Impact of COVID-19 Pandemic Exacerbation of Depressive Symptoms for Social Frailty from the ORANGE Registry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Criteria for the Diagnosis of Social Frailty
2.3. Assessment and Outcome
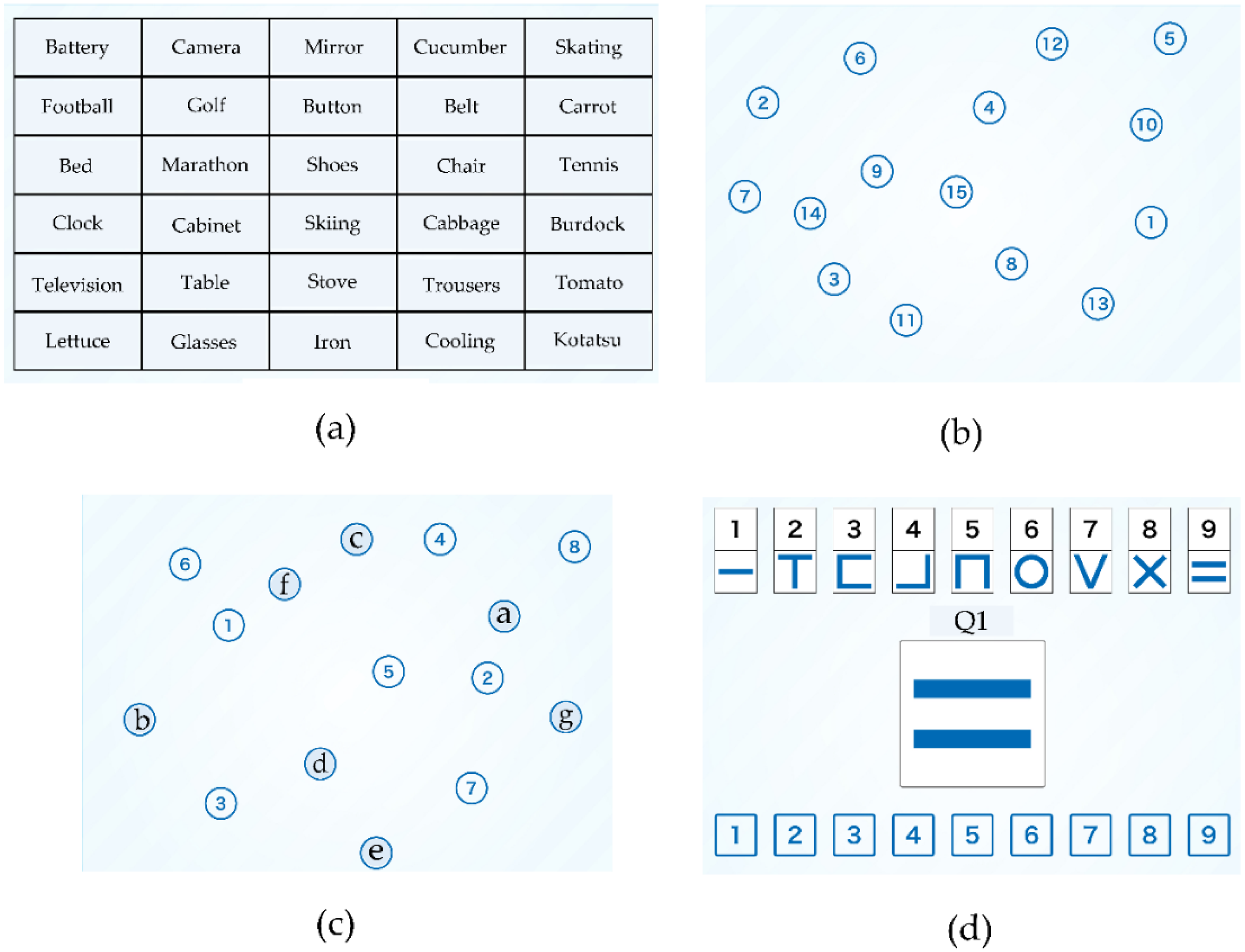
2.3.1. Components of NCGG-FAT
2.3.2. Components of TDAS
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baker, M.G.; Wilson, N.; Anglemyer, A. Successful Elimination of Covid-19 Transmission in New Zealand. N. Engl. J. Med. 2020, 383, e56. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.K.; Akl, E.A.; Duda, S.; Solo, K.; Yaacuab, S.; Schunemann, H.J. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: A systematic review and meta-analysis. Lancet 2020, 395, 1973–1987. [Google Scholar] [CrossRef]
- el-Zoghby, S.M.; Soltan, E.M.; Salama, H.M. Impact of the COVID-19 Pandemic on Mental Health and Social Support among Adult Egyptians. J. Community Health 2020, 45, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Bäuerle, A.; Teufel, M.; Musche, V.; Weismüller, B.; Kohler, H.; Hetkamp, M.; Dörrie, N.; Schweda, A.; Skoda, E.-M. Increased generalized anxiety, depression and distress during the COVID-19 pandemic: A cross-sectional study in Germany. J. Public Health 2020, 42, 672–678. [Google Scholar] [CrossRef]
- Alkhamees, A.A.; Alrashed, S.A.; Alzunnaydi, A.A.; Almohimeed, A.S.; Aljohani, M.S. The psyshological impact COVID-19 pandemic on the general population of Saudi Arabia. Compr. Psychiatry 2020, 102, 152192. [Google Scholar] [CrossRef]
- Beck, F.; Leger, D.; Fressard, L.; Peretti-watel, P.; Verger, P.; Coconel, G. Covid-19 health crisis and lockdown associated with high level of sleep complaints and hypnotic uptake at the population level. J. Sleep Res. 2021, 30, e13119. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, T.; Saida, K.; Tanaka, S.; Murayama, A. Rapid response: Impact of the COVID-19 pandemic on frailty in the elderly citizen; corona-frailty. BMJ 2020, 369, m1543. [Google Scholar]
- Rabassa, M.; Zamora-Ros, R.; Urpi-Sarda, M.; Bandinelli, S.; Ferrucci, L.; Andres-Lacueva, C.; Cherubini, A. Association of habitual dietary resveratrol exposure with the development of frailty in older age: The Invecchiare in Chianti study. Am. J. Clin. Nutr. 2015, 102, 1534–1542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, B.; Chen, H.; Huangm, L.; Ruan, Y.; Qi, S.; Guo, Y.; Huang, Z.; Sun, S.; Chen, X.; Shi, Y.; et al. Changes in frailty among community-dwelling Chinese older adults and its predictors: Evidence from a two-year longitudinal study. BMC Geriatr. 2020, 20, 130. [Google Scholar] [CrossRef] [Green Version]
- Shinohara, T.; Saida, K.; Tanaka, S.; Murayama, A. Actual Frailty Conditions and Lifestyle Changes in Community-Dwelling Older Adults Affected by Coronavirus Disease 2019 Countermeasures in Japan: A Cross-Sectional Study. SAGE Open Nurs. 2021, 7, 237796082110251. [Google Scholar] [CrossRef]
- Cudjoe, T.K.; Kotwal, A.A. “Social distancing” amidst a crisis in social isolation and loneliness. J. Am. Geriatr. Soc. 2006, 50, 776–780. [Google Scholar]
- Vindegaard, N.; Benros, M.E. COVID-19pandemic and mental health consequences: Systematic review of the current evidence. Brain Behav. Immun. 2020, 20, S0889–S1591. [Google Scholar]
- Bunt, S.; Steverink, N.; Olthof, J.; van der Schans, C.P.; Hobbelen, J.S.M. Social frailty in older adults: A scoping review. Eur. J. Ageing 2017, 14, 323–334. [Google Scholar] [CrossRef]
- Ding, Y.Y.; Kuha, J.; Murphy, M. Pathways from physical frailty to activity limitation in older people: Identifying moderators and mediators in the English Longitudinal Study of Ageing. Exp. Gerontol. 2017, 98, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Makizako, H.; Shimada, H.; Tsutsumimoto, K.; Lee, S.; Doi, T.; Nakakubo, S.; Hotta, R.; Suzuki, T. Social Frailty in Community-Dwelling Older Adults as a Risk Factor for Disability. J. Am. Med. Dir. Assoc. 2015, 16, 1003.e7–1003.e11. [Google Scholar] [CrossRef] [PubMed]
- Saji, N.; Sakurai, T.; Suzuki, K.; Mizusawa, H.; TobaM, K. ORANGE investigators ORANGE’s challenge: Developing wide-ranging dementia research in Japan. Lancet Neurol. 2016, 15, 661–662. [Google Scholar] [CrossRef] [Green Version]
- Makizako, H.; Shimada, H.; Park, H.; Doi, T.; Yoshida, D.; Uemura, K.; Tsutsumimoto, K.; Suzuki, T. Evaluation of multidimensional neurocognitive function using a tablet personal computer: Test-retest reliability and validity in community-dwelling older adults. Geriatr. Gerontol. Int. 2013, 13, 860–866. [Google Scholar] [CrossRef]
- Inoue, M.; Jimbo, D.; Taniguchi, M.; Urakami, K. Touch Panel-type Dementia Assessment Scale: A new computer-based rating scale for Alzheimer’s disease. Psychogeriatrics 2011, 11, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumimoto, K.; Doi, T.; Makizako, H.; Hotta, R.; Nakakubo, S.; Makino, K.; Suzuki, T.; Shimada, H. Association of Social Frailty with Both Cognitive and Physical Deficits Among Older People. J. Am. Med. Dir. Assoc. 2017, 18, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mao, G.; Leng, S.X. Frailty syndrome: An overview. Clin. Interv. Aging 2014, 9, 433–441. [Google Scholar]
- Makizako, H.; Doi, T.; Shimada, H.; Park, H.; Uemura, K.; Yoshida, D.; Tsutsumimoto, K.; Anan, Y.; Suzuki, T. Relationship between going outdoors daily and activation of the prefrontal cortex during verbal fluency tasks (VFTs) among older adults: A near-infrared spectroscopy study. Arch. Gerontol. Geriatr. 2013, 56, 118–123. [Google Scholar] [CrossRef]
- Tsutsumimoto, K.; Doi, T.; Makizako, H.; Hotta, R.; Nakakubo, S.; Kim, M.; Kurita, S.; Suzuki, T.; Shimada, H. Social Frailty Has a Stronger Impact on the Onset of Depressive Symptoms than Physical Frailty or Cognitive Impairment: A 4-Year Follow-up Longitudinal Cohort Study. J. Am. Med. Dir. Assoc. 2018, 19, 504–510. [Google Scholar] [CrossRef]
- Peerenboom, L.; Collard, R.M.; Naarding, P.; Comijs, H.C. The association between depression and emotional and social loneliness in older persons and the influence of social support, cognitive functioning and personality: A cross-sectional study. J. Affect Disord. 2015, 182, 26–31. [Google Scholar] [CrossRef]
- Clarke, C.L.; Sniehotta, F.F.; Vadiveloo, T.; Argo, I.S.; Donnan, P.T.; McMurdo, M.E.T.; Witham, M.D. Factors associated with change in objectively measured physical activity in older people—Data from the physical activity cohort Scotland study. BMC Geriatr. 2017, 17, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAuley, E.; Blissmer, B.; Marquez, D.X.; Jerome, G.J.; Kramer, A.F.; Katula, J. Social relations, physical activity, and well-being in older adults. Prev. Med. 2000, 31, 608–617. [Google Scholar] [CrossRef]
- Ma, L.; Sun, F.; Tang, Z. Social Frailty Is Associated with Physical Functioning, Cognition, and Depression, and Predicts Mortality. J. Nutr. Health Aging 2018, 22, 989–995. [Google Scholar] [CrossRef]
- Tsiboe, A.K. Describing the experiences of older persons with visual impairments during COVID-19 in rural Ghana. J. Adult Prot. 2020, 22, 371–383. [Google Scholar] [CrossRef]
- Heid, A.R.; Cartwright, F.; Wilson-Genderson, M.; Pruchno, R. Challenges Experienced by Older People During the Initial Months of the COVID-19 Pandemic. Gerontologist 2021, 61, 48–58. [Google Scholar] [CrossRef]
- Shrira, A.; Hoffman, Y.; Bodner, E.; Palgi, Y. COVID-19-Related Loneliness and Psychiatric Symptoms among Older Adults: The Buffering Role of Subjective Age. Am. J. Geriatr. Psychiatry 2020, 28, 1200–1204. [Google Scholar] [CrossRef]
- Hamm, M.E.; Brown, P.J.; Karp, J.F.; Lenard, E.; Cameron, F.; Dawdani, A.; Lavretsky, H.; Miller, J.P.; Mulsant, B.H.; Pham, V.T.; et al. Experiences of American Older Adults with Pre-existing Depression during the Beginnings of the COVID-19 Pandemic: A Multicity, Mixed-Methods Study. Am. J. Geriatr. Psychiatry 2020, 28, 924–932. [Google Scholar] [CrossRef]
- Krendl, A.C.; Perry, B.L. The Impact of Sheltering in Place During the COVID-19 Pandemic on Older Adults’ Social and Mental Well-Being. J. Gerontol. B Psychol. Sci. Soc. Sci. 2021, 76, e53–e58. [Google Scholar] [CrossRef] [PubMed]
- Theo, G.; Van, T.; Stephanie, S.; Elske, S.; Henriette, R.; Daniel, H. Loneliness and Mental Health during the COVID-19 Pandemic: A Study among Dutch Older Adults. J. Gerontol. 2021, 76, e249–e255. [Google Scholar]
- Van Dijk, S.D.M.; Bouman, R.; Folmer, E.H.; Held, R.C.; Warringa, J.E.; Marijnissen, R.M.; Voshaar, R.C.O. (Vi)-rushed into Online Group Schema Therapy Based Day-Treatment for Older Adults by the COVID-19 Outbreak in the Netherlands. Am. J. Geriat. Psychiatry 2020, 28, 983–988. [Google Scholar] [CrossRef] [PubMed]
- The National Center for Geriatrics and Gerontology. Online Applications for in the Place of Attendance. Available online: http://www.ncgg.jp/ri/news/20200605.html (accessed on 12 August 2021).


| Status | Robust | Social Prefrailty | Social Frailty | p Value | |||
|---|---|---|---|---|---|---|---|
| n = 63 | n = 29 | n = 11 | |||||
| Median | IQR | Median | IQR | Median | IQR | ||
| Age (years) | 73.5 | 8.0 | 74.5 | 10.0 | 71.0 | 8.0 | 0.380 |
| Gender (n) | Male 46, Female 17 | Male 20, Female 9 | Male 6, Female 5 | 0.464 | |||
| Medication (n) | 2.0 | 4.0 | 3.0 | 4.0 | 3.5 | 2.0 | 0.265 |
| Education (years) | 12.0 | 3.0 | 12.0 | 3.0 | 12.5 | 3.0 | 0.363 |
| UWS (m/s) | 1.16 | 0.27 | 1.15 | 0.31 | 1.11 | 0.19 | 0.528 |
| GS (kg) | 23.6 | 8.7 | 24.7 | 8.8 | 24.9 | 10.3 | 0.898 |
| WM (score) | 12.7 | 4.3 | 11.7 | 5.3 | 13.0 | 6.3 | 0.170 |
| TMT-A (s) | 20.0 | 7.0 | 24.0 | 12.0 | 19.5 | 6.0 | 0.306 |
| TMT-B (s) | 37.5 | 27.0 | 40.0 | 21.0 | 33.0 | 15.0 | 0.454 |
| SDST (score) | 41.5 | 14.0 | 38.0 | 24.0 | 41.5 | 11.0 | 0.341 |
| TDAS (score) | 3.0 | 4.0 | 3.0 | 6.0 | 4.5 | 6.0 | 0.266 |
| GDS-15 (score) | 2.0 | 2.0 | 2.0 | 3.0 | 6.0 | 6.0 | 0.021 * |
| Robust | Social Prefrailty | Social Frailty | Total | |
|---|---|---|---|---|
| Number of the participants (n) | 63 | 29 | 11 | 103 |
| Living alone (% yes) | 0 | 6.9 | 0 | 1.9 |
| Talking with someone everyday (% no) | 0 | 6.9 | 9.1 | 2.9 |
| Feeling helpful to friends or family (% no) | 0 | 0 | 100 | 10.7 |
| Going out less frequently compared with last year (% yes) | 0 | 86.2 | 27.3 | 27.2 |
| Visiting friends sometimes (% no) | 0 | 0 | 100 | 10.7 |
| Groups | Social Prefrail (n = 29) | Social Frailty (n = 11) | ||||||
|---|---|---|---|---|---|---|---|---|
| Odds | 95%CI | p Value | Odds | 95%CI | p Value | |||
| Age (years) | 0.96 | 0.86 | 1.08 | 0.53 | 0.94 | 0.77 | 1.14 | 0.54 |
| Gender (female/male) | 3.30 | 0.60 | 1.82 | 0.17 | 3.25 | 0.22 | 1.47 | 0.39 |
| Medication (n) | 1.01 | 0.85 | 1.20 | 0.90 | 1.11 | 0.82 | 1.51 | 0.50 |
| Education (years) | 0.97 | 0.75 | 1.27 | 0.84 | 1.27 | 0.80 | 2.04 | 0.31 |
| UWS (m/s) | 0.37 | 0.02 | 5.68 | 0.48 | 0.89 | 0.06 | 6.53 | 0.31 |
| GS (kg) | 0.93 | 0.84 | 1.05 | 0.24 | 0.95 | 0.80 | 1.14 | 0.59 |
| WM (score) | 0.83 | 0.67 | 1.04 | 0.11 | 1.23 | 0.86 | 1.74 | 0.26 |
| TMT-A (s) | 1.05 | 0.96 | 1.14 | 0.31 | 0.96 | 0.78 | 1.19 | 0.73 |
| TMT-B (s) | 0.98 | 0.94 | 1.02 | 0.23 | 0.98 | 0.92 | 1.04 | 0.45 |
| SDST (score) | 0.99 | 0.92 | 1.06 | 0.73 | 0.98 | 0.88 | 1.09 | 0.69 |
| TDAS (score) | 0.91 | 0.78 | 1.07 | 0.25 | 1.20 | 0.95 | 1.51 | 0.12 |
| GDS-15 (score) | 0.91 | 0.71 | 1.18 | 0.48 | 1.57 | 1.15 | 2.13 | 0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kodama, A.; Kume, Y.; Lee, S.; Makizako, H.; Shimada, H.; Takahashi, T.; Ono, T.; Ota, H. Impact of COVID-19 Pandemic Exacerbation of Depressive Symptoms for Social Frailty from the ORANGE Registry. Int. J. Environ. Res. Public Health 2022, 19, 986. https://doi.org/10.3390/ijerph19020986
Kodama A, Kume Y, Lee S, Makizako H, Shimada H, Takahashi T, Ono T, Ota H. Impact of COVID-19 Pandemic Exacerbation of Depressive Symptoms for Social Frailty from the ORANGE Registry. International Journal of Environmental Research and Public Health. 2022; 19(2):986. https://doi.org/10.3390/ijerph19020986
Chicago/Turabian StyleKodama, Ayuto, Yu Kume, Sangyoon Lee, Hyuma Makizako, Hiroyuki Shimada, Tomoko Takahashi, Tsuyoshi Ono, and Hidetaka Ota. 2022. "Impact of COVID-19 Pandemic Exacerbation of Depressive Symptoms for Social Frailty from the ORANGE Registry" International Journal of Environmental Research and Public Health 19, no. 2: 986. https://doi.org/10.3390/ijerph19020986








