Health Risk Assessment during In Situ Remediation of Cr(VI)-Contaminated Groundwater by Permeable Reactive Barriers: A Field-Scale Study
Abstract
:1. Introduction
2. Materials and Methods
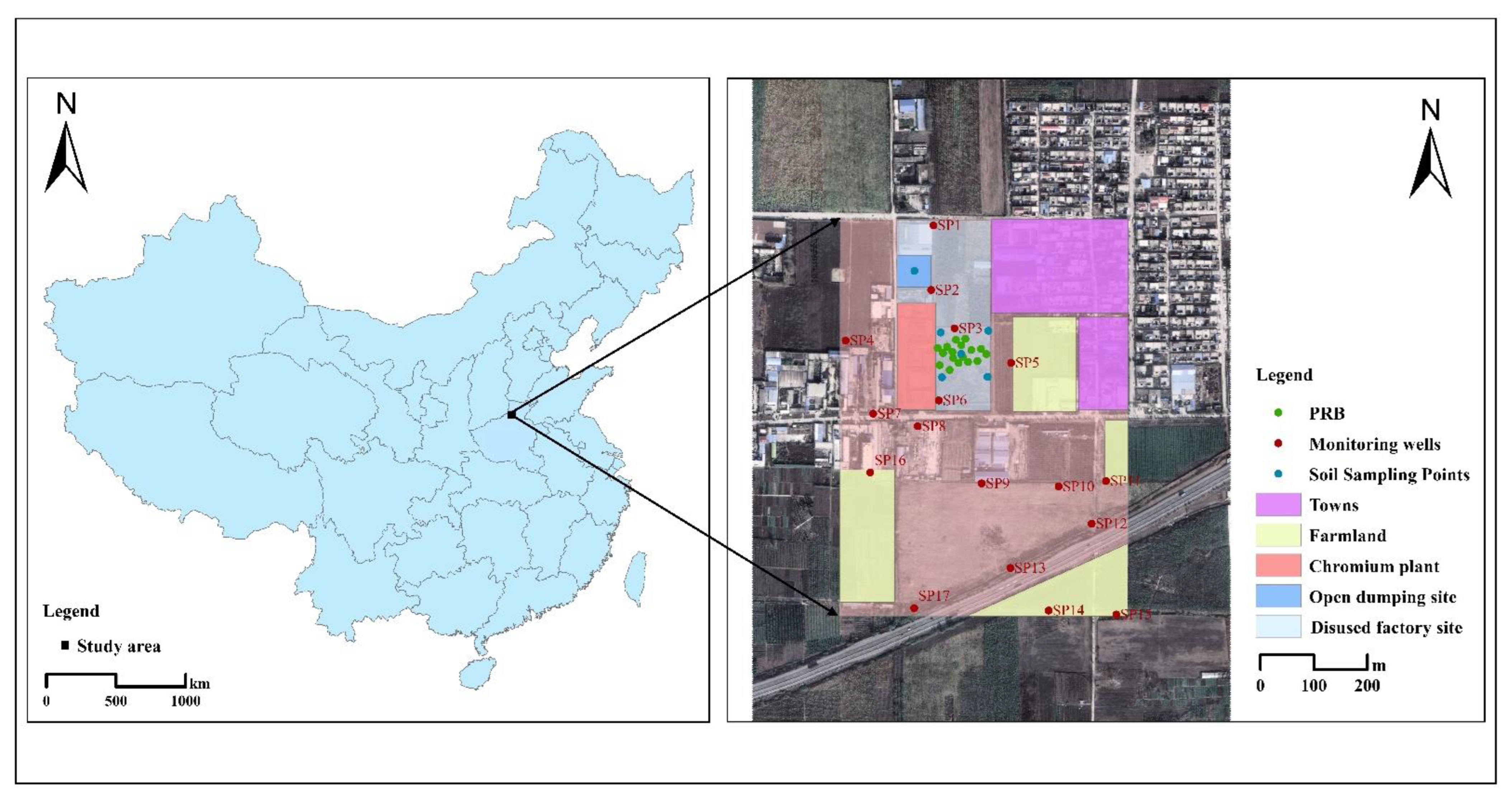
2.1. Study Area
2.2. Sample Collection and Analysis
2.3. Human Health Risk Assessment
2.3.1. Exposure Routes and Calculating Exposure
2.3.2. Risk Characterization and Contribution Rates
2.4. Sensitivity Analysis
2.5. Spatial Distribution
3. Results
3.1. Characterization of Cr(Ⅵ) Contamination
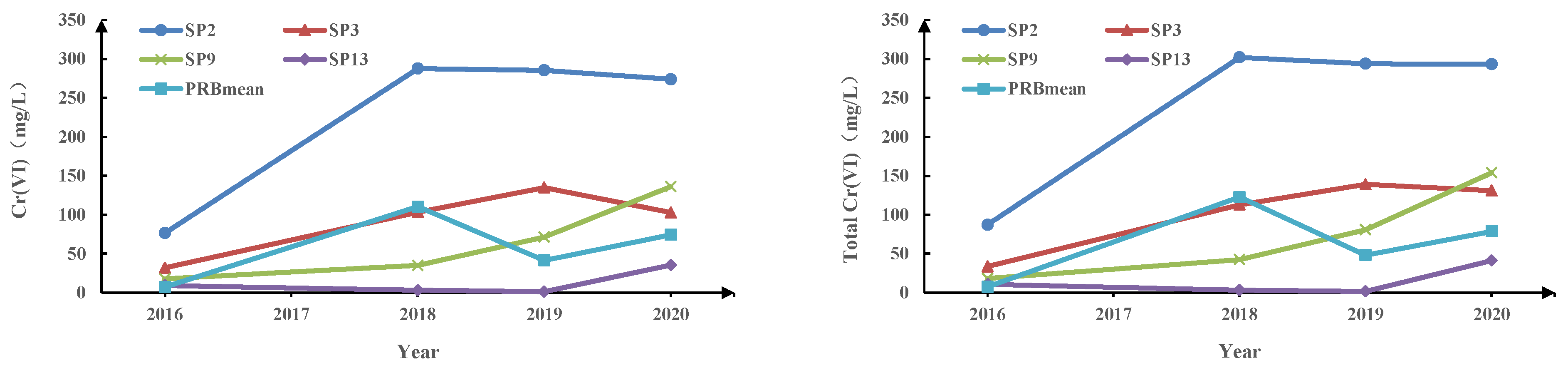
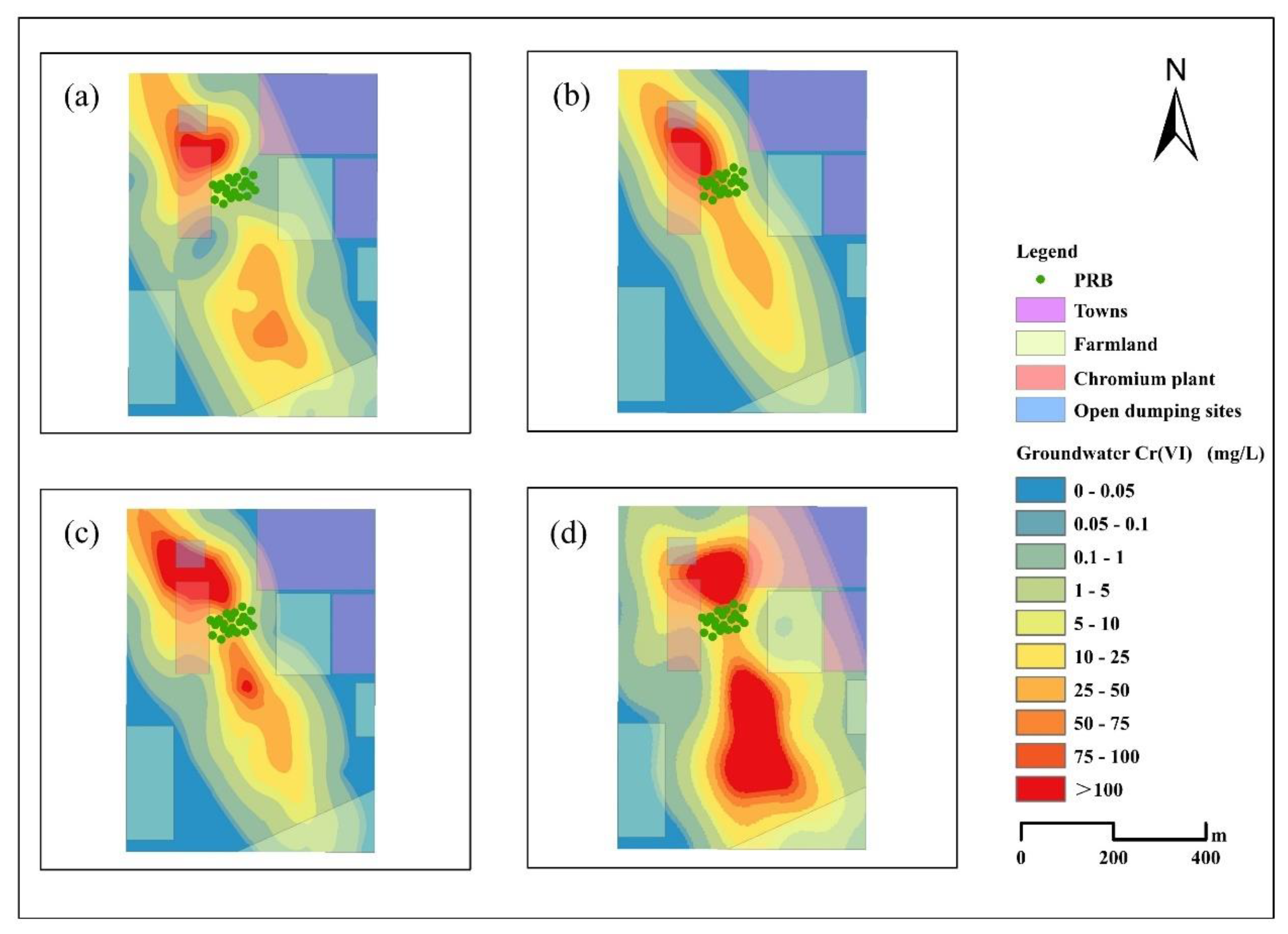
3.2. Spatial and Temporal Effects of PRB on Cr(VI)
3.3. Characterization of the Health Risk
3.4. Sensitivity Analysis for Carcinogenic Risk Assessment
4. Discussion
4.1. Characterization of Cr(Ⅵ) Contamination
4.2. Spatial and Temporal Effects of PRB on Cr(VI)
4.3. Characterization of the Health Risk
4.4. Sensitivity Analysis for Carcinogenic Risk Assessment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, W.; Chai, J.; Li, S.; Wang, X.; Wu, S.; Liang, Z.; Baloch, M.Y.J.; Silva, L.F.O.; Zhang, D. Physiological characteristics, geochemical properties and hydrological variables influencing pathogen migration in subsurface system: What we know or not? Geosci. Front. 2022, 13, 101346. [Google Scholar] [CrossRef]
- Jat Baloch, M.Y.; Zhang, W.; Chai, J.; Li, S.; Alqurashi, M.; Rehman, G.; Tariq, A.; Talpur, S.A.; Iqbal, J.; Munir, M.; et al. Shallow Groundwater Quality Assessment and Its Suitability Analysis for Drinking and Irrigation Purposes. Water 2021, 13, 3361. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, T.; Chen, N.; Feng, C. Changes in microbial community diversity, composition, and functions upon nitrate and Cr(VI) contaminated groundwater. Chemosphere 2022, 288, 132476. [Google Scholar] [CrossRef]
- Li, D.; Li, G.; He, Y.; Zhao, Y.; Miao, Q.; Zhang, H.; Yuan, Y.; Zhang, D. Key Cr species controlling Cr stability in contaminated soils before and chemical stabilization at a remediation engineering site. J. Hazard. Mater. 2022, 424, 127532. [Google Scholar] [CrossRef]
- Lian, G.; Wang, B.; Lee, X.; Li, L.; Liu, T.; Lyu, W. Enhanced removal of hexavalent chromium by engineered biochar composite fabricated from phosphogypsum and distillers grains. Sci. Total Environ. 2019, 697, 134119. [Google Scholar] [CrossRef]
- Yoshinaga, M.; Ninomiya, H.; Al Hossain, M.M.A.; Sudo, M.; Akhand, A.A.; Ahsan, N.; Alim, M.A.; Khalequzzaman, M.; Iida, M.; Yajima, I. A comprehensive study including monitoring, assessment of health effects and development of a remediation method for chromium pollution. Chemosphere 2018, 201, 667–675. [Google Scholar] [CrossRef]
- Ge, S.; Ge, S.C. Simultaneous Cr(VI) reduction and Zn(II) biosorption by Stenotrophomonas sp. and constitutive expression of related genes. Biotechnol. Lett. 2016, 38, 877–884. [Google Scholar] [CrossRef]
- Fuoco, I.; Figoli, A.; Criscuoli, A.; Brozzo, G.; De Rosa, R.; Gabriele, B.; Apollaro, C. Geochemical modeling of chromium release in natural waters and treatment by RO/NF membrane processes. Chemosphere 2020, 254, 126696. [Google Scholar] [CrossRef]
- Li, Z.; Wang, G.; Cai, W.; Liu, F.; HUANG, D. Reactive materials and structural design of PRB for remediation of a Cr(Ⅵ)contaminated site. Earth Sci. Front. 2021, 28, 186–196. [Google Scholar]
- Gómez, V.; Callao, M.P. Chromium determination and speciation since 2000. TrAC Trends Anal. Chem. 2006, 25, 1006–1015. [Google Scholar] [CrossRef]
- Yang, X.; Liu, P.; Yao, M.; Sun, H.; Liu, R.; Xie, J.; Zhao, Y. Mechanism and enhancement of Cr(VI) contaminated groundwater remediation by molasses. Sci. Total Environ. 2021, 780, 146580. [Google Scholar] [CrossRef]
- Stambulska, U.Y.; Bayliak, M.M.; Lushchak, V.I. Chromium(VI) Toxicity in Legume Plants: Modulation Effects of Rhizobial Symbiosis. BioMed Res. Int. 2018, 2018, 8031213. [Google Scholar] [CrossRef]
- Prasad, S.; Yadav, K.K.; Kumar, S.; Gupta, N.; Cabral-Pinto, M.M.S.; Rezania, S.; Radwan, N.; Alam, J. Chromium contamination and effect on environmental health and its remediation: A sustainable approaches. J. Environ. Manag. 2021, 285, 112174. [Google Scholar] [CrossRef]
- Tseng, C.-H.; Lee, I.-H.; Chen, Y.-C. Evaluation of hexavalent chromium concentration in water and its health risk with a system dynamics model. Sci. Total Environ. 2019, 669, 103–111. [Google Scholar] [CrossRef]
- Mishra, S.; Bharagava, R. Toxic and genotoxic effects of hexavalent chromium in environment and its bioremediation strategies. J. Environ. Sci. Health PART C-Environ. Carcinog. Ecotoxicol. Rev. 2016, 34, 1–32. [Google Scholar] [CrossRef]
- Jiang, B.; Li, G.; Xing, Y.; Zhang, D.; Jia, J.; Cui, Z.; Luan, X.; Tang, H. A whole-cell bioreporter assay for quantitative genotoxicity evaluation of environmental samples. Chemosphere 2017, 184, 384–392. [Google Scholar] [CrossRef] [Green Version]
- Bharagava, R.N.; Mishra, S. Hexavalent chromium reduction potential of Cellulosimicrobium sp. isolated from common effluent treatment plant of tannery industries. Ecotoxicol. Environ. Saf. 2018, 147, 102–109. [Google Scholar] [CrossRef]
- Ou, J.-H.; Sheu, Y.-T.; Tsang, D.C.W.; Sun, Y.-J.; Kao, C.-M. Application of iron/aluminum bimetallic nanoparticle system for chromium-contaminated groundwater remediation. Chemosphere 2020, 256, 127158. [Google Scholar] [CrossRef]
- Song, X.; Wang, Q.; Jin, P.; Chen, X.; Tang, S.; Wei, C.; Li, K.; Ding, X.; Tang, Z.; Fu, H. Enhanced biostimulation coupled with a dynamic groundwater recirculation system for Cr(VI) removal from groundwater: A field-scale study. Sci. Total Environ. 2021, 772, 145495. [Google Scholar] [CrossRef]
- Gibert, O.; Assal, A.; Devlin, H.; Elliot, T.; Kalin, R.M. Performance of a field-scale biological permeable reactive barrier for in-situ remediation of nitrate-contaminated groundwater. Sci. Total Environ. 2019, 659, 211–220. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Mou, H.; Chen, L.; Mirza, Z.A.; Liu, L. Cr(VI)-contaminated groundwater remediation with simulated permeable reactive barrier (PRB) filled with natural pyrite as reactive material: Environmental factors and effectiveness. J. Hazard. Mater. 2015, 298, 83–90. [Google Scholar] [CrossRef]
- Song, J.; Huang, G.; Han, D.; Hou, Q.; Gan, L.; Zhang, M. A review of reactive media within permeable reactive barriers for the removal of heavy metal(loid)s in groundwater: Current status and future prospects. J. Clean. Prod. 2021, 319, 128644. [Google Scholar] [CrossRef]
- Maamoun, I.; Eljamal, O.; Falyouna, O.; Eljamal, R.; Sugihara, Y. Multi-objective optimization of permeable reactive barrier design for Cr(VI) removal from groundwater. Ecotoxicol. Environ. Saf. 2020, 200, 110773. [Google Scholar] [CrossRef]
- Wang, Q.; Song, X.; Wei, C.; Jin, P.; Chen, X.; Tang, Z.; Li, K.; Ding, X.; Fu, H. In situ remediation of Cr(VI) contaminated groundwater by ZVI-PRB and the corresponding indigenous microbial community responses: A field-scale study. Sci. Total Environ. 2022, 805, 150260. [Google Scholar] [CrossRef]
- Cao, R.; Liu, S.; Yang, X.; Wang, C.; Wang, Y.; Wang, W.; Pi, Y. Enhanced remediation of Cr(VI)-contaminated groundwater by coupling electrokinetics with ZVI/Fe3O4/AC-based permeable reactive barrier. J. Environ. Sci. 2022, 112, 280–290. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, M.; Li, X. Improved longevity of nanoscale zero-valent iron with a magnesium hydroxide coating shell for the removal of Cr(VI) in sand columns. Environ. Int. 2019, 133, 105249. [Google Scholar] [CrossRef]
- Panichev, N.; Mabasa, W.; Ngobeni, P.; Mandiwana, K.; Panicheva, S. The oxidation of Cr(III) to Cr(VI) in the environment by atmospheric oxygen during the bush fires. J. Hazard. Mater. 2008, 153, 937–941. [Google Scholar] [CrossRef]
- Fallahzadeh, R.A.; Khosravi, R.; Dehdashti, B.; Ghahramani, E.; Omidi, F.; Adli, A.; Miri, M. Spatial distribution variation and probabilistic risk assessment of exposure to chromium in ground water supplies; a case study in the east of Iran. Food Chem. Toxicol. 2018, 115, 260–266. [Google Scholar] [CrossRef]
- Kaur, L.; Rishi, M.S.; Siddiqui, A.U. Deterministic and probabilistic health risk assessment techniques to evaluate non-carcinogenic human health risk (NHHR) due to fluoride and nitrate in groundwater of Panipat, Haryana, India. Environ. Pollut. 2020, 259, 113711. [Google Scholar] [CrossRef]
- Wang, H.-Z.; Cai, L.-M.; Wang, Q.-S.; Hu, G.-C.; Chen, L.-G. A comprehensive exploration of risk assessment and source quantification of potentially toxic elements in road dust: A case study from a large Cu smelter in central China. CATENA 2021, 196, 104930. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, R.; Zhang, L.; Zheng, R.; Zhong, M. Improving the regulatory health risk assessment of mercury-contaminated sites. J. Hazard. Mater. 2021, 402, 123493. [Google Scholar] [CrossRef]
- Eslami, H.; Esmaeili, A.; Razaeian, M.; Salari, M.; Hosseini, A.N.; Mobinia, M.; Barani, A. Potentially toxic metal concentration, spatial distribution, and health risk assessment in drinking groundwater resources of southeast Iran. Geosci. Front. 2022, 13, 133–143. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, X.; Fang, X.; Zhang, N.; Xu, X.; Li, L.; Liu, Y.; Su, X.; Xia, Y. Characterization of drinking groundwater quality in rural areas of Inner Mongolia and assessment of human health risks. Ecotoxicol. Environ. Saf. 2022, 234, 113360. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wu, J. Hydrogeochemical Characteristics, Groundwater Quality, and Health Risks from Hexavalent Chromium and Nitrate in Groundwater of Huanhe Formation in Wuqi County, Northwest China. Expo. Health 2019, 11, 125–137. [Google Scholar] [CrossRef]
- Karunanidhi, D.; Aravinthasamy, P.; Kumar, D.; Subramani, T.; Roy, P.D. Sobol sensitivity approach for the appraisal of geomedical health risks associated with oral intake and dermal pathways of groundwater fluoride in a semi-arid region of south India. Ecotoxicol. Environ. Saf. 2020, 194, 110438. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Singh, A.; Jha, R.K.; Sahoo, S.K.; Jha, V. A Variance Decomposition Approach for Risk Assessment of Groundwater Quality. Expo. Health 2019, 11, 139–151. [Google Scholar] [CrossRef]
- Karunanidhi, D.; Aravinthasamy, P.; Subramani, T.; Kumar, D.; Setia, R. Investigation of health risks related with multipath entry of groundwater nitrate using Sobol sensitivity indicators in an urban-industrial sector of south India. Environ. Res. 2021, 200, 111726. [Google Scholar] [CrossRef]
- Mukherjee, I.; Singh, U.K. Characterization of groundwater nitrate exposure using Monte Carlo and Sobol sensitivity approaches in the diverse aquifer systems of an agricultural semiarid region of Lower Ganga Basin, India. Sci. Total Environ. 2021, 787, 147657. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, A.; Kumar, P.; Jha, R.K.; Sahoo, S.K.; Jha, V. Sobol sensitivity analysis for risk assessment of uranium in groundwater. Environ. Geochem. Health 2020, 42, 1789–1801. [Google Scholar] [CrossRef]
- Sanchez-Hachair, A.; Hofmann, A. Hexavalent chromium quantification in solution: Comparing direct UV–visible spectrometry with 1,5-diphenylcarbazide colorimetry. Comptes Rendus Chim. 2018, 21, 890–896. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, F.; Li, J.; Liu, Q.; Tu, C.; Suzuki, Y.; Huang, C. Spatial Distribution, Potential Sources, and Risk Assessment of Trace Metals of Groundwater in the North China Plain. Hum. Ecol. RISK Assess. 2015, 21, 726–743. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Shafieizadeh, M.; Taher, M.A.; Opoku, F.; Kiarii, E.M.; Govender, P.P.; Ranjbari, S.; Rezapour, M.; Orooji, Y. The role of magnetite/graphene oxide nano-composite as a high-efficiency adsorbent for removal of phenazopyridine residues from water samples, an experimental/theoretical investigation. J. Mol. Liq. 2020, 298, 112040. [Google Scholar] [CrossRef]
- Yang, J.; Ye, M.; Tang, Z.; Jiao, T.; Song, X.; Pei, Y.; Liu, H. Using cluster analysis for understanding spatial and temporal patterns and controlling factors of groundwater geochemistry in a regional aquifer. J. Hydrol. 2020, 583, 124594. [Google Scholar] [CrossRef]
- Bux, R.K. Natural and anthropogenic origin of metallic contamination and health risk assessment: A hydro-geochemical study of Sehwan Sharif, Pakistan. Chemosphere 2022, 300, 134611. [Google Scholar] [CrossRef]
- USEPA. Review of Background Concentrations for Metals; USEPA: Washington, DC, USA, 2007.
- WHO. Guidelines for drinking-water quality. In Guidelines for Drinking-Water Quality, 4th ed.; World Health Organisation: Geneva, Switzerland, 2011. Available online: https://apps.who.int/iris/handle/10665/44584 (accessed on 16 July 2012).
- Thacker, U.; Parikh, R.; Shouche, Y. Madamwar, Reduction of chromate by cell-free extract of Brucella sp. isolated from Cr(VI) contaminated sites. Bioresour. Technol. 2007, 98, 1541–1547. [Google Scholar] [CrossRef]
- Němeček, J.; Lhotský, O.; Cajthaml, T. Nanoscale zero-valent iron application for in situ reduction of hexavalent chromium and its effects on indigenous microorganism populations. Sci. Total Environ. 2014, 485, 739–747. [Google Scholar] [CrossRef]
- Wei, Y.; Xu, X.; Zhao, L.; Chen, X.; Qiu, H.; Gao, B.; Cao, X. Migration and transformation of chromium in unsaturated soil during groundwater table fluctuations induced by rainfall. J. Hazard. Mater. 2021, 416, 126229. [Google Scholar] [CrossRef]
- Slejko, F.; Petrini, R.; Lutman, A.; Forte, C.; Ghezzi, L. Chromium isotopes tracking the resurgence of hexavalent chromium contamination in a past-contaminated area in the Friuli Venezia Giulia Region, northern Italy. Isot. Environ. Health Stud. 2019, 55, 56–69. [Google Scholar] [CrossRef]
- MEEPRC. The Technical Guidelines for Risk Assessment of Soil Contamination of Land for Construction. 2019. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/trhj/201912/t20191224_749893.shtml (accessed on 5 December 2019).
- Bhattacharya, M.; Shriwastav, A.; Bhole, S.; Silori, R.; Mansfeldt, T.; Kretzschmar, R.; Singh, A. Processes Governing Chromium Contamination of Groundwater and Soil from a Chromium Waste Source. ACS Earth Space Chem. 2020, 4, 35–49. [Google Scholar] [CrossRef]
- Lyu, H.; Tang, J.; Huang, Y.; Gai, L.; Zeng, E.Y.; Liber, K.; Gong, Y. Removal of hexavalent chromium from aqueous solutions by a novel biochar supported nanoscale iron sulfide composite. Chem. Eng. J. 2017, 322, 516–524. [Google Scholar] [CrossRef]
- Thakur, A.K.; Vithanage, M.; Das, D.B.; Kumar, M. A review on design, material selection, mechanism, and modelling of permeable reactive barrier for community-scale groundwater treatment. Environ. Technol. Innov. 2020, 19, 100917. [Google Scholar] [CrossRef]
- Huang, Y.; Zeng, Q.; Hu, L.; Xiong, D.; Zhong, H.; He, Z. Column study of enhanced Cr(Ⅵ) removal and removal mechanisms by Sporosarcina saromensis W5 assisted bio-permeable reactive barrier. J. Hazard. Mater. 2021, 405, 124115. [Google Scholar] [CrossRef]
- Giri, R.K.V.; Raju, L.S.; Nancharaiah, Y.V.; Pulimi, M.; Chandrasekaran, N.; Mukherjee, A. Anaerobic nano zero-valent iron granules for hexavalent chromium removal from aqueous solution. Environ. Technol. Innov. 2019, 16, 100495. [Google Scholar] [CrossRef]
- Zhu, F.; Tan, X.; Zhao, W.; Feng, L.; He, S.; Wei, L.; Yang, L.; Wang, K.; Zhao, Q. Efficiency assessment of ZVI-based media as fillers in permeable reactive barrier for multiple heavy metal-contaminated groundwater remediation. J. Hazard. Mater. 2022, 424, 127605. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, B.; Yin, H.; Meng, L.; Jin, W.; Wang, F.; Xu, J.; Al-Tabbaa, A. Application of zeolites in permeable reactive barriers (PRBs) for in-situ groundwater remediation: A critical review. Chemosphere 2022, 308, 136290. [Google Scholar] [CrossRef]
- Medawela, S.; Indraratna, B.; Athuraliya, S.; Lugg, G.; Nghiem, L.D. Monitoring the performance of permeable reactive barriers constructed in acid sulfate soils. Eng. Geol. 2022, 296, 106465. [Google Scholar] [CrossRef]
- Lü, Y.; Li, Z.; Li, J.; Chen, K.; Dong, H.; Shou, J.; Li, Y. Synergetic effect of pyrite on Cr(VI) removal by zero valent iron in column experiments: An investigation of mechanisms. Chem. Eng. J. 2018, 349, 522–529. [Google Scholar] [CrossRef]
- Obiri-Nyarko, F.; Duah, A.A.; Karikari, A.Y.; Agyekum, W.A.; Manu, E.; Tagoe, R. Assessment of heavy metal contamination in soils at the Kpone landfill site, Ghana: Implication for ecological and health risk assessment. Chemosphere 2021, 282, 131007. [Google Scholar] [CrossRef]

| Concentration (mg/kg) | CR | HQ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total Cr | Cr (VI) | Ingestion | Dermal Contact | Inhalation | Total CR | Ingestion | Dermal contact | Inhalation | Total HQ | |
| Max | 592.81 | 566.2 | 2.73 × 10−6 | 6.76 × 10−6 | 7.82 × 10−5 | 8.77 × 10−5 | 1.0068 | 2.4978 | 0.9378 | 4.4423 |
| Min | 2.58 | 2.53 | 1.22 × 10−8 | 3.02 × 10−8 | 3.49 × 10−7 | 3.92 × 10−7 | 0.0045 | 0.0112 | 0.0042 | 0.0199 |
| Mean | 102.04 | 97.3 | 4.69 × 10−7 | 1.16 × 10−6 | 1.34 × 10−5 | 1.51 × 10−5 | 0.173 | 0.4292 | 0.1611 | 0.7634 |
| Periods | Total Cr (mg/L) | Cr (VI) (mg/L) | CR | HQ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Max | Min | Mean | Max | Min | Mean | Max | Min | Mean | Max | Min | Mean | |
| 2016.09 | 87.23 | 0.01 | 9.07 | 76.52 | 0.01 | 7.85 | 3.68 × 10−3 | 4.82 × 10−7 | 3.78 × 10−4 | 1360.3 | 0.18 | 139.54 |
| 2018.04 | 487.56 | 0.04 | 82.29 | 464.34 | 0.03 | 70.48 | 2.23 × 10−2 | 1.4410−6 | 3.39 × 10−3 | 8250.69 | 0.53 | 1253.22 |
| 2019.01 | 293.86 | 0.01 | 40.72 | 285.15 | 0.01 | 35.61 | 1.37 × 10−2 | 4.82 × 10−7 | 1.72 × 10−3 | 5073.11 | 0.18 | 633.28 |
| 2020.05 | 476.51 | 0.01 | 64.56 | 362.42 | 0.01 | 54.03 | 1.75 × 10−2 | 4.82 × 10−7 | 2.60 × 10−3 | 6444.36 | 0.18 | 960.72 |
| Parameters | Unit | Value ± SD |
|---|---|---|
| Body weight of adults(BWa) | kg | 64.2 ± 9.63 |
| Daily groundwater consumption rate of adults(GWCRa) | L·d−1 | 1 ± 0.15 |
| Exposure frequency of adults(EFa) | d·a−1 | 250 ± 37.5 |
| Skin exposure ratio of adults(SERa) | - | 0.18 ± 0.027 |
| Concentration(C) | mg/L | min:0.01 max:464.3 |
| Daily oral ingestion rate of soils of adults(OSIRa) | mg·d−1 | 100 ± 15 |
| Height of adults(Ha) | cm | min: 161.5 max: 185 |
| Content of inhalable particulates in ambient air(PM10) | mg·m−3 | 0.119 ± 0.018 |
| Daily air inhalation rate of adults(DAIRa) | m3·d−1 | 14.5 ± 2.175 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Zhu, Y.; Gu, R.; Liang, Z.; Xu, W.; Jat Baloch, M.Y. Health Risk Assessment during In Situ Remediation of Cr(VI)-Contaminated Groundwater by Permeable Reactive Barriers: A Field-Scale Study. Int. J. Environ. Res. Public Health 2022, 19, 13079. https://doi.org/10.3390/ijerph192013079
Zhang W, Zhu Y, Gu R, Liang Z, Xu W, Jat Baloch MY. Health Risk Assessment during In Situ Remediation of Cr(VI)-Contaminated Groundwater by Permeable Reactive Barriers: A Field-Scale Study. International Journal of Environmental Research and Public Health. 2022; 19(20):13079. https://doi.org/10.3390/ijerph192013079
Chicago/Turabian StyleZhang, Wenjing, Yifan Zhu, Ruiting Gu, Zhentian Liang, Wenyan Xu, and Muhammad Yousuf Jat Baloch. 2022. "Health Risk Assessment during In Situ Remediation of Cr(VI)-Contaminated Groundwater by Permeable Reactive Barriers: A Field-Scale Study" International Journal of Environmental Research and Public Health 19, no. 20: 13079. https://doi.org/10.3390/ijerph192013079







