Disease Progression, Clinical Features, and Risk Factors for Pneumonia in Unvaccinated Children and Adolescents with Measles: A Re-Emerging Disease in Romania
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics
2.2. Patient Inclusion and Variables
2.3. Statistical Analysis
3. Results
3.1. Background Analysis
3.2. Clinical Profile and Biological Parameters
3.3. Risk Factor Analysis
4. Discussion
4.1. Current Findings and Existing Evidence
4.2. Limitations and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krupka, M.; Matusu, T.; Sutova, H.; Wezdenkova, K.; Vecerova, R.; Smesna, Y.; Kolar, M.; Frankova, H.B.; Krivankova, J.; Jorenek, M.; et al. Seroprevalence of Measles Antibodies in the Population of the Olomouc Region, Czech Republic—Comparison of the Results of Four Laboratories. Vaccines 2022, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Misin, A.; Antonello, R.M.; Di Bella, S.; Campisciano, G.; Zanotta, N.; Giacobbe, D.R.; Comar, M.; Luzzati, R. Measles: An Overview of a Re-Emerging Disease in Children and Immunocompromised Patients. Microorganisms 2020, 8, 276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalziel, B.D.; Bjørnstad, O.N.; van Panhuis, W.G.; Burke, D.S.; Metcalf, C.J.; Grenfell, B.T. Persistent Chaos of Measles Epidemics in the Prevaccination United States Caused by a Small Change in Seasonal Transmission Patterns. PLoS Comput Biol. 2016, 12, e1004655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, M.J.; Grais, R.F.; Bharti, N.; Conlan, A.J.; Bjornstad, O.N.; Wolfson, L.J.; Guerin, P.J.; Djibo, A.; Grenfell, B.T. The dynamics of measles in sub-saharan Africa. Nature 2008, 451, 679–684. [Google Scholar] [CrossRef] [Green Version]
- Kamada, M.; Kenzaka, T. A Case of Rubella Caused by Rubella Vaccination. Vaccines 2021, 9, 1040. [Google Scholar] [CrossRef]
- Laksono, B.M.; De Vries, R.D.; McQuaid, S.; Duprex, W.P.; De Swart, R.L. Measles Virus Host Invasion and Pathogenesis. Viruses 2016, 8, 210. [Google Scholar] [CrossRef] [Green Version]
- López-Perea, N.; Fernández-García, A.; Echevarría, J.E.; de Ory, F.; Pérez-Olmeda, M.; Masa-Calles, J. Measles in Vaccinated People: Epidemiology and Challenges in Surveillance and Diagnosis in the Post-Elimination Phase. Spain, 2014–2020. Viruses 2021, 13, 1982. [Google Scholar] [CrossRef]
- Ferren, M.; Horvat, B.; Mathieu, C. Measles Encephalitis: Towards New Therapeutics. Viruses 2019, 11, 1017. [Google Scholar] [CrossRef] [Green Version]
- Yano, H.; Suetake, M.; Endo, H.; Takayanagi, R.; Numata, M.; Ohyama, K.; Sagai, S.; Okitsu, N.; Okamoto, M.; Nishimura, H.; et al. Isolation of measles virus from middle ear fluid of infants with acute otitis media. J Infect. 2005, 51, e237–e240. [Google Scholar] [CrossRef]
- Kasundriya, S.K.; Dhaneria, M.; Mathur, A.; Pathak, A. Incidence and Risk Factors for Severe Pneumonia in Children Hospitalized with Pneumonia in Ujjain, India. Int. J. Environ. Res. Public Health 2020, 17, 4637. [Google Scholar] [CrossRef]
- Manolescu, D.; Timar, B.; Bratosin, F.; Rosca, O.; Citu, C.; Oancea, C. Predictors for COVID-19 Complete Remission with HRCT Pattern Evolution: A Monocentric, Prospective Study. Diagnostics 2022, 12, 1397. [Google Scholar] [CrossRef]
- Bogdan, I.; Citu, C.; Bratosin, F.; Malita, D.; Romosan, I.; Gurban, C.V.; Bota, A.V.; Turaiche, M.; Bratu, M.L.; Pilut, C.N.; et al. The Impact of Multiplex PCR in Diagnosing and Managing Bacterial Infections in COVID-19 Patients Self-Medicated with Antibiotics. Antibiotics 2022, 11, 437. [Google Scholar] [CrossRef]
- Storr, C.; Sanftenberg, L.; Schelling, J.; Heininger, U.; Schneider, A. Measles Status-Barriers to Vaccination and Strategies for Overcoming Them. Dtsch Arztebl Int. 2018, 115, 723–730. [Google Scholar] [CrossRef]
- Griffith, B.C.; Ulrich, A.K.; Becker, A.B.; Nederhoff, D.; Koch, B.; Awan, F.A.; Basta, N.E. Does education about local vaccination rates and the importance of herd immunity change US parents’ concern about measles? Vaccine 2020, 38, 8040–8048. [Google Scholar] [CrossRef]
- Hao, L.; Glasser, J.W.; Su, Q.; Ma, C.; Feng, Z.; Yin, Z.; Goodson, J.L.; Wen, N.; Fan, C.; Yang, H.; et al. Evaluating vaccination policies to accelerate measles elimination in China: A meta-population modelling study. Int. J. Epidemiol. 2019, 48, 1240–1251. [Google Scholar] [CrossRef]
- Local Burden of Disease Vaccine Coverage Collaborators. Mapping routine measles vaccination in low- and middle-income countries. Nature 2021, 589, 415–419. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Number of Measles Cases by Month and Notification Rate per Million Population by Country, January 2020–December 2020. 2021. Available online: https://www.ecdc.europa.eu/en/publications-data/number-measles-cases-month-and-notification-rate-million-population-country-27 (accessed on 5 July 2022).
- Donadel, M.; Stanescu, A.; Pistol, A.; Stewart, B.; Butu, C.; Jankovic, D.; Paunescu, B.; Zimmerman, L. Risk factors for measles deaths among children during a Nationwide measles outbreak-Romania, 2016–2018. BMC Infect. Dis. 2021, 21, 279. [Google Scholar] [CrossRef]
- Plans-Rubió, P. Vaccination Coverage for Routine Vaccines and Herd Immunity Levels against Measles and Pertussis in the World in 2019. Vaccines 2021, 9, 256. [Google Scholar] [CrossRef]
- Davitoiu, A.M.; Spatariu, L.; Plesca, D.A.; Dimitriu, M.; Cirstoveanu, C.G.; Chindris, S. Review of the measles epidemic in children from Central Eastern Europe in the third millennium. Exp. Ther. Med. 2021, 22, 816. [Google Scholar] [CrossRef]
- Centers for Disease Control. Classification of measles cases and categorization of measles elimination program. Morb. Mortal. Wkly. Rep. 1983, 31, 707–711. [Google Scholar]
- Sawyer, S.M.; Azzopardi, P.S.; Wickremarathne, D.; Patton, G.C. The age of adolescence. Lancet Child. Adolesc. Health 2018, 2, 223–228. [Google Scholar] [CrossRef]
- Broască, L.; Trușculescu, A.A.; Ancușa, V.M.; Ciocârlie, H.; Oancea, C.-I.; Stoicescu, E.-R.; Manolescu, D.L. A Novel Method for Lung Image Processing Using Complex Networks. Tomography 2022, 8, 1928–1946. [Google Scholar] [CrossRef]
- Malinverni, S.; Lazzaroni, S.; Nuňez, M.; Preseau, T.; Cotton, F.; Martiny, D.; Bouazza, F.; Collot, V.; Konopnicki, D.; Alard, S.; et al. Diagnostic Accuracy of Procalcitonin upon Emergency Department Admission during SARS-CoV-2 Pandemic. Antibiotics 2022, 11, 1141. [Google Scholar] [CrossRef]
- Faneye, A.O.; Adeniji, J.A.; Olusola, B.A.; Motayo, B.O.; Akintunde, G.B. Measles Virus Infection Among Vaccinated and Unvaccinated Children in Nigeria. Viral Immunol. 2015, 28, 304–308. [Google Scholar] [CrossRef] [Green Version]
- Stoicescu, E.R.; Manolescu, D.L.; Iacob, R.; Cerbu, S.; Dima, M.; Iacob, E.R.; Ciuca, I.M.; Oancea, C.; Iacob, D. The Assessment of COVID-19 Pneumonia in Neonates: Observed by Lung Ultrasound Technique and Correlated with Biomarkers and Symptoms. J. Clin. Med. 2022, 11, 3555. [Google Scholar] [CrossRef]
- Human Development Index (HDI). Human Development Reports. United Nations Development Programme. Available online: https://hdr.undp.org (accessed on 15 March 2022).
- Horhat, R.M.; Vlaicu, B.; Bagiu, R.; Putnoky, S.; Bagiu, I.; Horhat, D.I.; Szuhanek, C.; Sinescu, C.; Negrutiu, M.L.; Nica, L. A Ten-year Time Laps, Regarding Drug Consumption in the Western Part of Romania. Rev. Chim. 2018, 69, 1371–1375. [Google Scholar] [CrossRef]
- Ashbaugh, H.R.; Cherry, J.D.; Hoff, N.A.; Doshi, R.H.; Alfonso, V.H.; Gadoth, A.; Mukadi, P.; Higgins, S.G.; Budd, R.; Randall, C.; et al. Association of Previous Measles Infection With Markers of Acute Infectious Disease Among 9- to 59-Month-Old Children in the Democratic Republic of the Congo. J. Pediatr. Infect. Dis. Soc. 2019, 8, 531–538. [Google Scholar] [CrossRef]
- Foolchand, A.; Ghazi, T.; Chuturgoon, A.A. Malnutrition and Dietary Habits Alter the Immune System Which May Consequently Influence SARS-CoV-2 Virulence: A Review. Int. J. Mol. Sci. 2022, 23, 2654. [Google Scholar] [CrossRef]
- Arima, Y.; Oishi, K. Letter to the editor: Measles cases among fully vaccinated persons. Euro Surveill. 2018, 23, 1800449. [Google Scholar] [CrossRef]
- Portnoy, A.; Jit, M.; Ferrari, M.; Hanson, M.; Brenzel, L.; Verguet, S. Estimates of case-fatality ratios of measles in low-income and middle-income countries: A systematic review and modelling analysis. Lancet Glob. Health 2019, 7, e472–e481. [Google Scholar] [CrossRef] [Green Version]
- Dimala, C.A.; Kadia, B.M.; Nji, M.A.M.; Bechem, N.N. Factors associated with measles resurgence in the United States in the post-elimination era. Sci Rep 2021, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Leong, W.Y.; Wilder-Smith, A.B. Measles Resurgence in Europe: Migrants and Travellers are not the Main Drivers. J. Epidemiol. Glob. Health 2019, 9, 294–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rota, P.A.; Moss, W.J.; Takeda, M.; De Swart, R.; Thompson, K.; Goodson, J.L. Measles. Nat. Rev. Dis. Prim. 2016, 2, 16049. [Google Scholar] [CrossRef] [PubMed]
- Aborode, A.T.; Babatunde, A.O.; Osayomwanbor, B.-A.S.; Fajemisin, E.A.; Inya, O.C.; Olajiga, O.; Uwandu-Uzoma, A.C. Measles outbreak amidst COVID-19 pandemic in Africa: Grappling with looming crises. Trop. Med. Health 2021, 49, 89. [Google Scholar] [CrossRef]
- Semba, R.D.; Bloem, M.W. Measles blindness. Surv. Ophthalmol. 2004, 49, 243–255. [Google Scholar] [CrossRef]
- Gilbert, C.; Bowman, R.; Malik, A.N. The epidemiology of blindness in children: Changing priorities. Community Eye Health 2017, 30, 74–77. [Google Scholar]
- Chovatiya, R.; Silverberg, J.I. Inpatient morbidity and mortality of measles in the United States. PLoS ONE 2020, 15, e0231329. [Google Scholar] [CrossRef]
- Kuehn, B. US Narrowly Preserves Measles Elimination Status. JAMA 2019, 322, 1949. [Google Scholar] [CrossRef]
- Zhou, F.; Reef, S.; Massoudi, M.; Papania, M.J.; Yusuf, H.R.; Bardenheier, B.; Zimmerman, L.; McCauley, M.M. An economic analysis of the current universal 2-dose mea-sles-mumps-rubella vaccination program in the United States. J. Infect. Dis. 2004, 189 (Suppl. S1), S131–S145. [Google Scholar] [CrossRef] [PubMed]
- Kohlmaier, B.; Holzmann, H.; Stiasny, K.; Leitner, M.; Zurl, C.; Strenger, V.; Kundi, M.; Zenz, W. Effectiveness and Safety of an Intravenous Immune Globulin (IVIG) Preparation in Post-exposure Prophylaxis (PEP) Against Measles in Infants. Front. Pediatr. 2021, 9, 762793. [Google Scholar] [CrossRef]
- McLean, H.Q.; Fiebelkorn, A.P.; Temte, J.L.; Wallace, G.S.; Centers for Disease Control and Prevention. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: Summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2013, 62, 1–34, Erratum in MMWR Recomm. Rep. 2015, 64, 259. [Google Scholar]
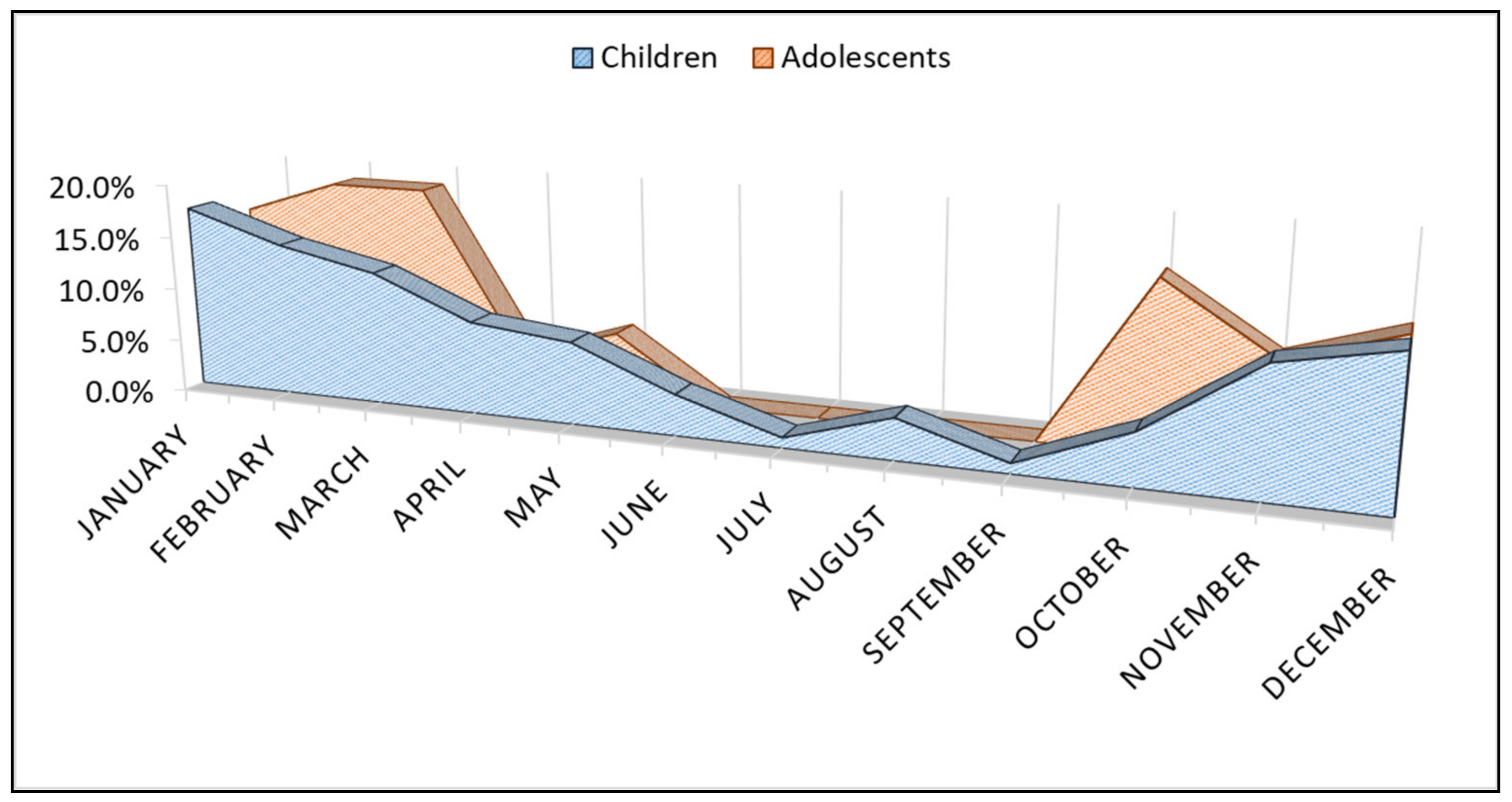


| Variables | Children (n = 104) | Adolescents (n = 32) | p-Value * |
|---|---|---|---|
| Background | |||
| Age (mean ± SD) | 2.4 ± 2.1 | 12.5 ± 2.2 | <0.001 |
| Sex—female | 52 (50.0%) | 15 (46.9%) | 0.757 |
| Born premature—yes | 17 (16.3%) | 3 (9.4%) | 0.330 |
| Place of origin—urban | 47 (45.2%) | 14 (43.8%) | 0.885 |
| Ethnicity—Roma | 44 (42.3%) | 13 (40.6%) | 0.866 |
| Infection source | 0.047 | ||
| Family | 42 (40.4%) | 7 (21.9%) | |
| Collective | 1 (1.0%) | 2 (6.3%) | |
| Isolated case | 61 (58.7%) | 23 (71.9%) | |
| Vaccination status | 0.530 | ||
| Unvaccinated | 97 (93.3%) | 29 (90.6%) | |
| Incomplete | 3 (2.9%) | 3 (9.4%) | |
| Complete | 4 (3.8%) | 0 (0.0%) | |
| Nutritional status | 0.040 | ||
| Poor | 32 (30.8%) | 4 (12.5%) | |
| Good | 72 (69.2%) | 28 (87.5%) | |
| Months from last MMR dose (mean ± SD) | 9.6 ± 6.2 | 70.2 ± 29.4 | <0.001 |
| Congenital immune deficiency | 4 (3.8%) | 0 (0.0%) | 0.363 |
| Variables | Children (n = 104) | Adolescents (n = 32) | p-Value * |
|---|---|---|---|
| Signs and Symptoms | |||
| Koplik’s spots | 21 (20.2%) | 12 (37.5%) | 0.045 |
| Maculopapular rash | 94 (90.4%) | 27 (84.4%) | 0.342 |
| Hyperpigmented rash | 10 (9.6%) | 5 (15.6%) | 0.342 |
| Fever | 101 (97.1%) | 30 (93.8%) | 0.376 |
| Coryza | 50 (48.1%) | 16 (50.0%) | 0.849 |
| Cough | 101 (97.1%) | 30 (93.8%) | 0.376 |
| Diarrhea | 29 (27.9%) | 8 (25.0%) | 0.748 |
| Complications | |||
| Conjunctivitis | 31 (29.8%) | 8 (25.0%) | 0.598 |
| Otitis media | 12 (11.5%) | 0 (0.0%) | 0.044 |
| Upper respiratory tract infection | 27 (26.0%) | 11 (34.4%) | 0.353 |
| Pneumonia | 63 (60.6%) | 26 (81.3%) | 0.032 |
| Acute respiratory failure | 3 (2.9%) | 4 (12.5%) | 0.031 |
| Thrombocytopenia | 4 (3.8%) | 1 (3.1%) | 0.849 |
| Sepsis | 2 (1.9%) | 1 (3.1%) | 0.685 |
| Liver damage | 11 (10.6%) | 6 (18.8%) | 0.221 |
| Seizure | 3 (26.9%) | 0 (0.0%) | 0.331 |
| Anemia | 26 (25.0%) | 10 (31.3%) | 0.483 |
| Chest X-ray | |||
| Bilateral consolidation | 61 (58.7%) | 25 (78.1%) | 0.045 |
| Interstitial pattern | 28 (26.9%) | 14 (43.8%) | 0.071 |
| Antibiotic treatment | |||
| None | 11 (10.6%) | 8 (25.0%) | 0.039 |
| Cephalosporins | 71 (68.3%) | 16 (50.0%) | 0.059 |
| Clarithromycin | 5 (4.8%) | 2 (6.3%) | 0.746 |
| Penicillins | 7 (6.7%) | 2 (6.3%) | 0.923 |
| Others | 10 (9.6%) | 4 (12.5%) | 0.638 |
| Hospital stay—days (median [IQR]) | |||
| All patients | 7 (4–9) | 7 (4–11) | 0.881 |
| Patients with pneumonia | |||
| ICU admission | 4 (3.8%) | 1 (3.1%) | 0.849 |
| Mortality | 0 (0.0%) | 0 (0.0%) | - |
| Variables | Normal Range | Children (n = 104) | Normal Range | Adolescents (n = 32) | p-Value * |
|---|---|---|---|---|---|
| WBC (thousands/mm3) | 5.0–15.0 | 8.8 ± 4.5 | 4.5–11.0 | 5.2 ± 2.6 | <0.001 |
| Lymphocytes (thousands/mm3) | 3.0–9.5 | 5.9 ± 1.7 | 1.0–4.8 | 4.7 ± 1.2 | 0.003 |
| RBC (millions/mm3) | 4.20–6.10 | 3.9 ± 1.1 | 4.35–5.65 | 4.4 ± 1.2 | 0.040 |
| Hemoglobin (g/dL) | 11.0–13.5 | 10.8 ± 1.5 | 13.0–17.0 | 12.3 ± 1.5 | <0.001 |
| Platelets (thousands/mm3) | 150–350 | 312 ± 148 | 150–450 | 212 ± 122 | <0.001 |
| ALT (U/L) | 10–40 | 52.6 ± 33.1 | 7–35 | 55.0 ± 40.1 | 0.731 |
| AST (U/L) | 10–35 | 28.9 ± 19.1 | 10–40 | 42.9 ± 16.0 | 0.076 |
| LDH (U/L) | 100–250 | 251 ± 106 | 140–280 | 268 ± 114 | 0.099 |
| Procalcitonin (ug/L) | 0–0.5 | 0.5 ± 0.3 | 0–0.5 | 0.9 ± 0.6 | <0.001 |
| CRP (mg/L) | 0–14 | 29.7 ± 14.3 | 0–10 | 29.9 ± 15.0 | 0.989 |
| Fibrinogen (g/L) | 2–4 | 9.1 ± 4.3 | 2–4 | 8.9 ± 4.1 | 0.127 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turaiche, M.; Grigoras, M.L.; Bratosin, F.; Bogdan, I.; Bota, A.V.; Cerbu, B.; Gurban, C.V.; Wulandari, P.H.; Gurumurthy, S.; Hemaswini, K.; et al. Disease Progression, Clinical Features, and Risk Factors for Pneumonia in Unvaccinated Children and Adolescents with Measles: A Re-Emerging Disease in Romania. Int. J. Environ. Res. Public Health 2022, 19, 13165. https://doi.org/10.3390/ijerph192013165
Turaiche M, Grigoras ML, Bratosin F, Bogdan I, Bota AV, Cerbu B, Gurban CV, Wulandari PH, Gurumurthy S, Hemaswini K, et al. Disease Progression, Clinical Features, and Risk Factors for Pneumonia in Unvaccinated Children and Adolescents with Measles: A Re-Emerging Disease in Romania. International Journal of Environmental Research and Public Health. 2022; 19(20):13165. https://doi.org/10.3390/ijerph192013165
Chicago/Turabian StyleTuraiche, Mirela, Mirela Loredana Grigoras, Felix Bratosin, Iulia Bogdan, Adrian Vasile Bota, Bianca Cerbu, Camelia Vidita Gurban, Prima Hapsari Wulandari, Srivathsava Gurumurthy, Kakarla Hemaswini, and et al. 2022. "Disease Progression, Clinical Features, and Risk Factors for Pneumonia in Unvaccinated Children and Adolescents with Measles: A Re-Emerging Disease in Romania" International Journal of Environmental Research and Public Health 19, no. 20: 13165. https://doi.org/10.3390/ijerph192013165
APA StyleTuraiche, M., Grigoras, M. L., Bratosin, F., Bogdan, I., Bota, A. V., Cerbu, B., Gurban, C. V., Wulandari, P. H., Gurumurthy, S., Hemaswini, K., Citu, C., & Marincu, I. (2022). Disease Progression, Clinical Features, and Risk Factors for Pneumonia in Unvaccinated Children and Adolescents with Measles: A Re-Emerging Disease in Romania. International Journal of Environmental Research and Public Health, 19(20), 13165. https://doi.org/10.3390/ijerph192013165










