Biomechanical Characteristics of the Knee Joint during Gait in Obese versus Normal Subjects
Abstract
:1. Introduction
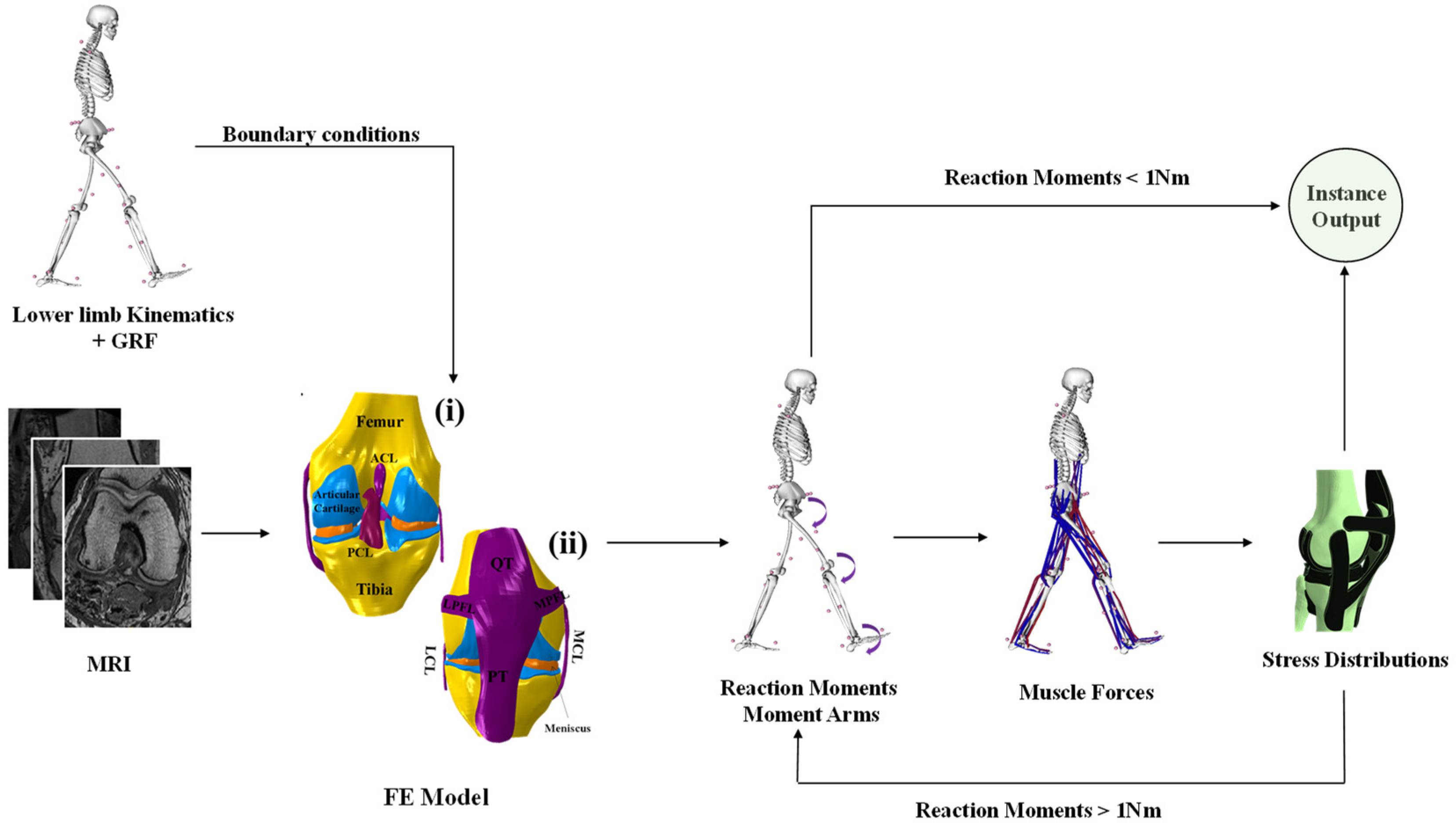
2. Materials and Methods
2.1. FE Model
2.2. Constitutive Models of the Soft Tissue
2.2.1. Cartilage
2.2.2. Meniscus
2.2.3. Ligaments
2.3. Muscles Optimization
2.4. Loading and Boundary Conditions
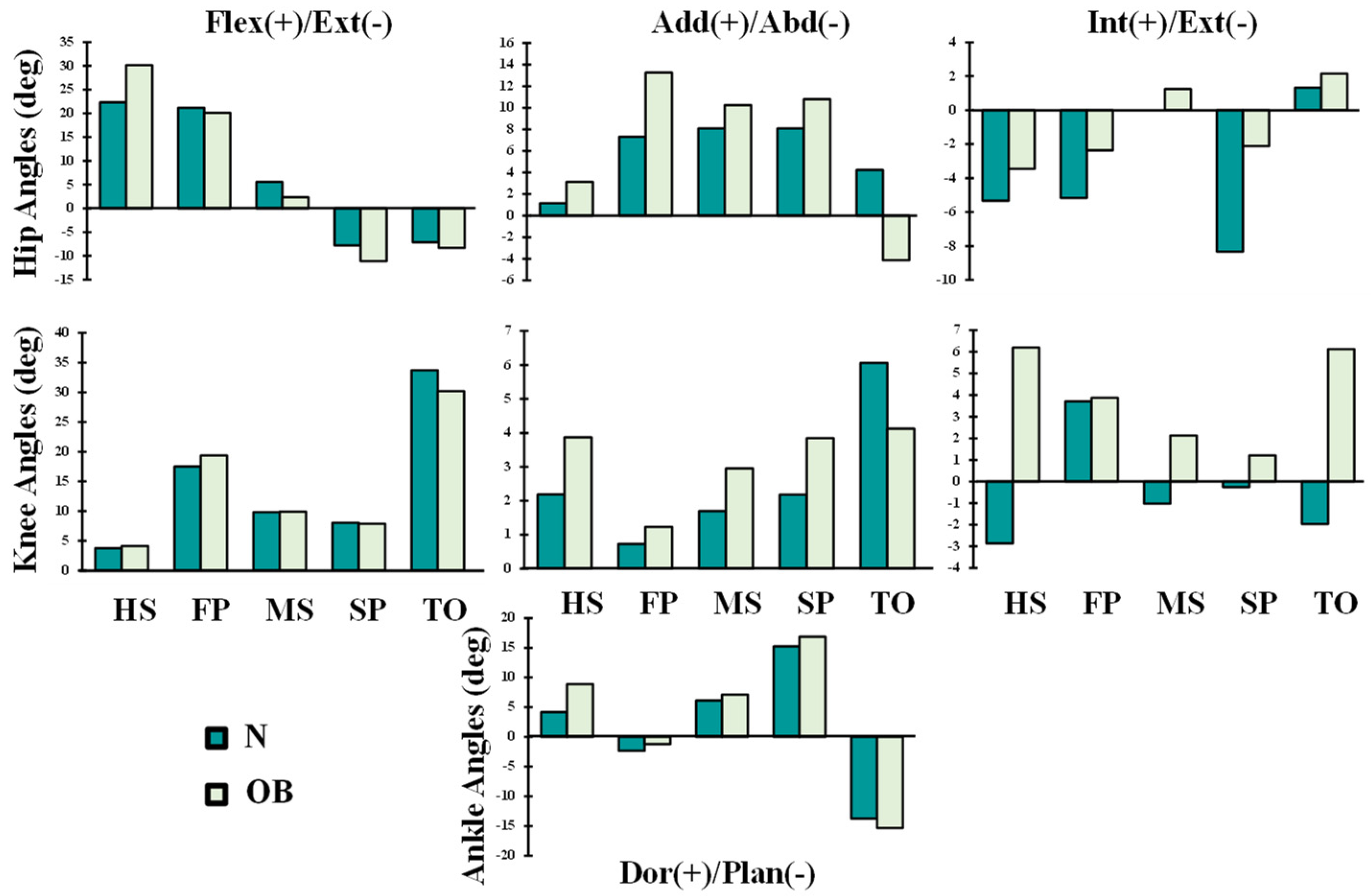
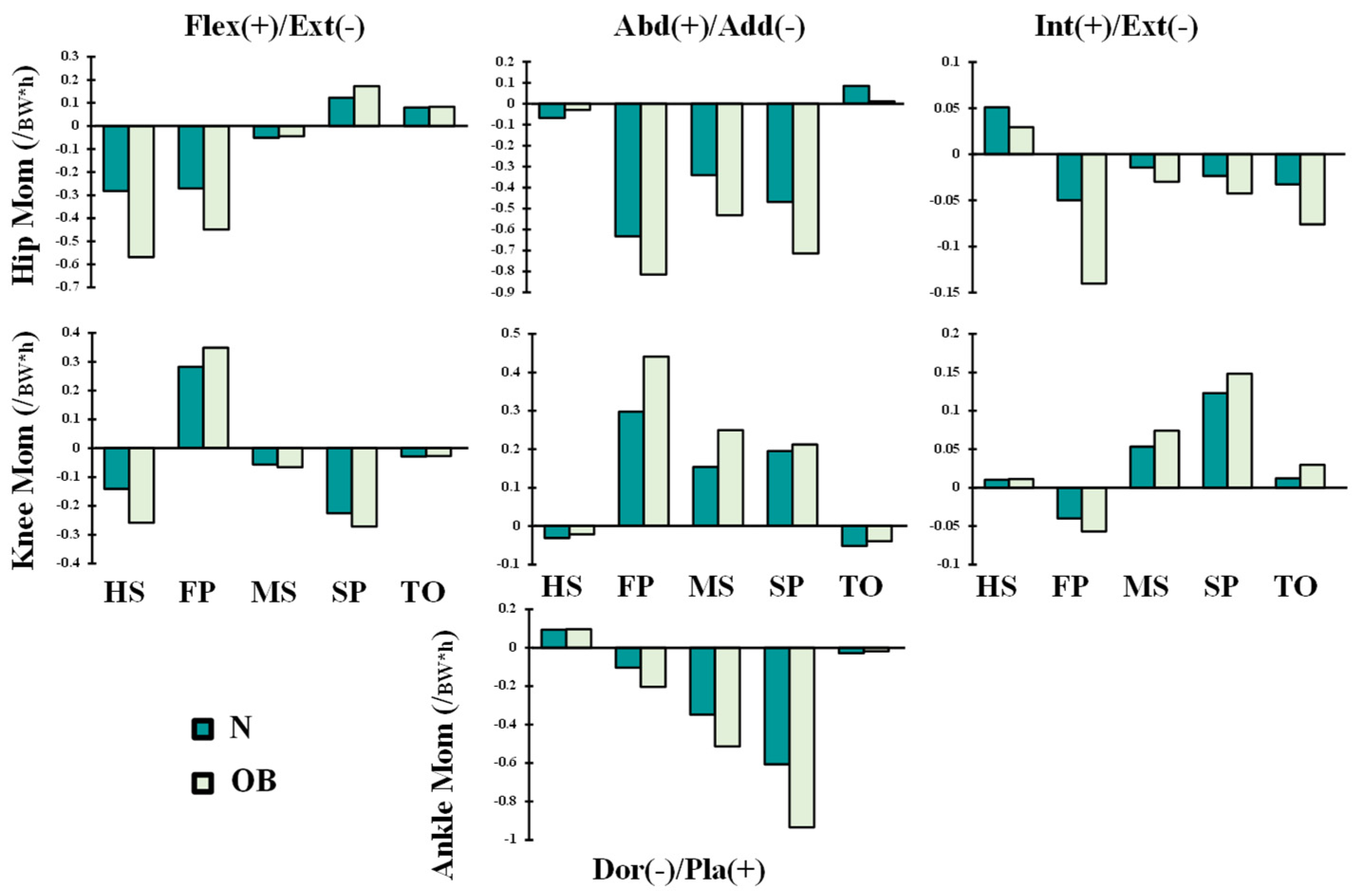
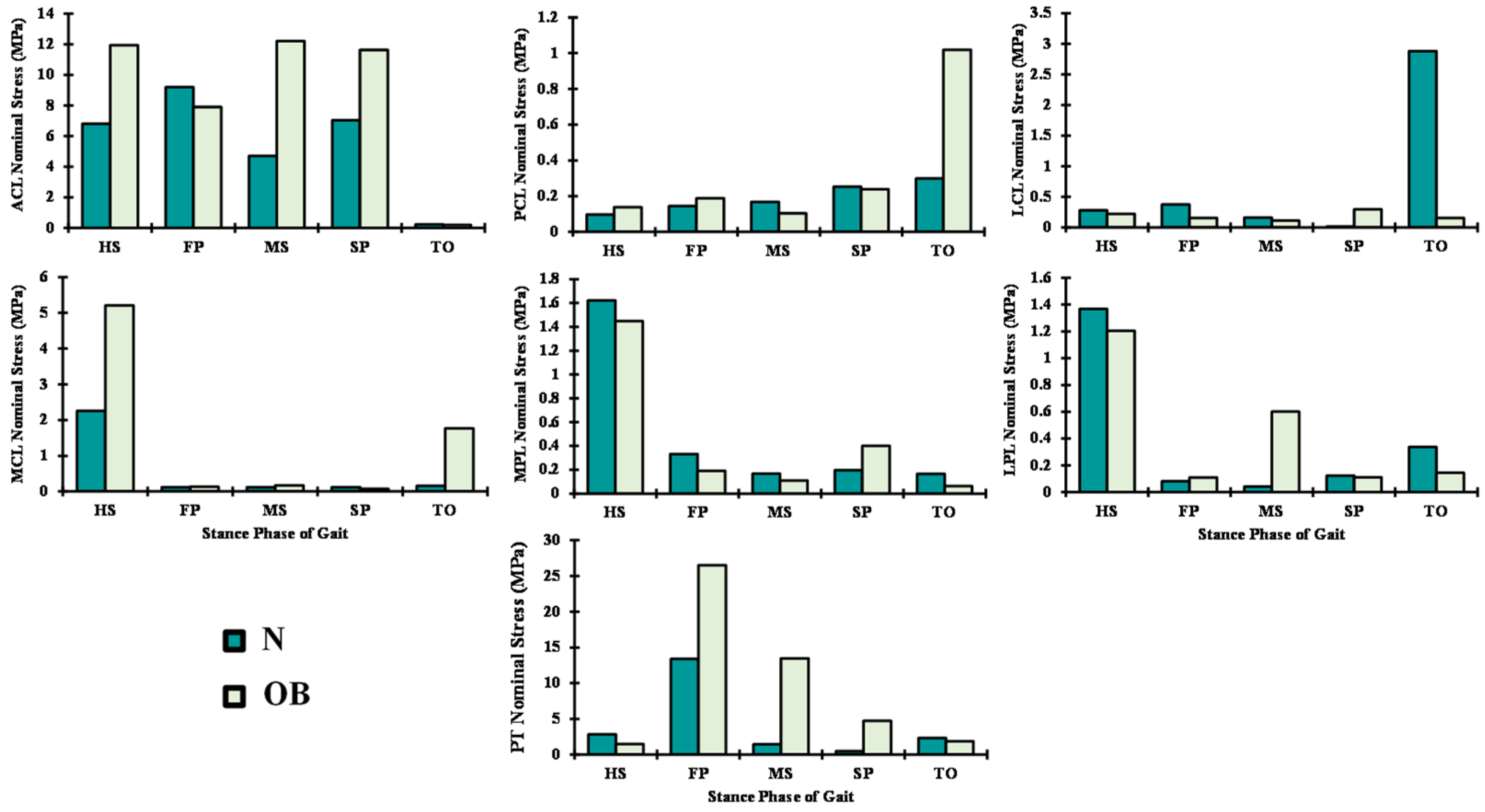
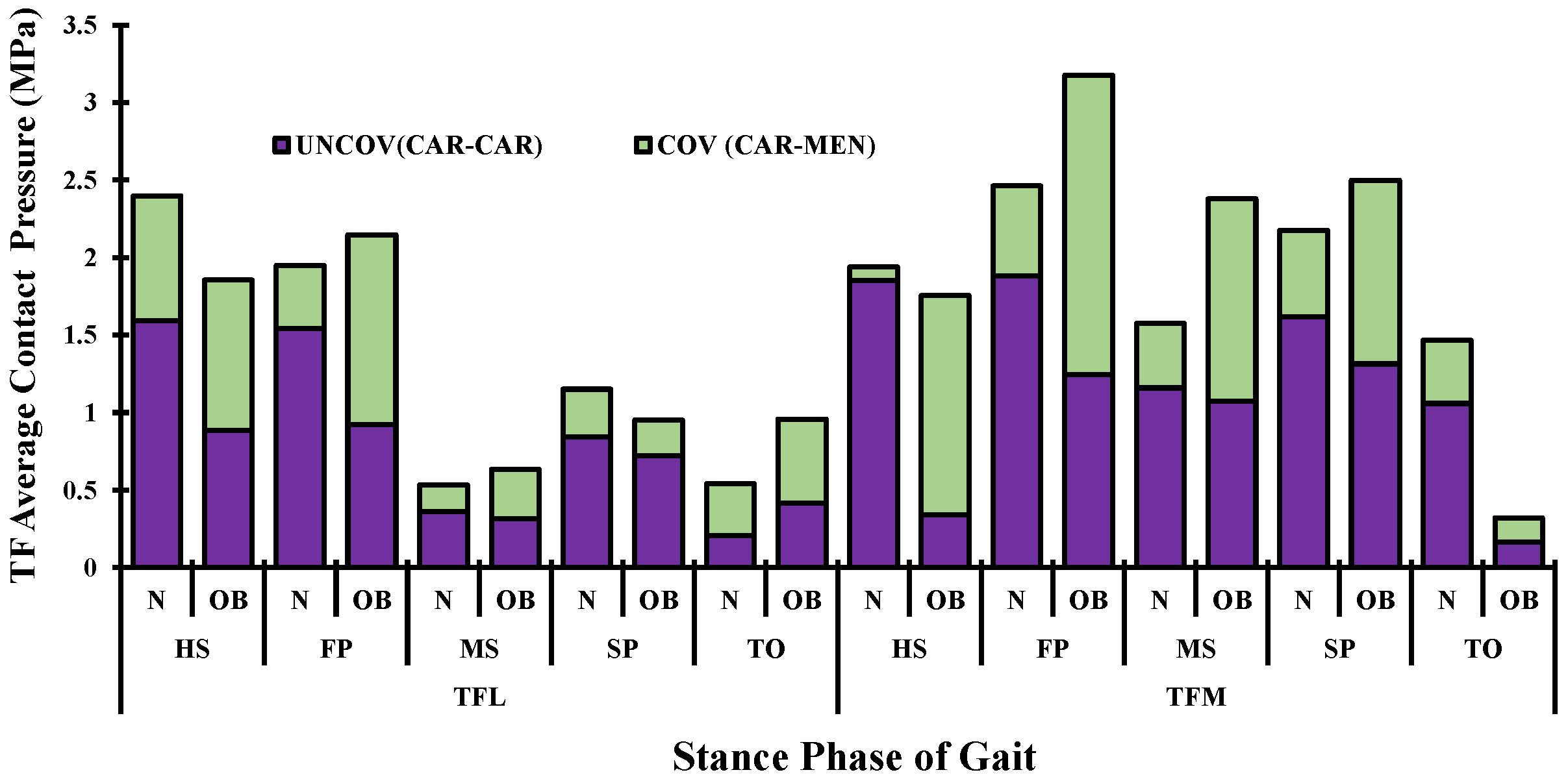
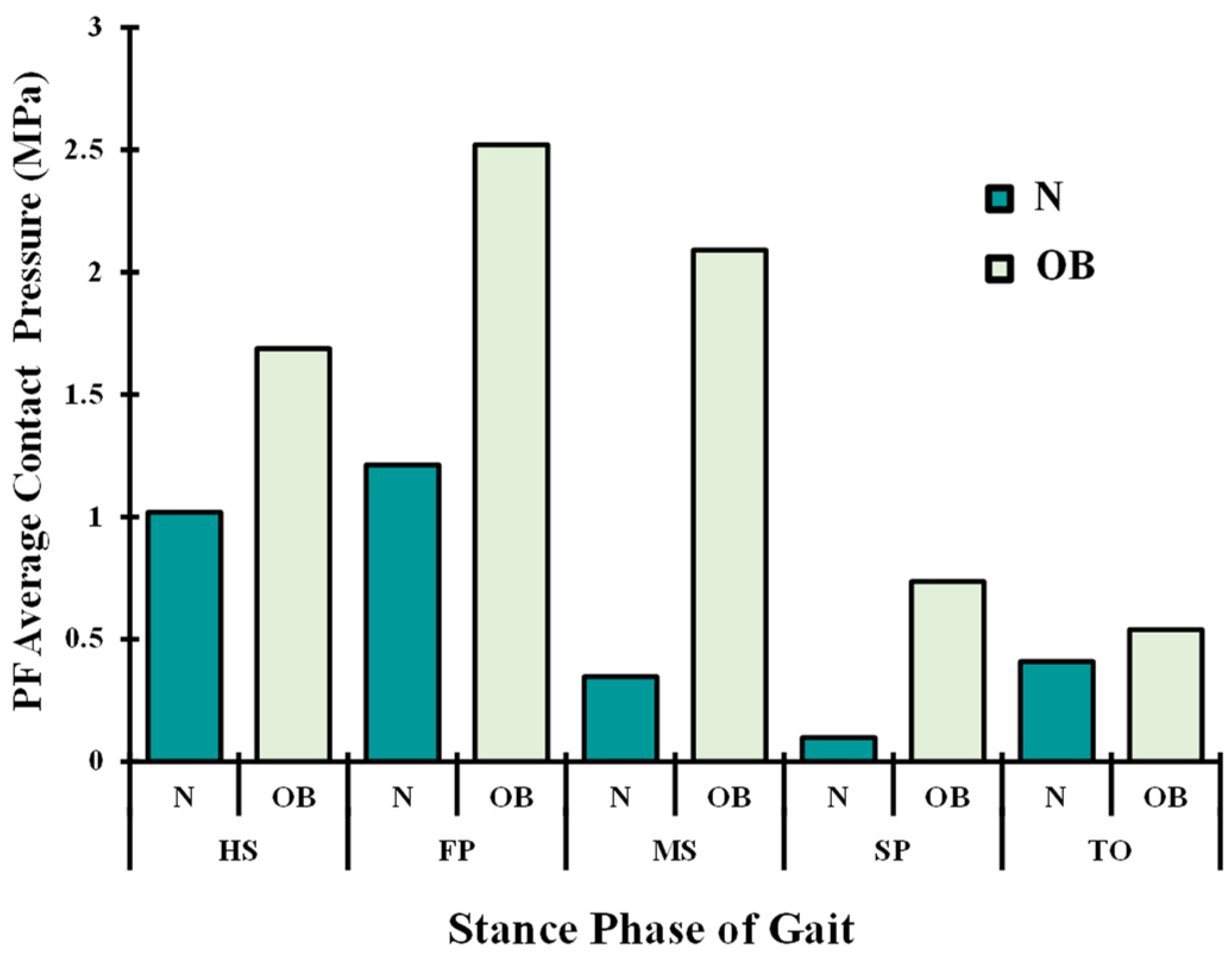
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leardini, G.; Salaffi, F.; Caporali, R.; Canesi, B.; Rovati, L.; Montanelli, R.; Italian Group for Study of the Costs of Arthritis. Direct and indirect costs of osteoarthritis of the knee. Clin. Exp. Rheumatol. 2004, 22, 699–706. [Google Scholar] [PubMed]
- Malaviya, A.N.; Shehab, D.; Bhargava, S.; Al-Jarallah, K.; Al-Awadi, A.; Sharma, P.N.; Al-Ghuriear, S.; Al-Shugayer, A. Characteristics of osteoarthritis among Kuwaitis: A hospital-based study. Clin. Rheumatol. 1998, 17, 210–213. [Google Scholar] [CrossRef] [PubMed]
- March, L.; Bachmeier, C. Economics of osteoarthritis: A global perspective. Occup. Health Ind. Med. 1998, 3, 154. [Google Scholar] [CrossRef]
- Woolf, A.D.; Pfleger, B. Burden of major musculoskeletal conditions. Bull. World Health Organ. 2003, 81, 646–656. [Google Scholar]
- Knecht, S.; Vanwanseele, B.; Stüssi, E. A review on the mechanical quality of articular cartilage—Implications for the diagnosis of osteoarthritis. Clin. Biomech. 2006, 21, 999–1012. [Google Scholar] [CrossRef]
- Lee, R.; Kean, W.F. Obesity and knee osteoarthritis. Inflammopharmacology 2012, 20, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Mora, J.C.; Przkora, R.; Cruz-Almeida, Y. Knee osteoarthritis: Pathophysiology and current treatment modalities. J. Pain Res. 2018, 11, 2189–2196. [Google Scholar] [CrossRef] [Green Version]
- Asay, J.L.; Erhart-Hledik, J.C.; Andriacchi, T.P. Changes in the total knee joint moment in patients with medial compartment knee osteoarthritis over 5 years. J. Orthop. Res. 2018, 36, 2373–2379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cudejko, T.; Van Der Esch, M.; Schrijvers, J.; Richards, R.; van den Noort, J.C.; Wrigley, T.; Van Der Leeden, M.; Roorda, L.D.; Lems, W.; Harlaar, J.; et al. The immediate effect of a soft knee brace on dynamic knee instability in persons with knee osteoarthritis. Rheumatology 2018, 57, 1735–1742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holsgaard-Larsen, A.; Clausen, B.; Søndergaard, J.; Christensen, R.; Andriacchi, T.; Roos, E. The effect on knee-joint load of instruction in analgesic use compared with neuromuscular exercise in patients with early knee osteoarthritis–A randomized, single-blind, controlled trial. Osteoarthr. Cartil. 2016, 24, S497–S498. [Google Scholar] [CrossRef] [Green Version]
- Shakoor, N.; Sengupta, M.; Foucher, K.; Wimmer, M.A.; Fogg, L.F.; Block, J. Effects of common footwear on joint loading in osteoarthritis of the knee. Arthritis Care Res. 2010, 62, 917–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blagojevic, M.; Jinks, C.; Jeffery, A.; Jordan, K. Risk factors for onset of osteoarthritis of the knee in older adults: A systematic review and meta-analysis. Osteoarthr. Cartil. 2010, 18, 24–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Browning, R.C.; Kram, R. Effects of Obesity on the Biomechanics of Walking at Different Speeds. Med. Sci. Sports Exerc. 2007, 39, 1632–1641. [Google Scholar] [CrossRef]
- DeVita, P.; Hortobágyi, T. Obesity is not associated with increased knee joint torque and power during level walking. J. Biomech. 2003, 36, 1355–1362. [Google Scholar] [CrossRef]
- Silvernail, J.F.; Milner, C.E.; Thompson, D.; Zhang, S.; Zhao, X. The influence of body mass index and velocity on knee biomechanics during walking. Gait Posture 2013, 37, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Heo, S.; Lee, G.; Park, W. Pre-obesity and obesity impacts on passive joint range of motion. Ergonomics 2018, 61, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Messier, S.P. Obesity and Osteoarthritis: Disease Genesis and Nonpharmacologic Weight Management. Rheum. Dis. Clin. North Am. 2008, 34, 713–729. [Google Scholar] [CrossRef] [PubMed]
- Runhaar, J.; Koes, B.W.; Clockaerts, S.; Bierma-Zeinstra, S.M.A. A systematic review on changed biomechanics of lower extremities in obese individuals: A possible role in development of osteoarthritis. Obes. Rev. 2011, 12, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
- Haight, D.J.; Lerner, Z.F.; Board, W.J.; Browning, R.C. A comparison of slow, uphill and fast, level walking on lower extremity biomechanics and tibiofemoral joint loading in obese and nonobese adults. J. Orthop. Res. 2014, 32, 324–330. [Google Scholar] [CrossRef]
- Harding, G.T.; Dunbar, M.J.; Hubley-Kozey, C.L.; Stanish, W.D.; Wilson, J.L.A. Obesity is associated with higher absolute tibiofemoral contact and muscle forces during gait with and without knee osteoarthritis. Clin. Biomech. 2016, 31, 79–86. [Google Scholar] [CrossRef]
- Harding, G.T.; Hubley-Kozey, C.; Dunbar, M.J.; Stanish, W.D.; Wilson, J.A. Body mass index affects knee joint mechanics during gait differently with and without moderate knee osteoarthritis. Osteoarthr. Cartil. 2012, 20, 1234–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horsak, B.; Schwab, C.; Baca, A.; Greber-Platzer, S.; Kreissl, A.; Nehrer, S.; Keilani, M.; Crevenna, R.; Kranzl, A.; Wondrasch, B. Effects of a lower extremity exercise program on gait biomechanics and clinical outcomes in children and adolescents with obesity: A randomized controlled trial. Gait Posture 2019, 70, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Lerner, Z.F.; Board, W.J.; Browning, R.C. Effects of obesity on lower extremity muscle function during walking at two speeds. Gait Posture 2014, 39, 978–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paterson, K.; Sosdian, L.; Hinman, R.; Wrigley, T.; Kasza, J.; Dowsey, M.; Choong, P.; Bennell, K. Effects of sex and obesity on gait biomechanics before and six months after total knee arthroplasty: A longitudinal cohort study. Gait Posture 2018, 61, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Russell, E.M.; Hamill, J. Lateral wedges decrease biomechanical risk factors for knee osteoarthritis in obese women. J. Biomech. 2011, 44, 2286–2291. [Google Scholar] [CrossRef] [PubMed]
- Verlaan, L.; Boekesteijn, R.J.; Oomen, P.W.; Liu, W.-Y.; Peters, M.J.M.; Witlox, M.A.; Emans, P.J.; van Rhijn, L.W.; Meijer, K. Biomechanical Alterations during Sit-to-Stand Transfer Are Caused by a Synergy between Knee Osteoarthritis and Obesity. Biomed. Res. Int. 2018, 2018, 3519498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yocum, D.; Weinhandl, J.T.; Fairbrother, J.T.; Zhang, S. Wide step width reduces knee abduction moment of obese adults during stair negotiation. J. Biomech. 2018, 75, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Marouane, H.; Adouni, M.; Shirazi-Adl, A. 3D active-passive response of human knee joint in gait is markedly altered when simulated as a planar 2D joint. Biomech. Model. Mechanobiol. 2017, 16, 693–703. [Google Scholar] [CrossRef]
- Erdemir, A. Open knee: Open source modeling & simulation to enable scientific discovery and clinical care in knee biomechanics. J. Knee Surg. 2016, 29, 107. [Google Scholar]
- Donahue, T.L.H.; Hull, M.L.; Rashid, M.M.; Jacobs, C.R. A Finite Element Model of the Human Knee Joint for the Study of Tibio-Femoral Contact. J. Biomech. Eng. 2002, 124, 273–280. [Google Scholar] [CrossRef]
- Sajjadinia, S.S.; Haghpanahi, M.; Razi, M. Computational simulation of the multiphasic degeneration of the bone-cartilage unit during osteoarthritis via indentation and unconfined compression tests. Proc. Inst. Mech. Eng. H 2019, 233, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.; Huyghe, J.M.; van Donkelaar, C.C. Depth-dependent Compressive Equilibrium Properties of Articular Cartilage Explained by its Composition. Biomech. Model. Mechanobiol. 2007, 6, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Tissakht, M.; Ahmed, A. Tensile stress-strain characteristics of the human meniscal material. J. Biomech. 1995, 28, 411–422. [Google Scholar] [CrossRef]
- Fithian, D.C.; A Kelly, M.; Mow, V.C. Material properties and structure-function relationships in the menisci. Clin. Orthop. Relat. Res. 1990, 1990, 19–31. [Google Scholar] [CrossRef]
- Proctor, C.S.; Schmidt, M.B.; Whipple, R.R.; Kelly, M.A.; Mow, V.C. Material properties of the normal medial bovine meniscus. J. Orthop. Res. 1989, 7, 771–782. [Google Scholar] [CrossRef]
- Stäubli, H.U.; Schatzmann, L.; Brunner, P.; Rincón, L.; Nolte, L.-P. Mechanical Tensile Properties of the Quadriceps Tendon and Patellar Ligament in Young Adults. Am. J. Sports Med. 1999, 27, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Dhaher, T.H.K.; Barry, M. The effect of connective tissue material uncertainties on knee joint mechanics under isolated loading conditions. J. Biomech. 2010, 43, 3118–3125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limbert, G.; Middleton, J. A transversely isotropic viscohyperelastic material: Application to the modeling of biological soft connective tissues. Int. J. Solids Struct. 2004, 41, 4237–4260. [Google Scholar] [CrossRef]
- Adouni, M.; Faisal, T.R.; Dhaher, Y.Y. Computational frame of ligament in situ strain in a full knee model. Comput. Biol. Med. 2020, 126, 104012. [Google Scholar] [CrossRef]
- Delp, S.L.; Anderson, F.C.; Arnold, A.S.; Loan, P.; Habib, A.; John, C.T.; Guendelman, E.; Thelen, D.G. OpenSim: Open-Source Software to Create and Analyze Dynamic Simulations of Movement. IEEE Trans. Biomed. Eng. 2007, 54, 1940–1950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroeder, M.J. A multi-domain synthesis of neuromechanical adaptations post anterior cruciate ligament reconstructive surgery. In Biomedical Engineering; Northwestern University: Evanston, IL, USA, 2014; p. 185. [Google Scholar]
- Schroeder, M.J. A computational framework to evaluate the efficacy of anterior cruciate ligament reconstruction procedures. In Biomedical Engineering; Northwestern University: Evanston, IL, USA, 2010; p. 106. [Google Scholar]
- Russell, E.M. Lateral Wedges and the Biomechanical Risk for Knee Osteoarthritis; ProQuest Dissertations Publishing: Ann Arbor, MI, USA, 2011. [Google Scholar]
- Adouni, M.; Shirazi-Adl, A. Partitioning of knee joint internal forces in gait is dictated by the knee adduction angle and not by the knee adduction moment. J. Biomech. 2014, 47, 1696–1703. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.; Richards, J.; Whittle, M.W. Whittle’s Gait Analysis-E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Astephen, J.L. Biomechanical factors in the progression of knee osteoarthritis. In School of Biomedical Engineering; Dalhousie University: Halifax, NS, Canada, 2007; p. 216. [Google Scholar]
- MacLean, K.F.; Callaghan, J.P.; Maly, M.R. Effect of obesity on knee joint biomechanics during gait in young adults. Cogent Med. 2016, 3, 1173778. [Google Scholar] [CrossRef] [Green Version]
- Wearing, S.C.; Hennig, E.M.; Byrne, N.; Steele, J.R.; Hills, A.P. Musculoskeletal disorders associated with obesity: A biomechanical perspective. Obes. Rev. 2006, 7, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Amiri, P.; Hubley-Kozey, C.; Landry, S.; Stanish, W.; Wilson, J.A. Obesity is associated with prolonged activity of the quadriceps and gastrocnemii during gait. J. Electromyogr. Kinesiol. 2015, 25, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.G.; Wolf, A. The effects of body weight unloading on kinetics and muscle activity of overweight males during Overground walking. Clin. Biomech. 2018, 52, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Perry, J. Gait analysis: Normal and pathological function. J. Pediatric Orthop. 1992, 12, 815. [Google Scholar] [CrossRef]
- Dufek, J.S.; Currie, R.L.; Gouws, P.-L.; Candela, L.; Gutierrez, A.P.; Mercer, J.A.; Putney, L.G. Effects of overweight and obesity on walking characteristics in adolescents. Hum. Mov. Sci. 2012, 31, 897–906. [Google Scholar] [CrossRef]
- Pflum, M.A.; Shelburne, K.B.; Torry, M.R.; Decker, M.J.; Pandy, M.G. Model Prediction of Anterior Cruciate Ligament Force during Drop-Landings. Med. Sci. Sports Exerc. 2004, 36, 1949–1958. [Google Scholar] [CrossRef] [PubMed]
- Orozco, G.A.; Tanska, P.; Mononen, M.; Halonen, K.S.; Korhonen, R. The effect of constitutive representations and structural constituents of ligaments on knee joint mechanics. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Shelburne, K.B.; Pandy, M.; Anderson, F.C.; Torry, M.R. Pattern of anterior cruciate ligament force in normal walking. J. Biomech. 2004, 37, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Shelburne, K.B.; Torry, M.R.; Pandy, M. Muscle, Ligament, and Joint-Contact Forces at the Knee during Walking. Med. Sci. Sports Exerc. 2005, 37, 1948–1956. [Google Scholar] [CrossRef] [Green Version]
- Lacy, K.W.; Cracchiolo, A.; Yu, S.; Goitz, H. Medial Femoral Condyle Cartilage Defect Biomechanics: Effect of Obesity, Defect Size, and Cartilage Thickness. Am. J. Sports Med. 2016, 44, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Gersing, A.S.; Solka, M.; Joseph, G.B.; Schwaiger, B.J.; Heilmeier, U.; Feuerriegel, G.; Nevitt, M.C.; McCulloch, C.E.; Link, T.M. Progression of cartilage degeneration and clinical symptoms in obese and overweight individuals is dependent on the amount of weight loss: 48-month data from the Osteoarthritis Initiative. Osteoarthr. Cartil. 2016, 24, 1126–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laberge, M.A.; Baum, T.; Virayavanich, W.; Nardo, L.; Nevitt, M.C.; Lynch, J.; McCulloch, C.E.; Link, T.M. Obesity increases the prevalence and severity of focal knee abnormalities diagnosed using 3T MRI in middle-aged subjects—Data from the Osteoarthritis Initiative. Skelet. Radiol. 2012, 41, 633–641. [Google Scholar] [CrossRef] [Green Version]
- Husni, E. A New Look at Obesity and Osteoarthritis. 2015. Available online: https://consultqd.clevelandclinic.org/a-new-look-at-obesity-and-osteoarthritis/ (accessed on 12 January 2022).
- Whittle, M.W. Clinical gait analysis: A review. Hum. Mov. Sci. 1996, 15, 369–387. [Google Scholar] [CrossRef]
- Messier, S.P.; Gutekunst, D.J.; Davis, C.; DeVita, P. Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis Rheum. 2005, 52, 2026–2032. [Google Scholar] [CrossRef] [PubMed]
- Mow, V.C.; Huiskes, R. Basic Orthopaedic Biomechanics & Mechano-Biology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005. [Google Scholar]
- Henriksen, M.; Christensen, R.; Danneskiold-Samsøe, B.; Bliddal, H. Changes in lower extremity muscle mass and muscle strength after weight loss in obese patients with knee osteoarthritis: A prospective cohort study. Arthritis Rheum. 2012, 64, 438–442. [Google Scholar] [CrossRef] [PubMed]



| Material Parameters | |
|---|---|
| : Initial collagen coefficients | 4.63 |
| : Strain-dep collagen coefficients | 3670 |
| : Shear modulus | 0.723 |
| : Collagen fibril volume fraction 1 | or |
| : Total depth-dependent collagen volume fraction 2 | |
| : Initial solid volume fraction | |
| : Incompressibility penalty parameter |
| Ec (MPa) | Et (MPa) | Gt (MPa) | ||
|---|---|---|---|---|
| 120 | 20 | 0.3 | 0.2 | 47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Khatib, F.; Gouissem, A.; Mbarki, R.; Adouni, M. Biomechanical Characteristics of the Knee Joint during Gait in Obese versus Normal Subjects. Int. J. Environ. Res. Public Health 2022, 19, 989. https://doi.org/10.3390/ijerph19020989
Al Khatib F, Gouissem A, Mbarki R, Adouni M. Biomechanical Characteristics of the Knee Joint during Gait in Obese versus Normal Subjects. International Journal of Environmental Research and Public Health. 2022; 19(2):989. https://doi.org/10.3390/ijerph19020989
Chicago/Turabian StyleAl Khatib, Fadi, Afif Gouissem, Raouf Mbarki, and Malek Adouni. 2022. "Biomechanical Characteristics of the Knee Joint during Gait in Obese versus Normal Subjects" International Journal of Environmental Research and Public Health 19, no. 2: 989. https://doi.org/10.3390/ijerph19020989





