? Neth. J. Med. 2014, 72, 73–79. [Google Scholar] [PubMed]
- Lataye, R.; Campo, P. Combined effects of a simultaneous exposure to noise and toluene on hearing function. Neurotoxicol. Teratol. 1997, 19, 373–382. [Google Scholar] [CrossRef]
- Campo, P.; Morata, T.C.; Hong, O. Chemical exposure and hearing loss. Dis. Mon. 2015, 59, 119–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vyskocil1, A.; Drolet, D.; El Majidi, N.; Lemay, F.; Aliyeva, E.; Baril, M.; Gagnon, F.; Audet, E.; Viau, C. Mise à Jour de L’utilitaire MIXIE, Interactions Toxicologiques; Université de Montréal: Montreal, QC, Canada, 2014. [Google Scholar]
- Bopp, S.K.; Kienzler, A.; Richarz, A.N.; ven der Linden, S.C. Regulatory assessment and risk management of chemical mixtures: Challenges and ways forward. Crit. Rev. Toxicol. 2019, 49, 174–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavoué, J.; Joseph, L.; Knott, P.; Davies, H.; Labrèche, F.; Clerc, F.; Mater, G.; Kirkham, T. Expostats: A Bayesian Toolkit to Aid the Interpretation of Occupational Exposure Measurements. Ann. Work Expo. Health 2019, 63, 267–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Toxicological Class | Toxic Effects | Toxicological Class | Toxic Effects |
|---|---|---|---|
| Ocular damage | Cataract Eye irritation Corneal edema Corneal necrosis | Cardiovascular damage | Cardiac damage Vascular system impairment Vasoconstriction Vasodilatation Other cardiovascular damage |
| Upper airway damage | Upper airway irritation Perforation of the nasal septum Other upper airway damage | Autonomic nervous system damage | Cholinesterase inhibition Muscular stimulation Other autonomic nervous system damage |
| Lower airway damage | Berylliosis Bronchitis Bronchopneumonia Pulmonary emphysema Pulmonary fibrosis Brazier’s disease Lower airway irritation Pulmonary edema Pneumoconiosis Other lower airway damage | Disruption of oxygen transport | Anemia Asphyxia Carboxyhemoglobinemia Blood-forming system disorder Hemolysis Cytochrome oxidase inhibition Heme synthesis inhibition Methemoglobinemia |
| Central nervous system damage | Central nervous system convulsion Central nervous system depression Other central nervous system damage | Peripheral nervous system damage | Peripheral neuropathy Other peripheral nervous system damage |
| Hematopoietic system disruption | Agranulocytosis Anemia Medullar aplasia Leukopenia Neutropenia Pancytopenia Thrombocytosis Thrombopenia Blood coagulation disorder | Ototoxicity | Cochlear damage Auditory nerve damage Vestibular damage Hyperacusis |
| Metabolic acidosis | Metabolic acidosis | Stimulation of basal metabolism | |
| Dental or bone damage | Bone damage Skeletal fluorosis Dental erosion Other dental or bone damage | Skin damage | Alopecia Chloracne Skin irritation Other skin damage (except sensitization) |
| Endocrine disrupter # | Antithyroid effect Other endocrine disrupter effect | Male reproductive system damage # | Testicular damage Impairment of male fertility Other male reproductive system damage |
| Immune system damage | Immune system damage | Female reproductive system damage # | Ovarian damage Impairment of female fertility Other female reproductive system damage |
| Hepatic damage | Liver necrosis Other hepatic damage | Spleen damage | Spleen damage |
| Developmental damage # | Embryonic damage Fetal damage Teratogenic effect Effect on offspring Mutagenic effect on germ cells Effect on offspring behavior Other developmental damage | Carcinogenicity and/or mutagenicity # | Bladder cancer Blood vessel cancer Laryngeal cancer Leukemia Liver cancer Lung cancer Mesothelioma Nasal cancer Nasopharyngeal cancer Prostate cancer Renal cancer Stomach cancer Sinonasal cancer Skin cancer Testicular cancer Upper respiratory tract cancer Mutagenic effect |
| Kidney damage | Glomerular damage Tubular damage Bladder damage Other kidney damage | Sensitization (skin or respiratory) # | Asthma Respiratory sensitization Contact dermatitis Skin sensitization |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Rocca, B.; Sarazin, P. MiXie, an Online Tool for Better Health Assessment of Workers Exposed to Multiple Chemicals. Int. J. Environ. Res. Public Health 2022, 19, 951. https://doi.org/10.3390/ijerph19020951
La Rocca B, Sarazin P. MiXie, an Online Tool for Better Health Assessment of Workers Exposed to Multiple Chemicals. International Journal of Environmental Research and Public Health. 2022; 19(2):951. https://doi.org/10.3390/ijerph19020951
Chicago/Turabian StyleLa Rocca, Bénédicte, and Philippe Sarazin. 2022. "MiXie, an Online Tool for Better Health Assessment of Workers Exposed to Multiple Chemicals" International Journal of Environmental Research and Public Health 19, no. 2: 951. https://doi.org/10.3390/ijerph19020951





