Evaluation of the Toxicity of Bisphenol A in Reproduction and Its Effect on Fertility and Embryonic Development in the Zebrafish (Danio rerio)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substance Preparation
2.2. Determination of BPA in the Water and in the Fish
2.3. Experiment Groups
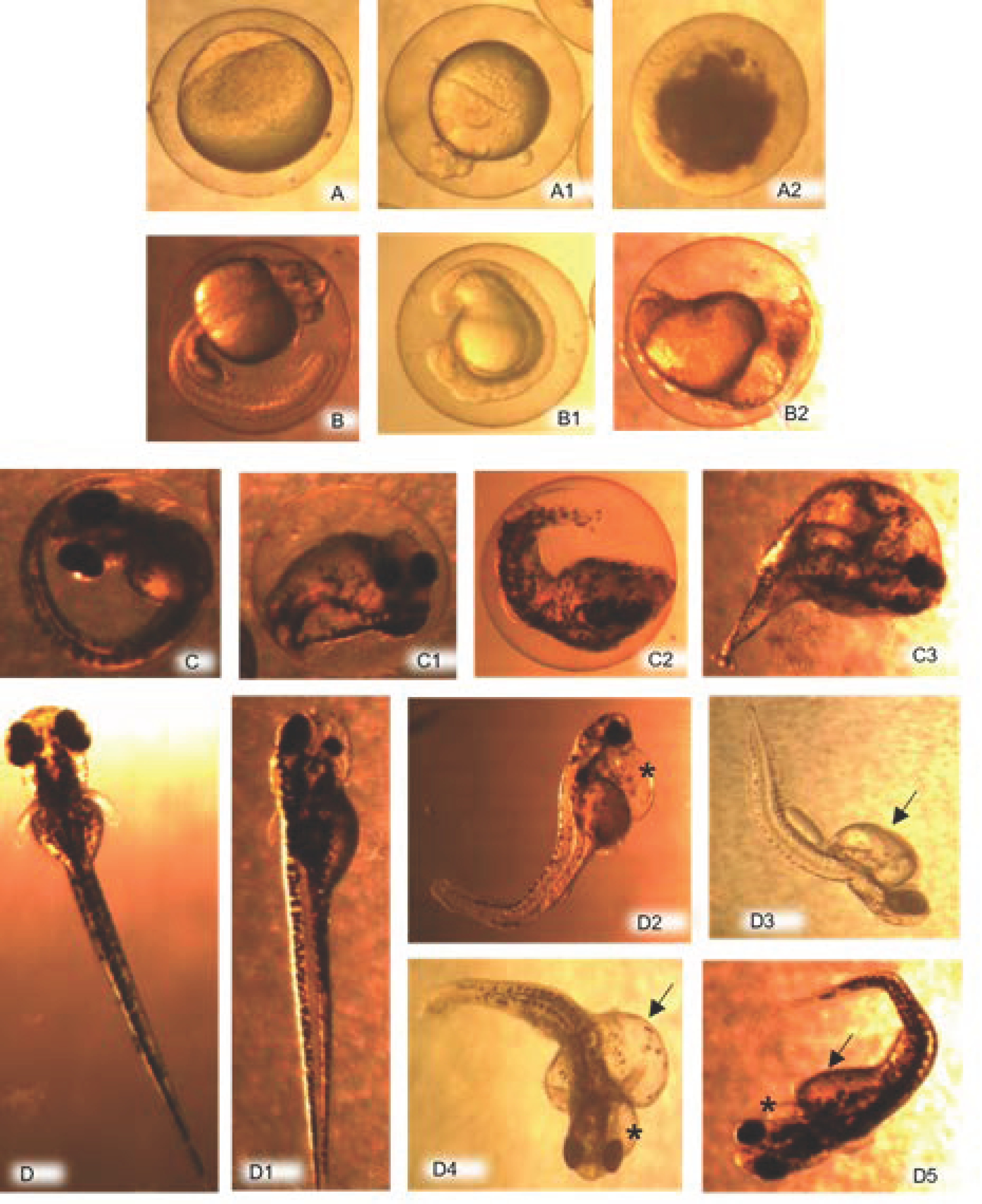
2.4. Evaluation of Embryos
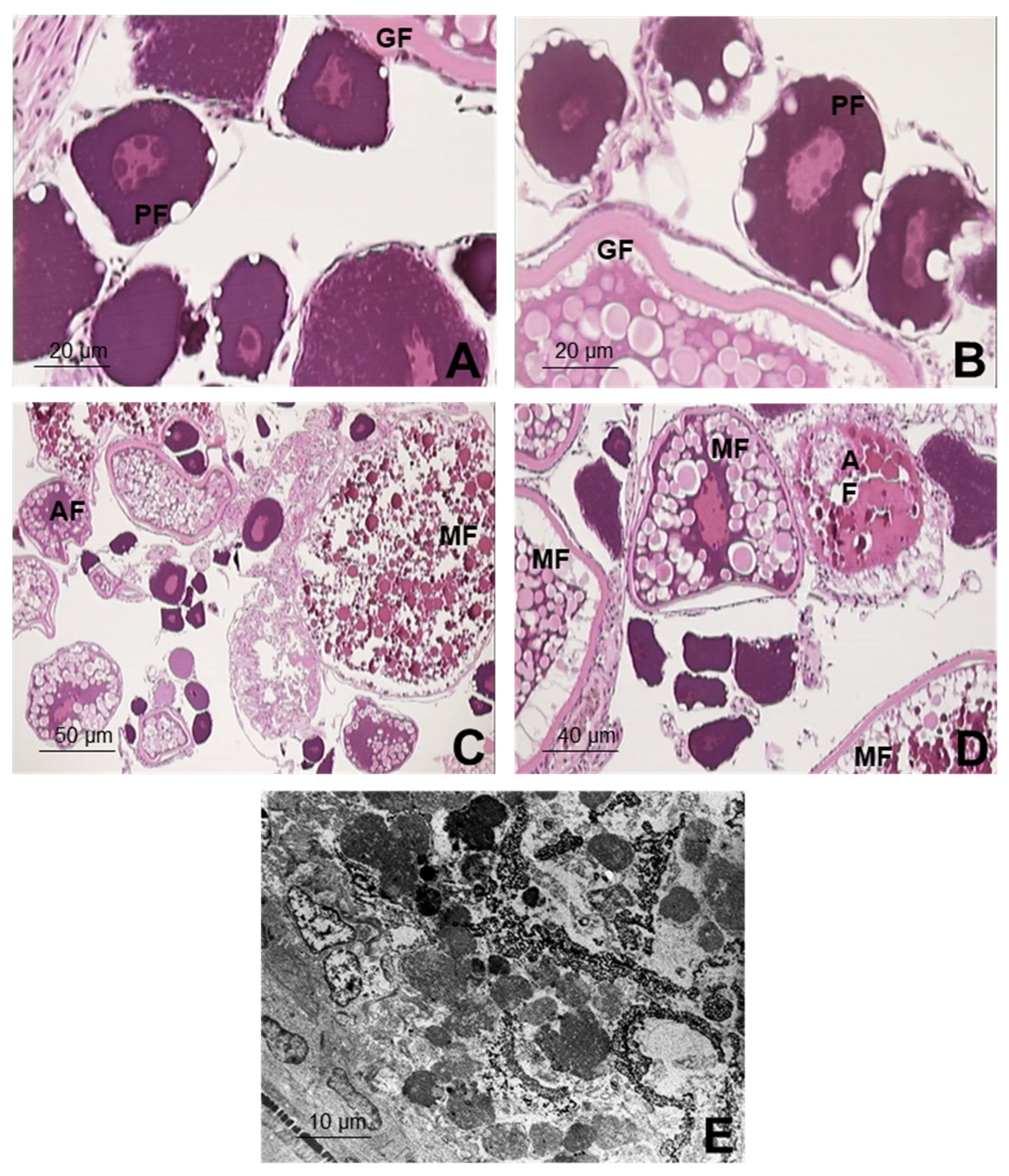
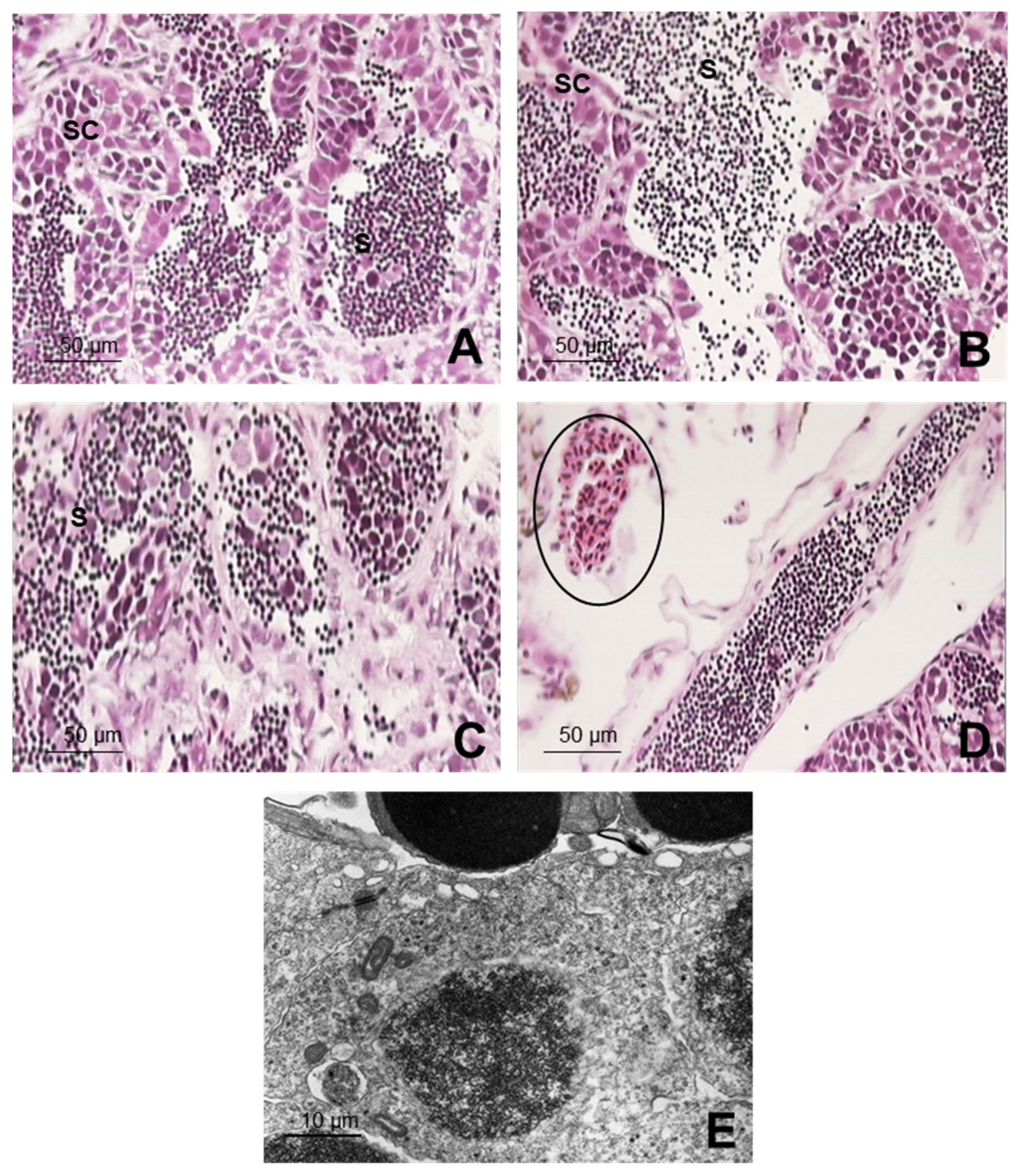
2.5. Structural and Ultrastructural Histopathological Study
2.6. Statistical Analysis
3. Results
3.1. BPA in the Water and in the Fish
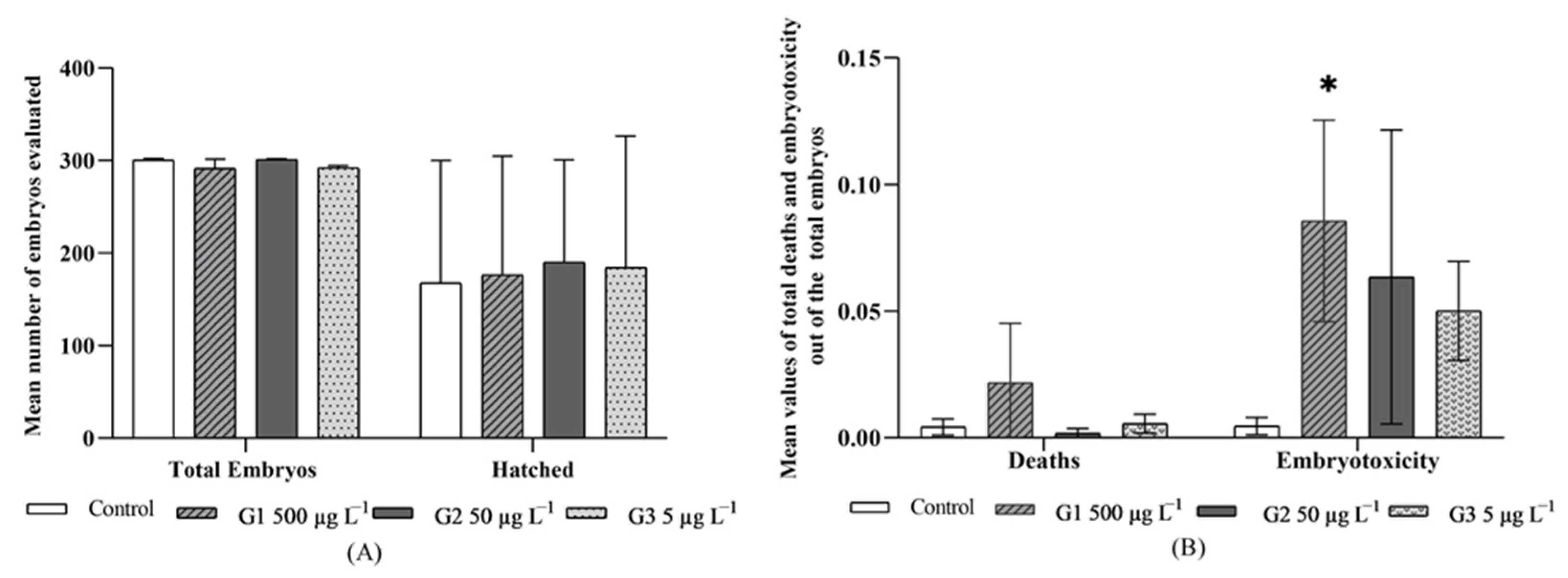
3.2. Embryo Evaluation
3.3. Structural and Ultrastructural Histopathological Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoekstra, E.J.; Simoneau, C. Release of Bisphenol A from Polycarbonate-A Review. Crit. Rev. Food Sci. Nutr. 2013, 53, 386–402. [Google Scholar] [CrossRef]
- Michałowicz, J. Bisphenol A—Sources, Toxicity and Biotransformation. Environ. Toxicol. Pharmacol. 2014, 37, 738–758. [Google Scholar] [CrossRef]
- PlasticsEurope—Association of Plastics Manufacturers Plastic—The Facts 2020: An Analysis of European Plastics Production, Demand and Waste Data. Available online: https://www.plasticseurope.org/en/resources/publications/4312-plastics-facts-2020 (accessed on 16 June 2021).
- Grand View Research Bisphenol A Market Size, Share & Trends Analysis Report by Application (Polycarbonates, Epoxy Resins) by Region, and Segment Forecasts, 2018–2025. Available online: https://www.grandviewresearch.com/industry-analysis/bisphenol-a-bpa-market (accessed on 17 June 2021).
- Geens, T.; Neels, H.; Covaci, A. Distribution of Bisphenol-A, Triclosan and n-Nonylphenol in Human Adipose Tissue, Liver and Brain. Chemosphere 2012, 87, 796–802. [Google Scholar] [CrossRef]
- Schug, T.T.; Janesick, A.; Blumberg, B.; Heindel, J.J. Endocrine Disrupting Chemicals and Disease Susceptibility. J. Steroid Biochem. Mol. Biol. 2011, 127, 204–215. [Google Scholar] [CrossRef] [Green Version]
- Goto, Y.; Kitamura, S.; Kashiwagi, K.; Oofusa, K.; Tooi, O.; Yoshizato, K.; Sato, J.; Ohta, S.; Kashiwagi, A. Suppression of amphibian metamorphosis by bisphenol A and related chemical substances. J. Health Sci. 2006, 52, 160–168. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.H.; Katayama, Y.; Kondo, F. Biodegradation or Metabolism of Bisphenol A: From Microorganisms to Mammals. Toxicology 2006, 217, 81–90. [Google Scholar] [CrossRef]
- Dai, Y.J.; Jia, Y.F.; Chen, N.; Bian, W.P.; Li, Q.K.; Ma, Y.B.; Chen, Y.L.; Pei, D.S. Zebrafish as a Model System to Study Toxicology. Environ. Toxicol. Chem. 2014, 33, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Howe, D.G.; Bradford, Y.M.; Eagle, A.; Fashena, D.; Frazer, K.; Kalita, P.; Mani, P.; Martin, R.; Moxon, S.T.; Paddock, H.; et al. The Zebrafish Model Organism Database: New Support for Human Disease Models, Mutation Details, Gene Expression Phenotypes and Searching. Nucleic Acids Res. 2017, 45, D758–D768. [Google Scholar] [CrossRef] [Green Version]
- Busquet, F.; Strecker, R.; Rawlings, J.M.; Belanger, S.E.; Braunbeck, T.; Carr, G.J.; Cenijn, P.; Fochtman, P.; Gourmelon, A.; Hübler, N.; et al. OECD Validation Study to Assess Intra- and Inter-Laboratory Reproducibility of the Zebrafish Embryo Toxicity Test for Acute Aquatic Toxicity Testing. Regul. Toxicol. Pharmacol. 2014, 69, 496–511. [Google Scholar] [CrossRef] [PubMed]
- Noyes, P.D.; Garcia, G.R.; Tanguay, R.L. Zebrafish as an: In Vivo Model for Sustainable Chemical Design. Green Chem. 2016, 18, 6410–6430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beekhuijzen, M.; de Koning, C.; Flores-Guillén, M.E.; de Vries-Buitenweg, S.; Tobor-Kaplon, M.; van de Waart, B.; Emmen, H. From Cutting Edge to Guideline: A First Step in Harmonization of the Zebrafish Embryotoxicity Test (ZET) by Describing the Most Optimal Test Conditions and Morphology Scoring System. Reprod. Toxicol. 2015, 56, 64–76. [Google Scholar] [CrossRef]
- OECD—Organisation for Economic Cooperation and Development. OECD Guidelines for the Testing of Chemi CALS 236—Fish Embryo Acute Toxicity (FET) Test; OECD: Paris, France, 2013.
- OECD Revised Guidance Document 150 on Standardised Test Guidelines for Evaluating Chemicals for Endocrine Disruption. In OECD Series on Testing and Assessment; OECD Publishing: Paris, France, 2018; pp. 415–419.
- Selderslaghs, I.W.T.; van Rompay, A.R.; de Coen, W.; Witters, H.E. Development of a Screening Assay to Identify Teratogenic and Embryotoxic Chemicals Using the Zebrafish Embryo. Reprod. Toxicol. 2009, 28, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Pelayo, S.; Oliveira, E.; Thienpont, B.; Babin, P.J.; Raldúa, D.; André, M.; Piña, B. Triiodothyronine-Induced Changes in the Zebrafish Transcriptome during the Eleutheroembryonic Stage: Implications for Bisphenol A Developmental Toxicity. Aquat. Toxicol. 2012, 110–111, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xiao, Y.; Gai, Z.; Li, R.; Zhu, Z.; Bai, C.; Tanguay, R.L.; Xu, X.; Huang, C.; Dong, Q. Reproductive Toxicity of Low Level Bisphenol A Exposures in a Two-Generation Zebrafish Assay: Evidence of Male-Specific Effects. Aquat. Toxicol. 2015, 169, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Santangeli, S.; Consales, C.; Pacchierotti, F.; Habibi, H.R.; Carnevali, O. Transgenerational Effects of BPA on Female Reproduction. Sci. Total Environ. 2019, 685, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- Akhter, A.; Rahaman, M.; Suzuki, R.-T.; Murono, Y.; Tokumoto, T. Next-Generation and Further Transgenerational Effects of Bisphenol A on Zebrafish Reproductive Tissues. Heliyon 2018, 4, 788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Directive 2020/2184 of the European Parliament and of the council of 16 December 2020 on the quality of water intended for human consumption. Off. J. Eur. Union 2020, 63, L435.
- EFSA—European Food Safety Authority Bisphenol, A. 2021. Available online: https://www.efsa.europa.eu/en/topics/topic/bisphenol (accessed on 17 October 2021).
- vom Saal, F.S.; Welshons, W.V. Large Effects from Small Exposures. II. The Importance of Positive Controls in Low-Dose Research on Bisphenol A. Environ. Res. 2006, 100, 50–76. [Google Scholar] [CrossRef]
- Wetherill, Y.B.; Akingbemi, B.T.; Kanno, J.; McLachlan, J.A.; Nadal, A.; Sonnenschein, C.; Watson, C.S.; Zoeller, R.T.; Belcher, S.M. In Vitro Molecular Mechanisms of Bisphenol A Action. Reprod. Toxicol. 2007, 24, 178–198. [Google Scholar] [CrossRef]
- Ji, K.; Hong, S.; Kho, Y.; Choi, K. Effects of Bisphenol S Exposure on Endocrine Functions and Reproduction of Zebrafish. Environ. Sci. Technol. 2013, 47, 8793–8800. [Google Scholar] [CrossRef]
- Schmidt, K.; Stachel, C.; Gowik, P. Development and In-House Validation of an LC-MS/MS Method for the Determination of Stilbenes and Resorcylic Acid Lactones in Bovine Urine. Anal. Bioanal. Chem. 2008, 391, 1199–1210. [Google Scholar] [CrossRef]
- Molina, A.M.; Abril, N.; Morales-Prieto, N.; Monterde, J.G.; Lora, A.J.; Ayala, N.; Moyano, R. Evaluation of Toxicological Endpoints in Female Zebrafish after Bisphenol A Exposure. Food Chem. Toxicol. 2018, 112, 19–25. [Google Scholar] [CrossRef]
- OECD—Organisation for Economic Cooperation and Development. OECD Guideline for the Testing of Chemicals 229—Fish Short Term Reproduction Assay; OCDE: Paris, France, 2012.
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Off. J. Eur. Union 2010, 53, L276.
- Brand, M.; Granato, M.; Nüsslein-Volhard, C. Chapter 1 Keeping and Raising Zebrafish. Nusslein-Volhard, C., Dahm, R., Eds.; Oxford University Press: Oxford, UK, 2002; pp. 7–24. [Google Scholar]
- Lin, T.; Chen, Y.Q.; Chen, W. Impact of Toxicological Properties of Sulfonamides on the Growth of Zebrafish Embryos in the Water. Environ. Toxicol. Pharmacol. 2013, 36, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- GraphPadPrism; Version 9.0.0; GraphPad Software Inc.: San Diego, CA, USA, 2020.
- Richter, C.A.; Birnbaum, L.S.; Farabollini, F.; Newbold, R.R.; Rubin, B.S.; Talsness, C.E.; Vandenbergh, J.G.; Walser-Kuntz, D.R.; vom Saal, F.S. In Vivo Effects of Bisphenol A in Laboratory Rodent Studies. Reprod. Toxicol. 2007, 24, 199–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canesi, L.; Fabbri, E. Environmental Effects of BPA: Focus on Aquatic Species. Dose-Response 2015, 13, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, E.; Genco, M.C.; Megrelis, L.; Ruderman, J.V. Effects of Bisphenol A and Triclocarban on Brain-Specific Expression of Aromatase in Early Zebrafish Embryos. Proc. Natl. Acad. Sci. USA 2011, 108, 17732–17737. [Google Scholar] [CrossRef] [Green Version]
- Bhandari, R.K.; Deem, S.L.; Holliday, D.K.; Jandegian, C.M.; Kassotis, C.D.; Nagel, S.C.; Tillitt, D.E.; vom Saal, F.S.; Rosenfeld, C.S. Effects of the Environmental Estrogenic Contaminants Bisphenol A and 17α-Ethinyl Estradiol on Sexual Development and Adult Behaviors in Aquatic Wildlife Species. Gen. Comp. Endocrinol. 2015, 214, 195–219. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.H.; Hlaing, M.M.; Zhang, X.; Yan, C.; Duan, Z.; Zhu, L.; Ung, C.Y.; Mathavan, S.; Ong, C.N.; Gong, Z. Toxicogenomic and Phenotypic Analyses of Bisphenol-a Early-Life Exposure Toxicity in Zebrafish. PLoS ONE 2011, 6, e28273. [Google Scholar] [CrossRef] [Green Version]
- Tišler, T.; Krel, A.; Gerželj, U.; Erjavec, B.; Dolenc, M.S.; Pintar, A. Hazard Identification and Risk Characterization of Bisphenols A, F and AF to Aquatic Organisms. Environ. Pollut. 2016, 212, 472–479. [Google Scholar] [CrossRef]
- Duan, Z.; Zhu, L.; Zhu, L.; Kun, Y.; Zhu, X. Individual and Joint Toxic Effects of Pentachlorophenol and Bisphenol A on the Development of Zebrafish (Danio rerio) Embryo. Ecotoxicol. Environ. Saf. 2008, 71, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Gibert, Y.; Sassi-Messai, S.; Fini, J.B.; Bernard, L.; Zalko, D.; Cravedi, J.P.; Balaguer, P.; Andersson-Lendahl, M.; Demeneix, B.; Laudet, V. Bisphenol A Induces Otolith Malformations during Vertebrate Embryogenesis. BMC Dev. Biol. 2011, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, X.; Huang, Y.; Li, X.; Lei, Y.; Teng, M.; Li, X.; Wang, C.; Li, Y. Developmental Effects and Estrogenicity of Bisphenol A Alternatives in a Zebrafish Embryo Model. Environ. Sci. Technol. 2018, 52, 3222–3231. [Google Scholar] [CrossRef] [PubMed]
- Aluru, N.A.; Leatherland, J.F.; Vijayan, M.M. Bisphenol A in Oocytes Leads to Growth Suppression and Altered Stress Performance in Juvenile Rainbow Trout. PLoS ONE 2010, 5, e1074. [Google Scholar] [CrossRef] [Green Version]
- Wolstenholme, J.T.; Rissman, E.F.; Connelly, J.J. The Role of Bisphenol A in Shaping the Brain, Epigenome and Behavior. Horm. Behav. 2011, 59, 296–305. [Google Scholar] [CrossRef] [Green Version]
- Xin, F.; Susiarjo, M.; Bartolomei, M.S. Multigenerational and Transgenerational Effects of Endocrine Disrupting Chemicals: A Role for Altered Epigenetic Regulation? Semin. Cell Dev. Biol. 2015, 43, 66–75. [Google Scholar] [CrossRef] [Green Version]
- Matozzo, V.; Gagné, F.; Marin, M.G.; Ricciardi, F.; Blaise, C. Vitellogenin as a Biomarker of Exposure to Estrogenic Compounds in Aquatic Invertebrates: A Review. Environ. Int. 2008, 34, 531–545. [Google Scholar] [CrossRef]
- Tse, W.K.F.; Yeung, B.H.Y.; Wan, H.T.; Wong, C.K.C. Early Embryogenesis in Zebrafish Is Affected by Bisphenol A Exposure. Biol. Open 2013, 2, 466–471. [Google Scholar] [CrossRef] [Green Version]
- Cabaton, N.J.; Wadia, P.R.; Rubin, B.S.; Zalko, D.; Schaeberle, C.M.; Askenase, M.H.; Gadbois, J.L.; Tharp, A.P.; Whitt, G.S.; Sonnenschein, C.; et al. Perinatal Exposure to Environmentally Relevant Levels of Bisphenol A Decreases Fertility and Fecundity in CD-1 Mice. Environ. Health Perspect. 2011, 119, 547–552. [Google Scholar] [CrossRef] [Green Version]
- Markey, C.M.; Wadia, P.R.; Rubin, B.S.; Sonnenschein, C.; Soto, A.M. Long-Term Effects of Fetal Exposure to Low Doses of the Xenoestrogen Bisphenol-A in the Female Mouse Genital Tract 1. Biol. Reprod. 2005, 72, 1344–1351. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-De-Toro, M.; Markey, C.M.; Wadia, P.R.; Luque, E.H.; Rubin, B.S.; Sonnenschein, C.; Soto, A.M. Perinatal Exposure to Bisphenol-A Alters Peripubertal Mammary Gland Development in Mice. Endocrinology 2005, 146, 4138–4147. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Itoh, K.; Yaoi, T.; Fujiwara, Y.; Sugimoto, T.; Fushiki, S. Murine Neocortical Histogenesis Is Perturbed by Prenatal Exposure to Low Doses of Bisphenol A. J. Neurosci. Res. 2006, 84, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, M.D. Composition, Accumulation and Utilization of Yolk Lipids in Teleost Fish; Chapman & Hall: London, UK, 1996; Volume 6. [Google Scholar]
- Biran, J.; Levavi-Sivan, B. Endocrine Control of Reproduction, Fish. In Encyclopedia of Reproduction; Elsevier: Amsterdam, The Netherlands, 2018; pp. 362–368. ISBN 9780128151457. [Google Scholar]
- Flint, S.; Markle, T.; Thompson, S.; Wallace, E. Bisphenol A Exposure, Effects, and Policy: A Wildlife Perspective. J. Environ. Manag. 2012, 104, 19–34. [Google Scholar] [CrossRef]
- Wang, T.; Han, J.; Duan, X.; Xiong, B.; Cui, X.-S.; Kim, N.-H.; Liu, H.-L.; Sun, S.-C. The Toxic Effects and Possible Mechanisms of Bisphenol A on Oocyte Maturation of Porcine in Vitro. Oncotarget 2016, 7, 32554. [Google Scholar] [CrossRef] [Green Version]
- Valdebenito, I.; Paiva, L.; Berland, M. Atresia Folicular en Peces Teleósteos: Una Revisión / Follicular Atresia in Teleost Fish: A Review. Arch. Med. Vet. 2011, 43, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Abdollahpour, H.; Falahatkar, B.; Jafari, N.; Lawrence, C. Effect of Stress Severity on Zebrafish (Danio rerio) Growth, Gonadal Development and Reproductive Performance: Do Females and Males Respond Differently? Aquaculture 2020, 522, 735099. [Google Scholar] [CrossRef]
- Miura, T.; Miura, C.; Ohta, T.; Nader, M.R.; Todo, T.; Yamauchi, K. Estradiol-17β Stimulates the Renewal of Spermatogonial Stem Cells in Males. Biochem. Biophys. Res. Commun. 1999, 264, 230–234. [Google Scholar] [CrossRef]
- Schulz, R.W.; de França, L.R.; Lareyre, J.J.; LeGac, F.; Chiarini-Garcia, H.; Nobrega, R.H.; Miura, T. Spermatogenesis in Fish. Gen. Comp. Endocrinol. 2010, 165, 390–411. [Google Scholar] [CrossRef]
- Lahnsteiner, F.; Berger, B.; Kletzl, M.; Weismann, T. Effect of Bisphenol A on Maturation and Quality of Semen and Eggs in the Brown Trout, Salmo Trutta f. Fario. Aquat. Toxicol. 2005, 75, 213–224. [Google Scholar] [CrossRef]
- Sohoni, P.; Tyler, C.R.; Hurd, K.; Caunter, J.; Hetheridge, M.; Williams, T.; Woods, C.; Evans, M.; Toy, R.; Gragas, M.; et al. Reproductive Effects of Long-Term Exposure to Bisphenol A in the Fathead Minnow (Pimephales promelas). Environ. Sci. Technol. 2001, 35, 2917–2925. [Google Scholar] [CrossRef]
| BPA Concentration in Water (µg L−1) | ||||
|---|---|---|---|---|
| Exposure Days | G1 (500 µg L−1) | G2 (50 µg L−1) | G3 (5 µg L−1) | Control |
| 7 | 484.871 ± 0.001 | 47.312 ± 0.003 | 4.545 ± 0.005 | nd * |
| 14 | 482.442 ± 0.004 | 48.449 ± 0.012 | 4.694 ± 0.008 | nd * |
| 21 | 480.757 ± 0.002 | 47.357 ± 0.008 | 4.753 ± 0.011 | nd * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis, L.d.P.G.; Lora-Benítez, A.J.; Molina-López, A.M.; Mora-Medina, R.; Ayala-Soldado, N.; Moyano-Salvago, M.d.R. Evaluation of the Toxicity of Bisphenol A in Reproduction and Its Effect on Fertility and Embryonic Development in the Zebrafish (Danio rerio). Int. J. Environ. Res. Public Health 2022, 19, 962. https://doi.org/10.3390/ijerph19020962
Reis LdPG, Lora-Benítez AJ, Molina-López AM, Mora-Medina R, Ayala-Soldado N, Moyano-Salvago MdR. Evaluation of the Toxicity of Bisphenol A in Reproduction and Its Effect on Fertility and Embryonic Development in the Zebrafish (Danio rerio). International Journal of Environmental Research and Public Health. 2022; 19(2):962. https://doi.org/10.3390/ijerph19020962
Chicago/Turabian StyleReis, Lilian de Paula Gonçalves, Antonio Jesús Lora-Benítez, Ana Mª Molina-López, Rafael Mora-Medina, Nahúm Ayala-Soldado, and Mª del Rosario Moyano-Salvago. 2022. "Evaluation of the Toxicity of Bisphenol A in Reproduction and Its Effect on Fertility and Embryonic Development in the Zebrafish (Danio rerio)" International Journal of Environmental Research and Public Health 19, no. 2: 962. https://doi.org/10.3390/ijerph19020962






