Biomass, Carbon and Nitrogen Partitioning and Water Use Efficiency Differences of Five Types of Alpine Grasslands in the Northern Tibetan Plateau
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites and Conditions
2.2. Experimental Samples
2.3. Methods
2.3.1. Importance Value (IV)
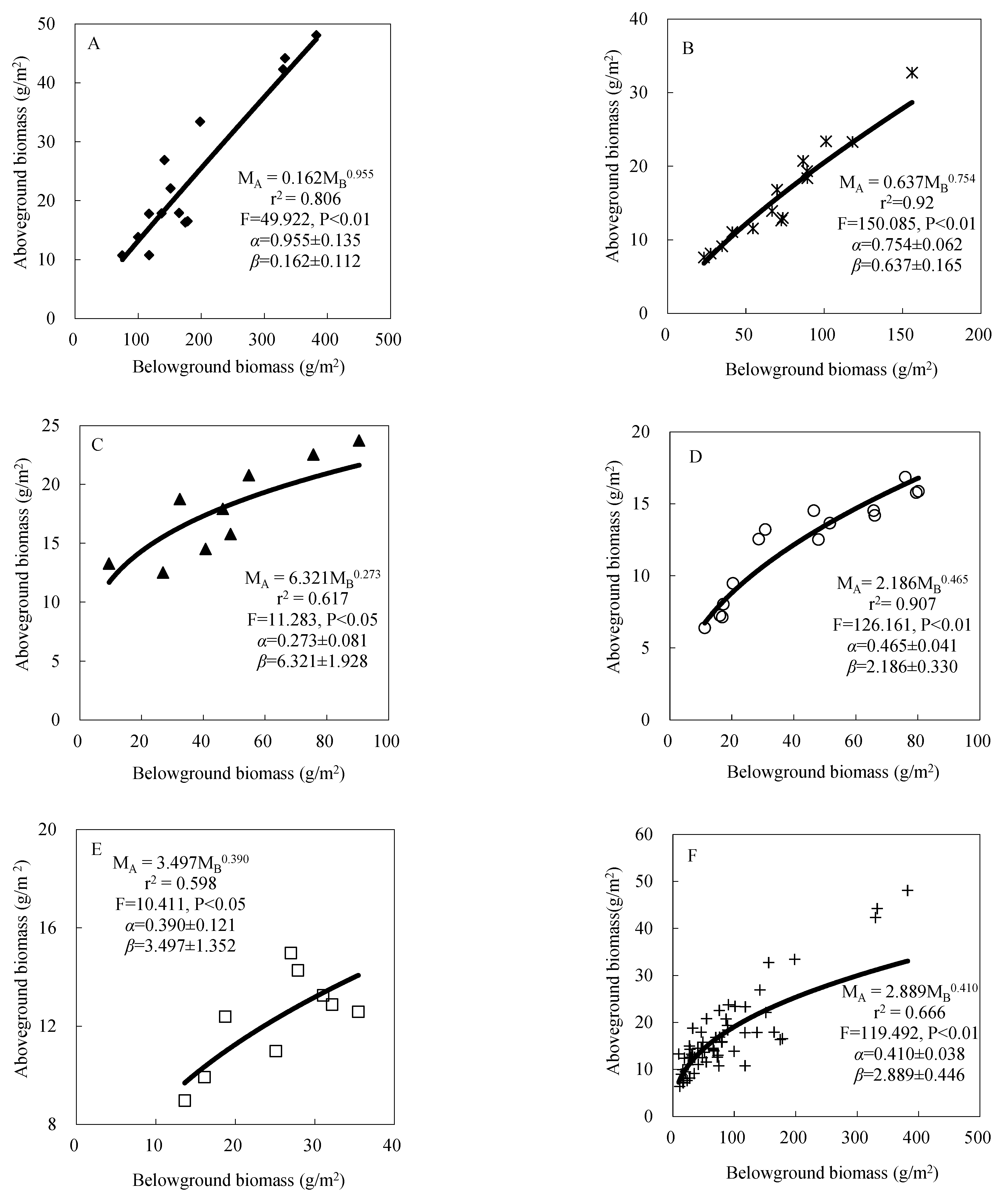
2.3.2. Biomass Allometric Method
2.3.3. Biomass and Soil Analysis Methods
2.3.4. Intrinsic Water Use Efficiency (WUEi) Calculation
2.3.5. Data Statistical Analysis
3. Results
3.1. Biomass
3.1.1. Biomass Characteristics
3.1.2. Biomass Partitioning
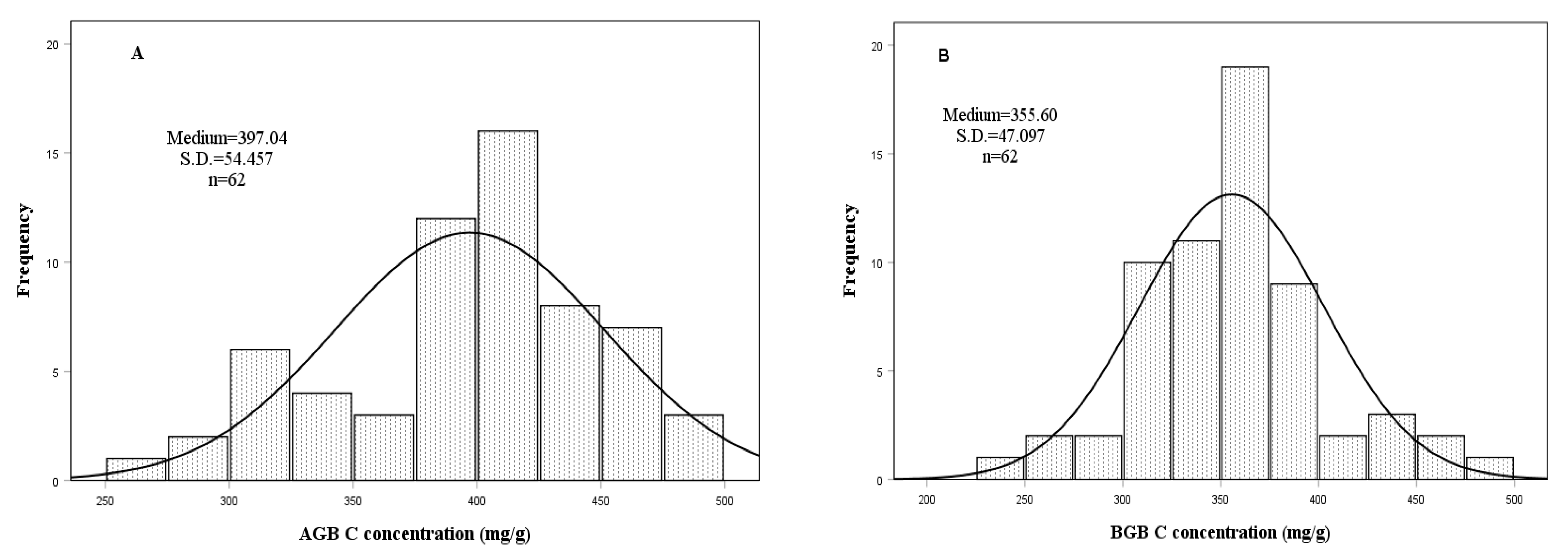
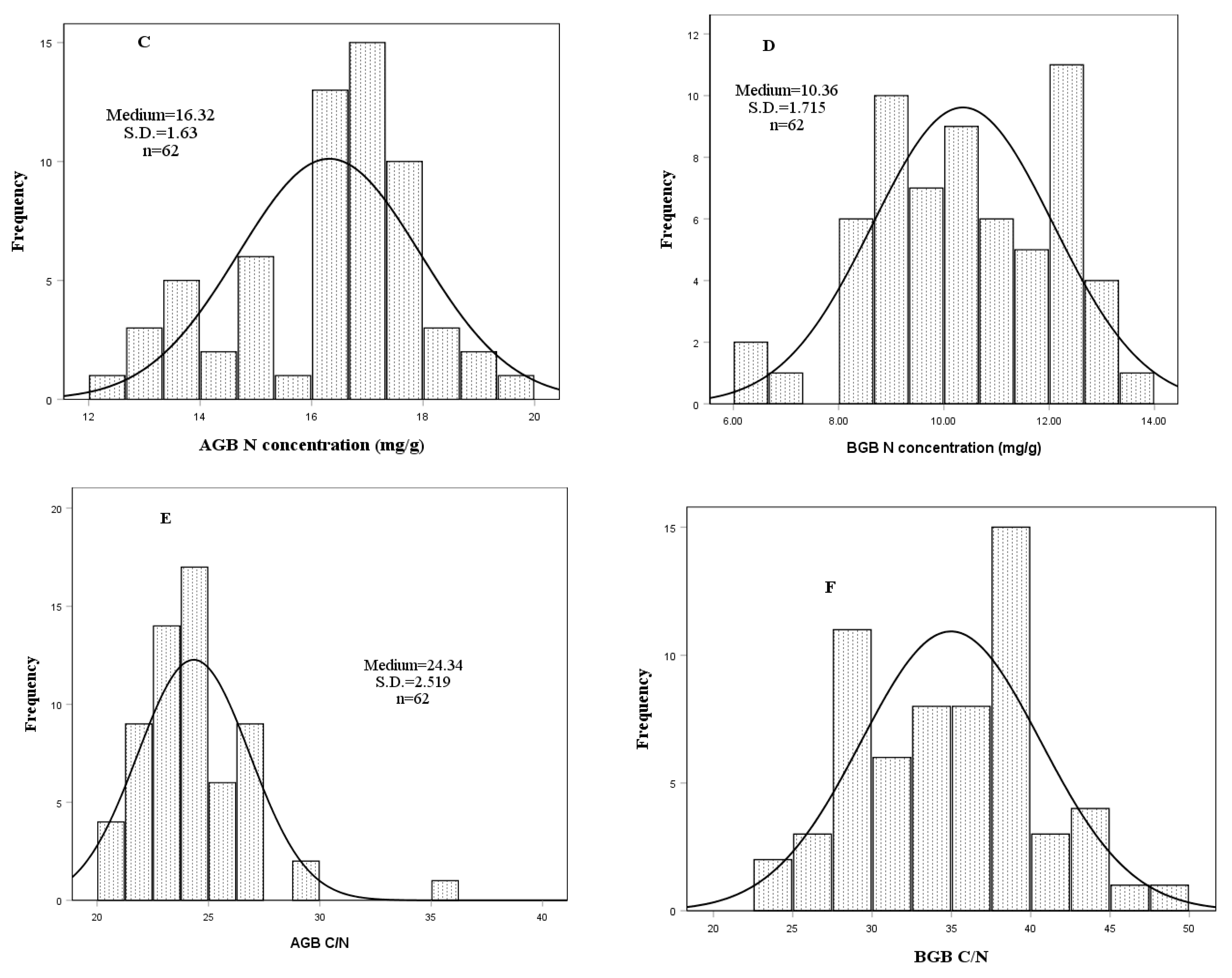
3.2. Biomass Organic Carbon (C), Nitrogen (N) Concentrations and Stocks
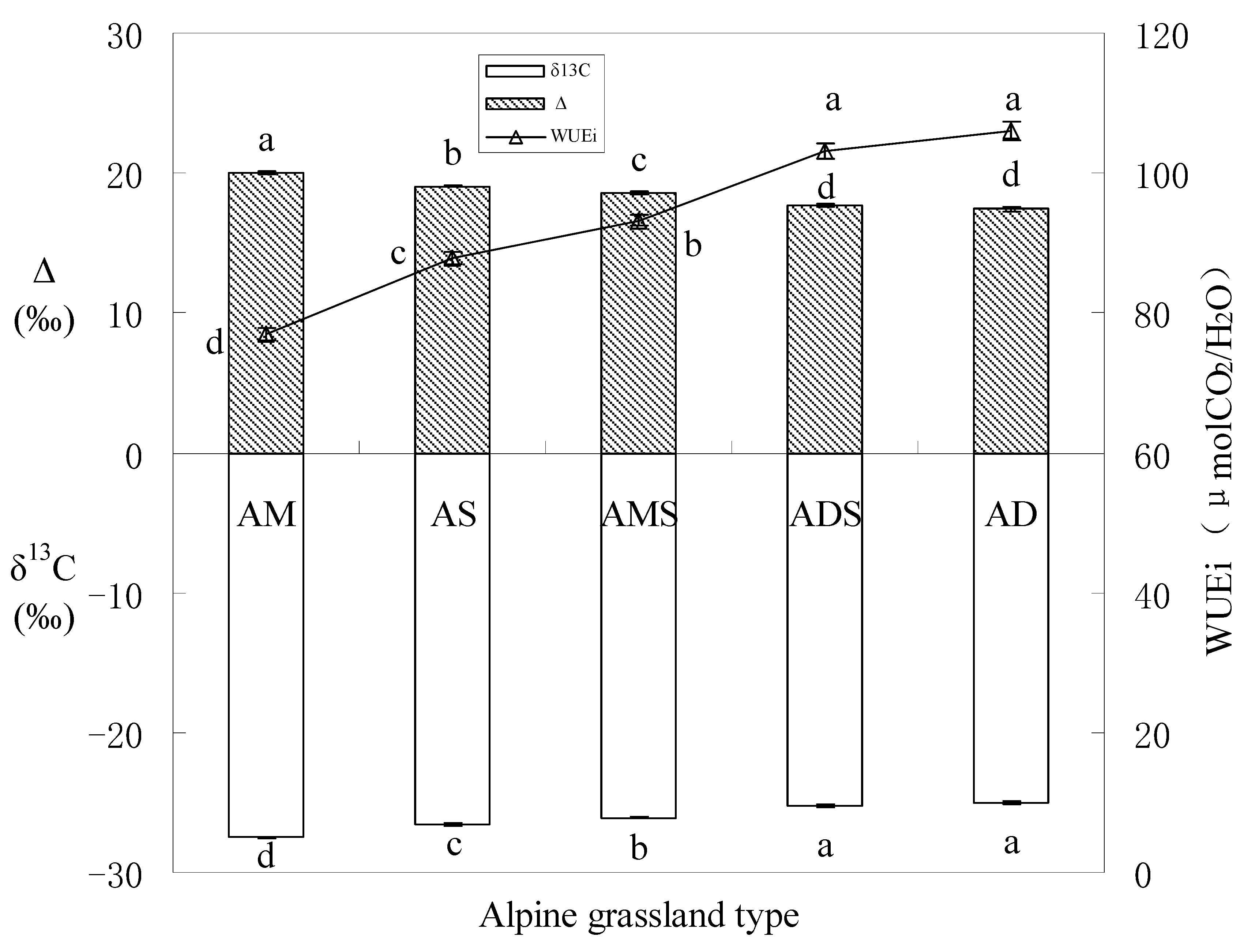
3.3. Soil Water Content (SWC) and Vegetation Water Use Efficiency (WUE)
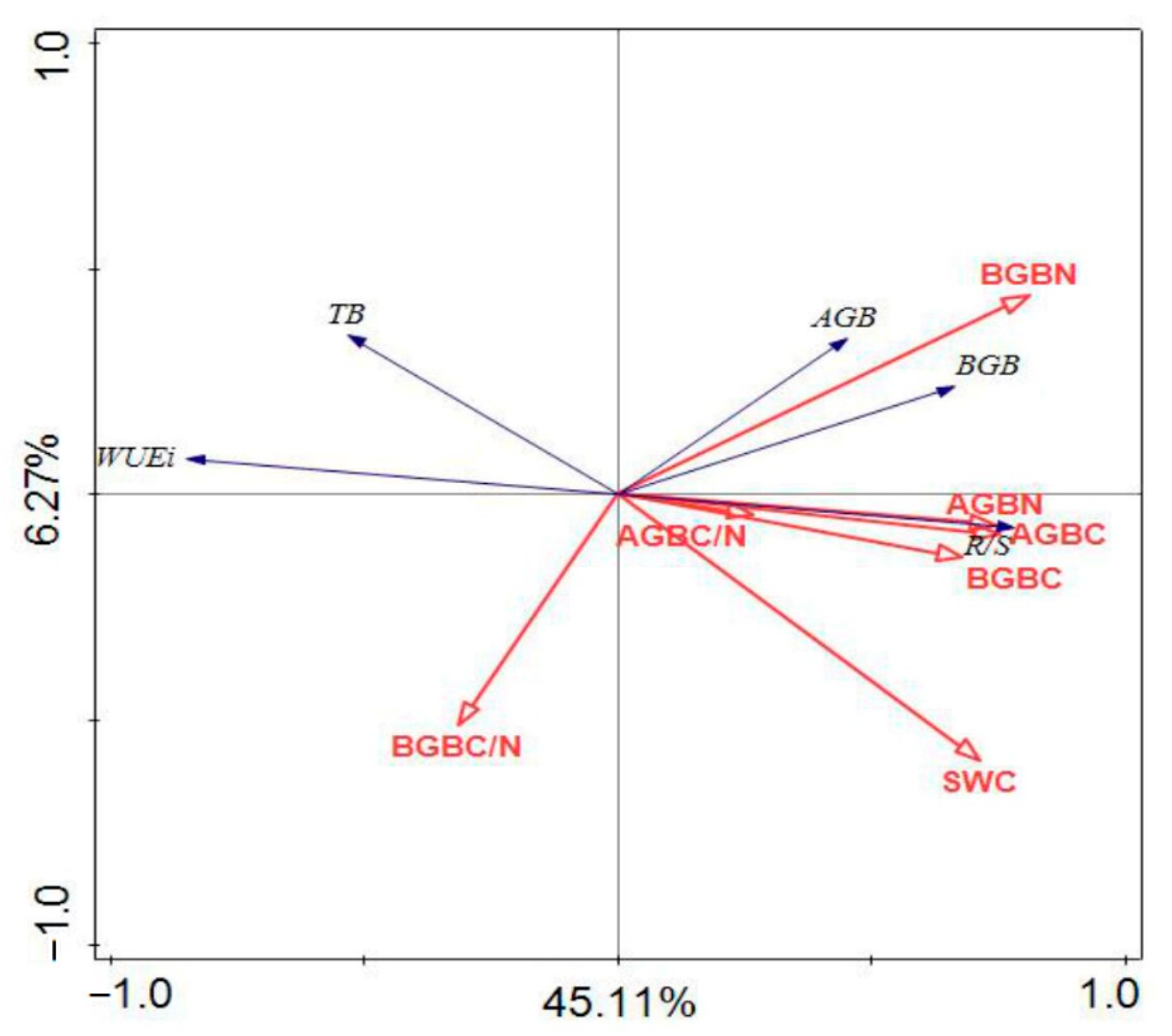
3.4. The Correlation among Biomass, C, N Concentrations, SWC and WUEi
4. Discussion
4.1. Biomass
4.2. C and N Stocks
4.3. Water Use Efficiency
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, J.; Cheng, G.W.; Li, W.P. Meta-analysis of relationships between environmental factors and aboveground biomass in the alpine grassland on the Tibetan Plateau. Biogeosciences 2013, 10, 1707–1715. [Google Scholar] [CrossRef] [Green Version]
- Chapin, F.S., III; Randerson, J.T.; McGuire, A.D.; Foley, J.A.; Field, C.B. Changing feedbacks in the climate-biosphere system. Front. Ecol. Environ. 2008, 6, 313–320. [Google Scholar] [CrossRef]
- Wang, X.; Li, M.-H.; Liu, S.; Liu, G. Fractal characteristics of soils under different land-use patterns in the arid and semiarid regions of the Tibetan Plateau, China. Geoderma 2006, 134, 56–61. [Google Scholar] [CrossRef]
- Mueller, C.; Kogel-Knabner, I. Soil organic carbon stocks, distribution, and composition affected by historic land use changes on adjacent sites. Biol. Fertil Soil 2009, 45, 347–359. [Google Scholar] [CrossRef]
- O’Mara, F.P. The role of grasslands in food security and climate change. Ann. Bot. 2012, 110, 1263–1270. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Bai, W.; Li, N.; Hu, H. Climate changes and its impact on tundra ecosystem in Qinghai-Tibet Plateau, China. Clim. Chang. 2010, 106, 463–482. [Google Scholar] [CrossRef]
- Beurskens, L.W.M.; Hekkenberg, M. Renewable energy projections as published in the National Renewable Energy Action Plans of the European Member States; Petten, N.L., Copenhagen, D.K., Eds.; Energy Research Centre of the Netherlands and European Environment Agency: Petten, The Netherlands, 2011; p. 244. [Google Scholar]
- Fang, J.; Yang, Y.; Ma, W.; Mohammat, A.; Shen, H. Ecosystem carbon stocks and their changes in China’s grasslands. Sci. China Life Sci. 2010, 53, 757–765. [Google Scholar] [CrossRef]
- Booth, M.S.; Stark, J.M.; Rastetter, E. Controls on nitrogen cycling in terrestrial ecosystems: A synthetic analysis of literature data. Ecol. Monogr. 2005, 75, 139–157. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Richardson, T.K.; Jain, A.K. Contributions of secondary forest and nitrogen dynamics to terrestrial carbon uptake. Biogeosciences 2010, 7, 3041–3050. [Google Scholar] [CrossRef] [Green Version]
- Impa, S.M.; Nadaradjan, S.; Boominathan, P.; Shashidhar, G.; Bindumadhava, H.; Sheshshayee, M.S. Carbon Isotope Discrimination Accurately Reflects Variability in WUE Measured at a Whole Plant Level in Rice. Crop Sci. 2005, 45, 2517–2522. [Google Scholar] [CrossRef]
- Stroosnijder, L. Modifying land management in order to improve efficiency of rainwater use in the African highlands. Soil Tillage Res. 2009, 103, 247–256. [Google Scholar] [CrossRef]
- Yang, H.; He, N.; He, Y.; Li, S.; Shi, P.; Zhang, X. Stable Water Use Efficiency of Tibetan Alpine Meadows in Past Half Century: Evidence from Wool δ13C Values. PLoS ONE 2015, 10, e0144752. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Zhang, X.; Shen, Z.; Shi, P.; Xu, X.; Li, X. Grazing-Exclusion Effects on Aboveground Biomass and Water-Use Efficiency of Alpine Grasslands on the Northern Tibetan Plateau. Rangel. Ecol. Manag. 2013, 66, 454–461. [Google Scholar] [CrossRef]
- Luo, T.; Zhang, L.; Zhu, H.; Daly, C.; Li, M.; Luo, J. Correlations between net primary productivity and foliar carbon isotope ratio across a Tibetan ecosystem transect. Ecography 2009, 32, 526–538. [Google Scholar] [CrossRef]
- Chen, T.; Yang, M.X.; Feng, H.Y. Spatial distribution of stable carbon isotope compositions of plant leaves in the north of the Tibetan Plateau. J. Glaciol. Geocryol. 2003, 25, 83–87. [Google Scholar]
- Wei, Y.X.; Chen, Q.G. Grassland classification and evaluation of grazing capacity in Naqu Prefecture, Tibet autonomous region, China. N. Zeal. J. Agr. Res. 2001, 44, 253–258. [Google Scholar]
- Chang, D.H.S.; Gauch, H.G., Jr. Multivariate analysis of plant communities and environmental factors in Ngari, Tibet. Ecology 1986, 67, 1568–1575. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Wu, J.; Shen, Z.; Zhang, Y.; Xu, X.; Fan, Y.; Zhao, Y.; Yan, W. Root biomass distribution in alpine ecosystems of the northern Tibetan Plateau. Environ. Earth Sci. 2011, 64, 1911–1919. [Google Scholar] [CrossRef]
- Chapin, F.S., III; Vitousek, P.M.; Bloom, A.J.; Field, C.B.; Waring, R.H. Plant responses to multiple environmental factors. Bioscience 1987, 37, 49–57. [Google Scholar] [CrossRef]
- McCarthy, M.C.; Enquist, B. Consistency between an allometric approach and optimal partitioning theory in global patterns of plant biomass allocation. Funct. Ecol. 2007, 21, 713–720. [Google Scholar] [CrossRef]
- Ingestad, T.; Agren, G.I. The Influence of Plant Nutrition on Biomass Allocation. Ecol. Appl. 1991, 1, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.Y.; Yan, Y.; Fan, J.H.; Cao, Y.Z.; Wang, X.D. Dynamics of above- and below-ground biomass and, C.; N, P accumulation in the alpine steppe of northern Tibet. J. Mt. Sci. 2011, 8, 838–844. [Google Scholar] [CrossRef]
- Peichl, M.; Leava, N.A.; Kiely, G. Above- and belowground ecosystem biomass, carbon and nitrogen allocation in recently afforested grassland and adjacent intensively managed grassland. Plant Soil 2011, 350, 281–296. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon isotope discrimination and photosynthesis. Ann. Rev. Plant Physiol. Mol. Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Hartmann, H.; Gleixner, G.; Thoma, M.; Schwab, V.F. Carbon isotope fractionation including photosynthetic and post-photosynthetic processes in C3 plants: Low [CO2] matters. Geochim. Cosmochim. Acta 2018, 245, 1–15. [Google Scholar] [CrossRef]
- Craufurd, P.; Austin, R.; Acevedo, E.; Hall, M. Carbon isotope discrimination and grain-yield in barley. Field Crop. Res. 1991, 27, 301–313. [Google Scholar] [CrossRef]
- Hu, L.U.; Yao, T.; Wang, D. Characters of vegetation in different disturbed habitats of alpine grassland in vulnerable ecological region. Grassl. Turf. 2012, 32, 42–48. [Google Scholar]
- Niu, K.; Choler, P.; Zhao, B.; Du, G. The allometry of reproductive biomass in response to land use in Tibetan alpine grasslands. Funct. Ecol. 2008, 23, 274–283. [Google Scholar] [CrossRef]
- Weiner, J. Allocation, plasticity and allometry in plants. Perspect. Plant Ecol. Evol. Syst. 2004, 6, 207–215. [Google Scholar] [CrossRef]
- Widagdo, F.R.A.; Xie, L.; Dong, L.; Li, F. Origin-based biomass allometric equations, biomass partitioning, and carbon concentration variations of planted and natural Larix gmelinii in northeast China. Glob. Ecol. Conserv. 2020, 23, e01111. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef]
- Krosby, M.; Breckheimer, I.; Pierce, D.J.; Singleton, P.H.; Hall, S.; Halupka, K.C.; Gaines, W.L.; Long, R.A.; McRae, B.H.; Cosentino, B.L.; et al. Focal species and landscape “naturalness” corridor models offer complementary approaches for connectivity conservation planning. Landsc. Ecol. 2015, 30, 2121–2132. [Google Scholar] [CrossRef]
- Shi, J.; Li, X.; Dong, S.; Zhuge, H.; Mu, Y. Trans-boundary conservation of Chiru by identifying its potential movement corridors in the alpine desert of Qinghai-Tibetan Plateau. Glob. Ecol. Conserv. 2018, 16, e00491. [Google Scholar] [CrossRef]
- Gao, Y.H.; Luo, P.; Wu, N.; Chen, H.; Wang, G.X. Impacts of grazing intensity on nitrogen pools and nitrogen pools in an alpine meadow on the eastern Tibetan Plateau. Appl. Ecol. Environ. Res. 2008, 6, 69–79. [Google Scholar] [CrossRef]
- Enquist, B.J. Cope’s Rule and the evolution of long-distance transport in vascular plants: Allometric scaling, biomass partitioning and optimization. Plant Cell Envir. 2003, 26, 151–161. [Google Scholar] [CrossRef] [Green Version]
- Kauffman, J.B.; Cummings, D.L.; Ellingson, L.J. Biomass, Carbon, and Nitrogen Pools in Mexican Tropical Dry Forest Landscapes. Ecosystems 2003, 6, 609–629. [Google Scholar] [CrossRef]
- Varik, M.; Aosaar, J.; Ostonen, I.; Lõhmus, K.; Uri, V. Carbon and nitrogen accumulation in belowground tree biomass in a chronosequence of silver birch stands. For. Ecol. Manag. 2013, 302, 62–70. [Google Scholar] [CrossRef]
- Finér, L.; Mannerkoski, H.; Piirainen, S.; Starr, M. Carbon and nitrogen pools in an old-growth, Norway spruce mixed forest in eastern Finland and changes associated with clear-cutting. For. Ecol. Manag. 2003, 174, 51–63. [Google Scholar] [CrossRef]
- Chapin, F.S., III; Vitousek, P.M.; Van Cleve, K. The nature of nutrient limitation in plant communities. Am. Nat. 1986, 127, 48–58. [Google Scholar] [CrossRef]
- Schuman, G.E.; Reeder, J.D.; Manley, J.T.; Hart, R.H.; Manley, W.A. Impact of grazing management on the carbon and nitrogen balance of a mixed-grass rangeland. Ecol Appl. 1999, 9, 65–71. [Google Scholar] [CrossRef]
- Hibbard, K.A.; Archer, S.; Schimel, D.S.; Valentine, D.W. Biogeochemical changes accompanying woody plant encroachment in a subtropical savanna. Ecology 2001, 82, 1999–2011. [Google Scholar] [CrossRef]
- Gao, Y.Z.; Giese, M.; Lin, S.; Sattelmacher, B.; Zhao, Y.; Brueck, H. Belowground net primary productivity and biomass allocation of a grassland in Inner Mongolia is affected by grazing intensity. Plant Soil 2008, 307, 41–50. [Google Scholar] [CrossRef]
- Milchunas, D.G.; Lauenroth, W.K. Quantitative Effects of Grazing on Vegetation and Soils Over a Global Range of Environments. Ecol. Monogr. 1993, 63, 327–366. [Google Scholar] [CrossRef]
- Dormaar, J.F.; Smoliak, S.; Willms, W.D. Distribution of Nitrogen Fractions in Grazed and Ungrazed Fescue Grassland Ah Horizons. Rangel. Ecol. Manag. 1990, 43, 6. [Google Scholar] [CrossRef]
- Ponton, S.; Flanagan, L.B.; Alstad, K.P.; Johnson, B.G.; Morgenstern, K.; Kljun, N.; Black, T.A.; Barr, A.G. Comparison of ecosystem water-use efficiency among Douglas-fir forest, aspen forest and grassland using eddy covariance and carbon isotope techniques. Glob. Chang. Biol. 2006, 12, 294–310. [Google Scholar] [CrossRef]
- Saurer, M.; Siegwolf, R.T.W.; Schweingruber, F.H. Carbon isotope discrimination indicates improving water-use efficiency of trees in northern Eurasia over the last 100 years. Glob. Chang. Biol. 2004, 10, 2109–2120. [Google Scholar] [CrossRef]
- Farquhar, G.D.; O’Leary, M.H.; Berry, J.A. On the Relationship Between Carbon Isotope Discrimination and the Intercellular Carbon Dioxide Concentration in Leaves. Funct. Plant Biol. 1982, 9, 121–137. [Google Scholar] [CrossRef]
- Sexton, T.M.; Steber, C.M.; Cousins, A.B. Leaf temperature impacts canopy water use efficiency independent of changes in leaf level water use efficiency. J. Plant Physiol. 2021, 258–259, 153357. [Google Scholar] [CrossRef]
- Tsialtas, J.T.; Handley, L.L.; Kassioumi, M.T.; Veresoglou, D.S.; Gagianas, A.A. Interspecific variation in potential water-use efficiency and its relation to plant species abundance in a water-limited grassland. Funct. Ecol. 2001, 15, 605–614. [Google Scholar] [CrossRef]
- Gándara, J.; Ross, S.; Quero, G.; Gonzalo, D.; Joaquín, D.; Figarola, G.; Viega, L. Differential water-use efficiency and growth among Eucalyptus grandis hybrids under two different rainfall conditions. For. Syst. 2020, 29, e006. [Google Scholar] [CrossRef]
- Martin, K.C.; Bruhn, D.; Lovelock, C.E.; Feller, I.C.; Evans, J.R.; Ball, M.C. Nitrogen fertilization enhances water-use efficiency in a saline environment. Plant Cell Environ. 2010, 33, 344–357. [Google Scholar] [CrossRef]
- Evans, J.R.; Poorter, H. Photosynthetic acclimation of plants to growth irradiance: The relative importance of specificleaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ. 2001, 24, 755–767. [Google Scholar] [CrossRef]
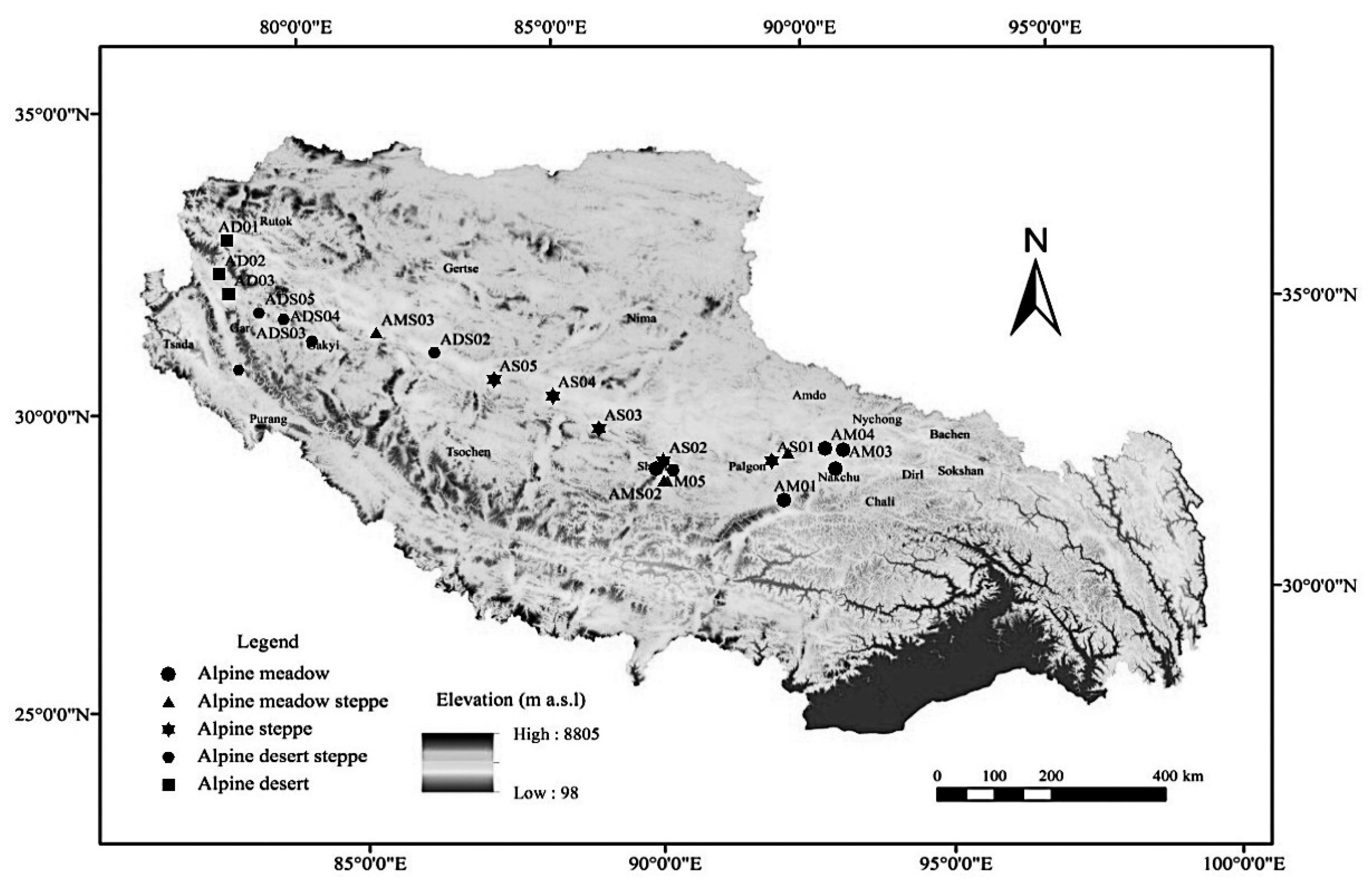
| Grasslands Type | Site | Latitude N | Longitude E | Elevation | Dominant Species | Important Value (IV) | Soil Water Content (SWC, %) |
|---|---|---|---|---|---|---|---|
| alpine meadow (AM) | Nam Co | 91.112 | 30.750 | 4812 | Carex spp. | 0.257 | 8.079 |
| Naqu | 91.980 | 31.377 | 4594 | Poa pratensis | 0.217 | 19.467 | |
| Nima | 92.070 | 31.729 | 4670 | Kobresia humilis | 0.174 | 13.666 | |
| Anduo | 91.730 | 31.716 | 4655 | Kobresia humilis | 0.248 | 14.737 | |
| Shenzha | 88.699 | 30.957 | 4654 | Edelweiss | 0.168 | 12.737 | |
| alpine steppe (AS) | Bange | 90.777 | 31.389 | 4619 | Carex spp. | 0.27 | 13.007 |
| Shenzha | 88.700 | 31.124 | 4735 | Potentilla chinensis | 0.228 | 10.106 | |
| Nima | 87.483 | 31.505 | 4648 | Stipa capillata Linn. | 0.277 | 12.105 | |
| Nima | 86.503 | 31.932 | 4718 | Carex spp. | 0.238 | 11.106 | |
| Gaize | 85.356 | 32.032 | 4785 | Stipa capillata Linn. | 0.19 | 9.206 | |
| alpine meadow steppe (AMS) | Bange | 91.058 | 31.552 | 4544 | Stipa capillata Linn. | 0.309 | 10.980 |
| Shenzha | 88.702 | 30.957 | 4664 | Carex spp. | 0.216 | 10.106 | |
| Gaize | 82.978 | 32.429 | 4421 | Artemisia argyi | 0.315 | 11.897 | |
| alpine desert steppe (ADS) | Shenzha | 88.712 | 30.960 | 4733 | Carex spp. | 0.257 | 8.765 |
| Nima | 84.133 | 32.291 | 4438 | Stipa capillata Linn. | 0.476 | 12.876 | |
| Gaize | 81.826 | 32.071 | 4606 | Ptilotricum wageri | 0.154 | 8.986 | |
| Gaize | 81.206 | 32.327 | 4543 | Potentilla chinensis | 0.153 | 14.176 | |
| Ge’gyai | 80.715 | 32.347 | 4628 | Oxytropis ochrocephala | 0.148 | 9.162 | |
| alpine desert (AD) | Rutog | 79.755 | 33.432 | 4266 | Suaeda corniculate | 0.358 | 2.201 |
| Rutog | 79.784 | 32.838 | 4443 | Stipa capillata Linn. | 0.391 | 3.562 | |
| Rutog | 80.061 | 32.544 | 4400 | Artemisia macilenta | 0.153 | 2.074 |
| Grassland type | AGB (g/m2) | BGB (g/m2) | TB (g/m2) | R/S |
|---|---|---|---|---|
| AM | 24.209 ± 3.402a | 185.760 ± 25.283a | 209.969 ± 28.490a | 7.952 ± 0.481a |
| AS | 16.127 ± 1.793b | 73.606 ± 9.217b | 89.733 ± 10.966b | 4.451 ± 0.198b |
| AMS | 17.767 ± 1.347b | 47.274 ± 8.196c | 65.042 ± 9.397bc | 2.544 ± 0.038cd |
| ADS | 12.133 ± 0.912b | 43.644 ± 6.513c | 55.777 ± 7.358bc | 3.322 ± 0.312c |
| AD | 12.251 ± 0.654b | 25.234 ± 2.520c | 37.485 ± 3.010c | 2.039 ± 0.156d |
| F Value | 5.921 ** | 20.515 ** | 18.319 ** | 49.423 * |
| Grass-land Type | AGB C Concentra-tion (mg/g) | BGB C Concentra-tion (mg/g) | AGB N Concentra-tion (mg/g) | BGB N Concentra-tion (mg/g) | AGB C/N | BGB C/N | Soil N Concentra-tion (mg/g) | Soil C Concentra-tion (mg/g) |
|---|---|---|---|---|---|---|---|---|
| AM | 448.892 ± 7.784a | 403.036 ± 11.778a | 17.511 ± 0.204a | 12.306 ± 0.208a | 25.630 ± 0.302a | 32.911 ± 1.185b | 1.819 ± 0.179a | 32.934 ± 1.466a |
| AS | 413.467 ± 5.530b | 366.600 ± 8.900b | 17.015 ± 0.312a | 10.310 ± 0.340b | 24.479 ± 0.570abc | 35.952 ± 1.169ab | 0.855 ± 0.054b | 15.150 ± 0.120b |
| AMS | 423.056 ± 9.950b | 324.056 ± 13.379c | 17.003 ± 0.187a | 10.098 ± 0.375b | 24.916 ± 0.708ab | 32.566 ± 2.037b | 0.442 ± 0.077c | 7.437 ± 0.229c |
| ADS | 372.467 ± 9.214c | 330.633 ± 9.990c | 15.748 ± 0.347b | 9.783 ± 0.412b | 23.848 ± 0.923bc | 34.567 ± 1.657b | 0.170 ± 0.001d | 4.035 ± 0.051d |
| AD | 303.944 ± 7.122d | 336.667 ± 5.891bc | 13.559 ± 0.147c | 8.651 ± 0.478c | 22.420 ± 0.495c | 39.616 ± 1.710a | 0.086 ± 0.016e | 1.818 ± 0.151e |
| F Value | 47.715 ** | 9.714 ** | 26.059 ** | 12.828 ** | 2.780 * | 2.821 * | 43.089 ** | 262.484 ** |
| Grassland Type | AGB C Stock (g C/m2) | BGB C Stock (g C/m2) | AGB N Stock (g N/m2) | BGB N Stock (g N/m2) | TB C Stock (g C/m2) | TB N Stock (g N/m2) |
|---|---|---|---|---|---|---|
| AM | 12.104 ± 1.701a | 92.880 ± 12.642a | 0.472 ± 0.067a | 2.915 ± 0.472a | 104.984 ± 14.245a | 3.387 ± 0.534b |
| AS | 8.064 ± 0.896b | 36.803 ± 4.609b | 0.338 ± 0.043ab | 1.063 ± 0.161b | 44.867 ± 5.483b | 1.400 ± 0.203b |
| AMS | 8.884 ± 0.674b | 23.637 ± 4.098bc | 0.360 ± 0.030ab | 0.768 ± 0.175b | 32.521 ± 4.699bc | 1.128 ± 0.200b |
| ADS | 6.067 ± 0.456b | 21.822 ± 3.256bc | 0.259 ± 0.022b | 0.790 ± 0.127b | 27.889 ± 3.679bc | 0.959 ± 0.146b |
| AD | 6.126 ± 0.327b | 12.617 ± 1.260c | 0.276 ± 0.019b | 0.328 ± 0.037b | 18.743 ± 1.505c | 0.604 ± 0.055b |
| F Value | 5.921 ** | 20.515 ** | 3.894 ** | 14.795 ** | 18.319 ** | 13.058 ** |
| Index | WUEi | AGB | BGB | TB | R/S |
|---|---|---|---|---|---|
| AGB C | −0.710 ** | 0.354 ** | 0.448 ** | 0.443 ** | 0.504 ** |
| BGB C | −0.592 ** | 0.247 | 0.441 ** | 0.426 ** | 0.518 ** |
| AGB N | −0.693 ** | 0.359 ** | 0.436 ** | 0.433 ** | 0.522 ** |
| BGB N | −0.632 ** | 0.508 ** | 0.638 ** | 0.632 ** | 0.643 ** |
| AGB C/N | −0.259 * | 0.119 | 0.167 | 0.163 | 0.163 |
| BGB C/N | 0.207 | −0.325 * | −0.298 | 0.162 | −0.265 * |
| SWC | −0.626 ** | 0.103 | 0.332 ** | 0.312 * | 0.656 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, L.; Zhang, B.; Zhang, H.; Li, J. Biomass, Carbon and Nitrogen Partitioning and Water Use Efficiency Differences of Five Types of Alpine Grasslands in the Northern Tibetan Plateau. Int. J. Environ. Res. Public Health 2022, 19, 13026. https://doi.org/10.3390/ijerph192013026
Cheng L, Zhang B, Zhang H, Li J. Biomass, Carbon and Nitrogen Partitioning and Water Use Efficiency Differences of Five Types of Alpine Grasslands in the Northern Tibetan Plateau. International Journal of Environmental Research and Public Health. 2022; 19(20):13026. https://doi.org/10.3390/ijerph192013026
Chicago/Turabian StyleCheng, Liping, Beibei Zhang, Hui Zhang, and Jiajia Li. 2022. "Biomass, Carbon and Nitrogen Partitioning and Water Use Efficiency Differences of Five Types of Alpine Grasslands in the Northern Tibetan Plateau" International Journal of Environmental Research and Public Health 19, no. 20: 13026. https://doi.org/10.3390/ijerph192013026
APA StyleCheng, L., Zhang, B., Zhang, H., & Li, J. (2022). Biomass, Carbon and Nitrogen Partitioning and Water Use Efficiency Differences of Five Types of Alpine Grasslands in the Northern Tibetan Plateau. International Journal of Environmental Research and Public Health, 19(20), 13026. https://doi.org/10.3390/ijerph192013026







