Influence of UGT1A1 Genetic Variants on Free Bilirubin Levels in Japanese Newborns: A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. Statistical Analyses
3. Results
3.1. UGT1A1 Variants
3.2. Odds Ratio
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of Hyperbilirubinemia in the Newborn Infant 35 or More Weeks of Gestation. Pediatrics 2004, 114, 297–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, L.; Bhutani, V.K. The Clinical Syndrome of Bilirubin-Induced Neurologic Dysfunction. Semin. Perinatol. 2011, 35, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Le Pichon, J.B.; Riordan, S.M.; Watchko, J.; Shapiro, S.M. The Neurological Sequelae of Neonatal Hyperbilirubinemia: Definitions, Diagnosis and Treatment of the Kernicterus Spectrum Disorders (KSDs). Curr. Pediatr. Rev. 2017, 13, 199–209. [Google Scholar] [PubMed]
- Diamond, I.; Schmid, R. Experimental Bilirubin Encephalopathy. The Mode of Entry of bilirubin-14C Into the Central Nervous System. J. Clin. Investig. 1966, 45, 678–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgess, G.H.; Oh, W.; Bratlid, D.; Brubakk, A.M.; Cashore, W.J.; Stonestreet, B.S. The Effects of Brain Blood Flow on Brain Bilirubin Deposition in Newborn Piglets. Pediatr. Res. 1985, 19, 691–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahlfors, C.E.; Wennberg, R.P.; Ostrow, J.D.; Tiribelli, C. Unbound (Free) Bilirubin: Improving the Paradigm for Evaluating Neonatal Jaundice. Clin. Chem. 2009, 55, 1288–1299. [Google Scholar] [CrossRef] [Green Version]
- Cashore, W.J.; Oh, W. Unbound Bilirubin and Kernicterus in Low-Birth-Weight Infants. Pediatrics 1982, 69, 481–485. [Google Scholar] [CrossRef]
- Funato, M.; Tamai, H.; Shimada, S.; Nakamura, H. Vigintiphobia, Unbound Bilirubin, and Auditory Brainstem Responses. Pediatrics 1994, 93, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Yonetani, M.; Uetani, Y.; Funato, M.; Lee, Y. Determination of Serum Unbound Bilirubin for Prediction of Kernicterus in Low Birthweight Infants. Acta Paediatr. Jpn. 1992, 34, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Hegyi, T.; Kleinfeld, A. Neonatal Hyperbilirubinemia and the Role of Unbound Bilirubin. J. Matern. Fetal Neonatal Med. 2021, 1–7. [Google Scholar] [CrossRef]
- Morioka, I.; Nakamura, H.; Koda, T.; Sakai, H.; Kurokawa, D.; Yonetani, M.; Morisawa, T.; Katayama, Y.; Wada, H.; Funato, M.; et al. Serum Unbound Bilirubin as a Predictor for Clinical Kernicterus in Extremely Low Birth Weight Infants at a Late Age in the Neonatal Intensive Care Unit. Brain Dev. 2015, 37, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Morioka, I. Hyperbilirubinemia in Preterm Infants in Japan: New Treatment Criteria. Pediatr. Int. 2018, 60, 684–690. [Google Scholar] [CrossRef] [Green Version]
- Abe, S.; Fujioka, K.; Nakasone, R.; Suga, S.; Ashina, M.; Nishida, K.; Wong, R.J.; Iijima, K. Bilirubin/Albumin (B/A) Ratios Correlate With Unbound Bilirubin Levels in Preterm Infants. Pediatr. Res. 2021, 89, 1427–1431. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, P.I.; Owens, I.S.; Burchell, B.; Bock, K.W.; Bairoch, A.; Bélanger, A.; Fournel-Gigleux, S.; Green, M.; Hum, D.W.; Iyanagi, T.; et al. The UDP Glycosyltransferase Gene Superfamily: Recommended Nomenclature Update Based on Evolutionary Divergence. Pharmacogenetics 1997, 7, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, Q.; Zheng, L.; Lin, M.; Zheng, X.B.; Lin, F.; Yang, L.Y. Multiple Genetic Modifiers of Bilirubin Metabolism Involvement in Significant Neonatal Hyperbilirubinemia in Patients of Chinese Descent. PLoS ONE 2015, 10, e0132034. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.H.; Chiu, Y.W.; Cheng, S.W.; Yang, C.Y. Risk Assessment of Gene Variants for Neonatal Hyperbilirubinemia in Taiwan. B.M.C. Pediatr. 2016, 16, 144. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.J.; Zhong, D.N.; Xie, X.Z.; Ye, D.Z.; Gao, Z.Y. UGT1A1 Gene Mutations and Neonatal Hyperbilirubinemia in Guangxi Heiyi Zhuang and Han Populations. Pediatr. Res. 2015, 78, 585–588. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.Y.; Lee, L.Y.; Ng, S.Y.; Hia, C.P.; Low, K.T.; Chong, Y.S.; Goh, D.L. UGT1A1 Haplotype Mutation Among Asians in Singapore. Neonatology 2009, 96, 150–155. [Google Scholar] [CrossRef]
- Akaba, K.; Kimura, T.; Sasaki, A.; Tanabe, S.; Wakabayashi, T.; Hiroi, M.; Yasumura, S.; Maki, K.; Aikawa, S.; Hayasaka, K. Neonatal Hyperbilirubinemia and a Common Mutation of the Bilirubin Uridine Diphosphate-Glucuronosyltransferase Gene in Japanese. J. Hum. Genet. 1999, 44, 22–25. [Google Scholar] [CrossRef]
- Sato, H.; Uchida, T.; Toyota, K.; Kanno, M.; Hashimoto, T.; Watanabe, M.; Nakamura, T.; Tamiya, G.; Aoki, K.; Hayasaka, K. Association of Breast-Fed Neonatal Hyperbilirubinemia With UGT1A1 Polymorphisms: 211G>A (G71R) Mutation Becomes a Risk Factor Under Inadequate Feeding. J. Hum. Genet. 2013, 58, 7–10. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, S.N.; Li, H.; Zha, W.; Peng, Q.; Li, S.; Chen, Y.; Jin, L. Quantitative Trait Analysis of Polymorphisms in Two Bilirubin Metabolism Enzymes to Physiologic Bilirubin Levels in Chinese Newborns. J. Pediatr. 2014, 165, 1154–1160.e1. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.K.; Kumar, P.; Rathi, R.; Sharma, N.; Das, R.; Prasad, R.; Narang, A. UGT1A1 Gene Polymorphisms in North Indian Neonates Presenting With Unconjugated Hyperbilirubinemia. Pediatr. Res. 2009, 65, 675–680. [Google Scholar] [CrossRef] [Green Version]
- Ergin, H.; Bican, M.; Atalay, O.E. A Causal Relationship Between UDP-Glucuronosyltransferase 1A1 Promoter Polymorphism and Idiopathic Hyperbilirubinemia in Turkish Newborns. Turk. J. Pediatr. 2010, 52, 28–34. [Google Scholar] [PubMed]
- Newman, T.B.; Easterling, M.J.; Goldman, E.S.; Stevenson, D.K. Laboratory Evaluation of Jaundice in Newborns. Frequency, Cost, and Yield. Am. J. Dis. Child. 1990, 144, 364–368. [Google Scholar] [CrossRef]
- Carmel, R.; Wong, E.T.; Weiner, J.M.; Johnson, C.S. Racial Differences in Serum Total Bilirubin Levels in Health and in Disease (Pernicious Anemia). JAMA 1985, 253, 3416–3418. [Google Scholar] [CrossRef] [PubMed]
- Ho, N.K. Neonatal Jaundice in Asia. Baillieres Clin. Haematol. 1992, 5, 131–142. [Google Scholar] [CrossRef]
- Tsao, P.C.; Yeh, H.L.; Chang, Y.C.; Chiang, P.H.; Shiau, Y.S.; Chiang, S.H.; Soong, W.J.; Jeng, M.J.; Hsiao, K.J. Outcomes of Neonatal Jaundice in Taiwan. Arch. Dis. Child. 2018, 103, 927–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkén, J.; Håkansson, S.; Ekéus, C.; Gustafson, P.; Norman, M. Rates of Extreme Neonatal Hyperbilirubinemia and Kernicterus in Children and Adherence to National Guidelines for Screening, Diagnosis, and Treatment in Sweden. JAMA Netw. Open 2019, 2, e190858. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.K.; Daito, Y.; Katayama, Y.; Minami, H.; Negishi, H. The Significance of Measurement of Serum Unbound Bilirubin Concentrations in High-Risk Infants. Pediatr. Int. 2009, 51, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Lee, Y. Microdetermination of Unbound Bilirubin in Icteric Newborn Sera: An Enzymatic Method Employing Peroxidase and Glucose Oxidase. Clin. Chim. Acta 1977, 79, 411–417. [Google Scholar] [PubMed]
- Shimabuku, R.; Nakamura, H. Total and Unbound Bilirubin Determination Using an Automated Peroxidase Micromethod. Kobe J. Med. Sci. 1982, 28, 91–104. [Google Scholar] [PubMed]
- Yokota, T.; Morioka, I.; Kodera, T.; Morisawa, T.; Sato, I.; Kawano, S.; Koda, T.; Matsuo, K.; Fujioka, K.; Morikawa, S.; et al. Novel Treatment Strategy for Japanese Newborns With High Serum Unbound Bilirubin. Pediatr. Int. 2013, 55, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Kringen, M.K.; Piehler, A.P.; Grimholt, R.M.; Opdal, M.S.; Haug, K.B.; Urdal, P. Serum Bilirubin Concentration in Healthy Adult North-Europeans Is Strictly Controlled by the UGT1A1 TA-Repeat Variants. PLoS ONE 2014, 9, e90248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebbesen, F.; Knudsen, A. The Possible Risk of Bilirubin Encephalopathy as Predicted by Plasma Parameters in Neonates With Previous Severe Asphyxia. Eur. J. Pediatr. 1992, 151, 910–912. [Google Scholar] [CrossRef] [PubMed]
- Tadaka, S.; Hishinuma, E.; Komaki, S.; Motoike, I.N.; Kawashima, J.; Saigusa, D.; Inoue, J.; Takayama, J.; Okamura, Y.; Aoki, Y.; et al. jMorp Updates in 2020: Large Enhancement of Multi-omics Data Resources on the General Japanese Population. Nucleic Acids Res. 2021, 49, D536–D544. [Google Scholar] [CrossRef] [PubMed]
- Akaba, K.; Kimura, T.; Sasaki, A.; Tanabe, S.; Ikegami, T.; Hashimoto, M.; Umeda, H.; Yoshida, H.; Umetsu, K.; Chiba, H.; et al. Neonatal Hyperbilirubinemia and Mutation of the Bilirubin Uridine Diphosphate-Glucuronosyltransferase Gene: A Common Missense Mutation Among Japanese, Koreans and Chinese. Biochem. Mol. Biol. Int. 1998, 46, 21–26. [Google Scholar] [CrossRef]
- Amin, S.B. Bilirubin Binding Capacity in the Preterm Neonate. Clin. Perinatol. 2016, 43, 241–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morioka, I.; Iwatani, S.; Koda, T.; Iijima, K.; Nakamura, H. Disorders of Bilirubin Binding to Albumin and Bilirubin-Induced Neurologic Dysfunction. Semin. Fetal Neonatal Med. 2015, 20, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Koiwai, O.; Nishizawa, M.; Hasada, K.; Aono, S.; Adachi, Y.; Mamiya, N.; Sato, H. Gilbert’s Syndrome Is Caused by a Heterozygous Missense Mutation in the Gene for Bilirubin UDP-Glucuronosyltransferase. Hum. Mol. Genet. 1995, 4, 1183–1186. [Google Scholar] [CrossRef]
- Long, J.; Zhang, S.; Fang, X.; Luo, Y.; Liu, J. Association of Neonatal Hyperbilirubinemia With Uridine Diphosphate-Glucuronosyltransferase 1A1 Gene Polymorphisms: Meta-analysis. Pediatr. Int. 2011, 53, 530–540. [Google Scholar] [CrossRef]
- Wei, N.; Chen, Z.; Xue, Z.; Zhu, Y. Polymorphism of VEGF Gene in Susceptibility to Chronic Immune-Mediated Inflammatory Diseases: A Meta-analysis. Rheumatol. Int. 2015, 35, 1351–1360. [Google Scholar] [CrossRef]
- Wang, W.; Ma, H.; Lu, L.; Sun, G.; Liu, D.; Zhou, Y.; Tong, Y.; Lu, Z. Association Between Thrombin-Activatable Fibrinolysis Inhibitor Gene Polymorphisms and Venous Thrombosis Risk: A Meta-analysis. Blood Coagul. Fibrinolysis 2016, 27, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Wang, J.; Yao, L.; Yu, X.J.; Yu, L.; Yu, L. Relationship Between XRCC3 T241M Polymorphism and Gastric Cancer Risk: A Meta-analysis. Med. Oncol. 2011, 28, 999–1003. [Google Scholar] [CrossRef]
- Johnson, A.D.; Kavousi, M.; Smith, A.V.; Chen, M.H.; Dehghan, A.; Aspelund, T.; Lin, J.P.; van Duijn, C.M.; Harris, T.B.; Cupples, L.A.; et al. Genome-Wide Association Meta-analysis for Total Serum Bilirubin Levels. Hum. Mol. Genet. 2009, 18, 2700–2710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanna, S.; Busonero, F.; Maschio, A.; McArdle, P.F.; Usala, G.; Dei, M.; Lai, S.; Mulas, A.; Piras, M.G.; Perseu, L.; et al. Common Variants in the SLCO1B3 Locus Are Associated With Bilirubin Levels and Unconjugated Hyperbilirubinemia. Hum. Mol. Genet. 2009, 18, 2711–2718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morioka, I.; Nakamura, H. Treatment Criteria for Infants With Hyperbilirubinemia in Japan. Semin. Perinatol. 2021, 45, 151352. [Google Scholar] [CrossRef]
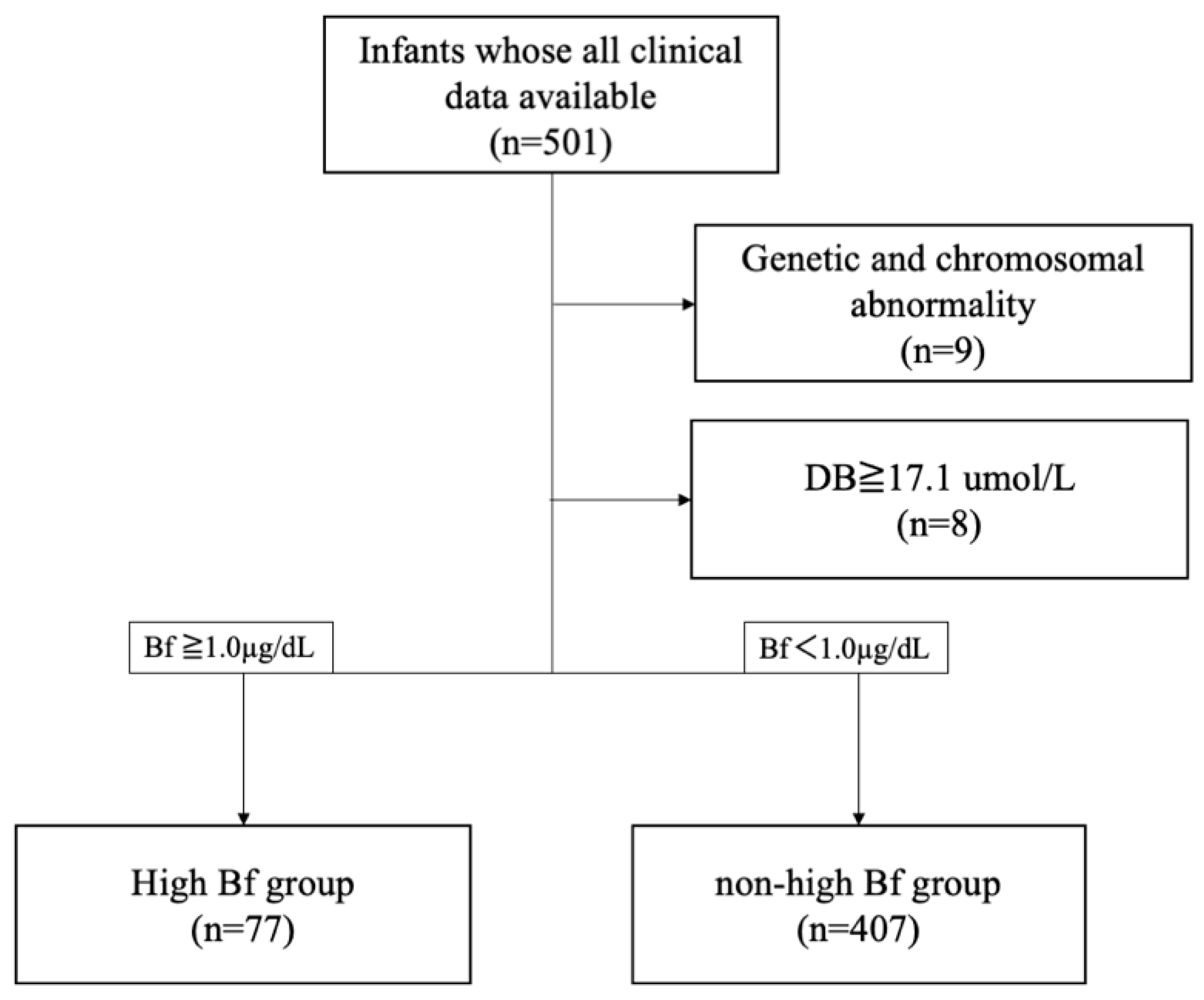
| High Bf (n = 77) | Non-High Bf (n = 407) | p-Value | |
|---|---|---|---|
| Male | 50 (64.9%) | 226 (55.5%) | 0.134 |
| Cesarean section | 29 (37.7%) | 258 (63.4%) | <0.001 |
| Birth weight (g) | 2622 (320, 4284) | 2076 (1036, 4732) | <0.001 |
| Gestational week (weeks) | 36 (22, 41) | 34 (26, 41) | 0.008 |
| Eextremely preterm infants (less than 28 gestational weeks) | 2 (2.6%) | 47 (11.5%) | 0.013 |
| Apgar score at 1 min < 7 | 10 (13.0%) | 105 (25.8%) | 0.019 |
| Apgar score at 5 min < 7 | 2 (2.6%) | 28 (6.9%) | 0.200 |
| Highest Bf (µg/dL) | 1.04 (1.00, 1.90) | 0.57 (0.02, 0.99) | <0.001 |
| Age at the highest Bf (day) | 4 (1, 39) | 3 (1, 39) | 0.086 |
| T-bil at the highest Bf (μmol/L) | 289 (181.3, 427.5) | 188.1 (35.9, 350.6) | <0.001 |
| D-bil at the highest Bf (μmol/L) | 5.1 (1.7, 5.1) | 3.4 (1.7, 5.1) | <0.001 |
| Alb at the highest Bf (g/dL) | 3.2 (2.4, 4.2) | 3.4 (1.9, 4.6) | 0.006 |
| High Bf | Non-High Bf | p-Value | |
|---|---|---|---|
| rs4148323 | |||
| GG | 42 (54.5%) | 287 (70.5%) | |
| GA | 25 (32.5%) | 108 (26.5%) | |
| AA | 10 (13.0%) | 12 (2.95%) | <0.001 |
| G allele | 109 (70.8%) | 682 (83.8%) | |
| A allele | 45 (29.2%) | 132 (16.2%) | <0.001 |
| rs3064744 | |||
| (TA)6/(TA)6 | 70 (90.9%) | 314 (77.1%) | |
| (TA)6/(TA)7 | 7 (9.1%) | 88 (21.6%) | |
| (TA)7/(TA)7 | 0 (0%) | 5 (1.2%) | 0.017 |
| (TA)6 allele | 147 (95.5%) | 716 (88.0%) | |
| (TA)7 allele | 7 (4.5%) | 98 (12.0%) | 0.044 |
| Factor | High Bf (n = 77) | Non-High Bf (n = 407) | OR (95% CI) | p | Adjusted OR (95% CI) | p |
|---|---|---|---|---|---|---|
| rs4148323 | ||||||
| GG | 42 (54.5%) | 287 (70.5%) | 1 | 1 | ||
| GA | 25 (32.5%) | 108 (26.5%) | 1.58 (0.92–2.72) | 0.098 | 1.29 (0.725–2.3) | 0.385 |
| AA | 10 (13.0%) | 12 (2.95%) | 5.69 (2.32–14.0) | <0.001 | 5.15 (1.95–13.6) | <0.001 |
| GA and AA | 35 (45.5%) | 120 (29.5%) | 1.99 (1.21–3.27) | 0.006 | 1.66 (0.980–2.82) | 0.060 |
| G | 109 (70.8%) | 682 (83.8%) | 1 | 1 | ||
| A | 45 (29.2%) | 132 (16.2%) | 2.02 (1.38–2.98) | 0.034 | 1.80 (1.19–2.72) | 0.005 |
| rs3064744 | ||||||
| (TA)6(TA)6 | 70 (90.9%) | 314 (77.1%) | 1 | 1 | ||
| (TA)6(TA)7 | 7 (9.1%) | 88 (21.6%) | 0.357 (0.158–0.804) | 0.013 | 0.446 (0.192–1.04) | 0.061 |
| (TA)7(TA)7 | 0 (0%) | 5 (1.2%) | N.D | N.D | ||
| (TA)6(TA)7 and (TA)7(TA)7 | 7 (9.1%) | 96 (22.9%) | 0.338 (0.150–0.759) | 0.009 | 0.426 (0183–0.989) | 0.047 |
| (TA)6 | 147 (95.5%) | 716 (88.0%) | 1 | 1 | ||
| (TA)7 | 7 (4.5%) | 98 (12.0%) | 0.342 (0.155–0.757) | 0.008 | 0.417 (0.183–0.952) | 0.038 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanafusa, H.; Abe, S.; Ohyama, S.; Kyono, Y.; Kido, T.; Nakasone, R.; Ashina, M.; Tanimura, K.; Nozu, K.; Fujioka, K. Influence of UGT1A1 Genetic Variants on Free Bilirubin Levels in Japanese Newborns: A Preliminary Study. Int. J. Environ. Res. Public Health 2022, 19, 13090. https://doi.org/10.3390/ijerph192013090
Hanafusa H, Abe S, Ohyama S, Kyono Y, Kido T, Nakasone R, Ashina M, Tanimura K, Nozu K, Fujioka K. Influence of UGT1A1 Genetic Variants on Free Bilirubin Levels in Japanese Newborns: A Preliminary Study. International Journal of Environmental Research and Public Health. 2022; 19(20):13090. https://doi.org/10.3390/ijerph192013090
Chicago/Turabian StyleHanafusa, Hiroaki, Shinya Abe, Shohei Ohyama, Yuki Kyono, Takumi Kido, Ruka Nakasone, Mariko Ashina, Kenji Tanimura, Kandai Nozu, and Kazumichi Fujioka. 2022. "Influence of UGT1A1 Genetic Variants on Free Bilirubin Levels in Japanese Newborns: A Preliminary Study" International Journal of Environmental Research and Public Health 19, no. 20: 13090. https://doi.org/10.3390/ijerph192013090





